Introduction
Photodynamic therapy (PDT) is widely used in the
therapy of gliomas at a biological level and in the clinic.
PDT-mediated generation of molecular oxygen or reactive oxygen
species (ROS) is assumed to be the main mechanism of cell death
(1), and plays an important role in
killing glioma cells. However, PDT has certain disadvantages; for
example, its initial killing effect is not satisfactory. The
effective depth of PDT is limited, as lasers are unable to
penetrate and reach deep tissues to activate the
photosensitizer.
Ultrasound has an appropriate tissue attenuation
coefficient for penetrating and reaching deep-seated tissues while
maintaining the ability to focus energy onto a small volume. This
unique advantage makes it more useful for noninvasive treatment of
deep-seated tumors when compared with electromagnetic modalities,
such as laser beams (2–4). These techniques may be applied to the
treatment of numerous types of cancer. However, a study has
reported that the delayed killing effect is unsatisfactory in
sonodynamic therapy (SDT), and advise that an alternative
therapeutic method should be combined with PDT (5).
It is of note that certain sensitizers can be
activated photochemically as well as sonochemically (6–8), such
as porphyrin sonosensitizers (3,4), as
well as others (9–11). PDT combined with SDT (SPDT) is a new
cancer therapy. The theory of the therapy is that by activating
these congenerous sensitizers with light and sound, more
cytotoxicity is generated to kill tumor cells. A number of scholars
have reported the effects of killing tumor cells by SPDT; for
example, Kessel et al(12)
demonstrated that photodamage following exposure to ultra-sound
decreased the viability of murine leukemia L1210 cells which had
survived ultrasonic treatment. Jin et al(13) studied SPDT for improving tumoricidal
effects in a transplantable mouse squamous cell carcinoma (SCC)
model, and showed that combination therapy induced tumor necrosis
2–3 times as deep as with either of the single modalities,
concluding that SPDT may be useful in the treatment of
non-superficial or nodular tumors. Kolarova et al(14) demonstrated that more ROS were
generated using SPDT than using a monotherapy of PDT or SDT.
Nonetheless, research in this domain is rare, and to
date, the effects of SPDT and the biological mechanisms by which it
kills different tumor cell lines are undefined. Therefore, in the
present study, we investigated the mechanisms by which PDT combined
with SDT activates hematoporphyrin monomethyl ether (HMME) to kill
C6 rat gliomas cells. We also studied the interactions between the
two modalities.
Materials and methods
Cell cultivation
Rat glioma C6 cells were obtained from the
Neurosurgery Institution of Harbin Medical University, China. C6
cells were maintained as monolayers in Roswell Park Memorial
Institute (RPMI)-1640 medium (Hyclone Lab, Logan, UT, USA) at 37°C
with 5% CO2 in a humidified incubator (Nuaire, Plymouth,
MN, USA). Cells in the exponential phase of growth were used for
all the following experiments. The study was approved by the Ethics
Committee of Haerbin Medical University, Harbin, China.
Sonodynamic and photodynamic
treatment
All operations were carried out at 37°C.
Exponentially growing cells were collected by centrifugation,
resuspended (5×106 cells/ml) in serum-free RPMI-1640
medium and incubated with HMME for 4 h. In the experiments, a
multifunction physical therapy ultrasound device (Tianshi
Technologies Ltd. Co, Beijing, China) was used to generate
ultrasound at 1 MHz in cells in the presence of 10 μg/ml
HMME. Ultrasonic intensities (0.5 W/cm2) were measured
by a stainless steel ball radiometer (diameter, 0.32 cm) (15). Further details are provided in a
previous study by Li et al(16). Following this, cells were irradiated
with a certain dose of light. For the irradiation, the wavelength
was limited to 630 nm by an interference filter. The light was set
to 100 mW/cm2 for subsequent irradiation up to different
doses of light using a semiconductor diode laser (Diomed 630,
Andover, MA, USA).
The samples were divided into different groups. In
the control group, the cells were not treated with HMME, nor with
ultrasound or light irradiation. In the HMME group, the cells were
treated with HMME but not with ultrasound or light irradiation. In
the ultrasound and light irradiation groups, the cells were treated
with ultrasound or light irradiation alone. In the SDT group, the
cells were treated with both HMME (10 μg/ml) and ultrasound.
In the PDT group, the cells were treated with both HMME (10
μg/ml) and light irradiation. In the SPDT group, the cells
were treated with HMME (10 μg/ml) and sonication at an
intensity of 0.5 W/cm2, followed by exposure of 90 sec;
subsequently, cells were irradiated with different doses of laser
light.
Cell survival assay
After the treatment cells were washed, re-suspended
in RPMI-1640 medium and subjected to MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay. MTT (20 μl; 5 mg/ml) was added. Four hours later, 100
μl DMSO was added to each well to dissolve the resulting
formazan crystals. Absorbance was read at 490 nm using an
enzyme-linked immunosorbent assay reader (SpectraMax; Molecular
Devices, Sunnyvale, CA, USA). Survival rate was calculated as:
Inhibition rate (%) = (1 − ODtreatment group /
ODcontrol group) × 100, where OD indicates optical
density.
Examination of apoptosis or necrosis
Following treatment, cells were re-incubated for up
to 4 h in the dark and washed twice with phosphate-buffered saline
(PBS). After adjusting the cell density to 1×106
cells/ml, 100 μl cell suspension was transferred to a
culture tube and mixed with 5 μl Annexin V-FITC (fluorescein
isothiocyanate; BD, Franklin Lakes, NJ, USA) and 5 μl
propidium iodide (PI; BD). The mixture was gently vortexed and
incubated at room temperature (25°C) in the dark for 15 min. After
adding 400 μl binding buffer, the apoptotic rate was
analyzed using a flow cytometer (BD).
Western blot analysis of caspase 3, 8 and
9 activation
Following treatment, cells were re-incubated for up
to 4 h in the dark. To examine caspase 3, 8 and 9 activation, cells
of different groups were separately washed, collected and
homogenized in a lysis buffer (10 mM Tris-HCl, pH 8, 0.32 mM
sucrose, 5 mM EDTA, 2 mM DTT, 1 mM phenylmethyl sulfonylfluoride
and 1% Triton X-100) and then centrifuged. Proteins in different
groups were separately electrophoresed on SDS polyacrylamide gel
(12%), the gel-separated proteins were transferred to nitropure
nitrocellulose membranes (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), and the membranes were probed overnight at 4°C with
primary antibodies. Each of the targeted proteins was immunostained
by distinct antibodies. The antibodies presented were: anti-actin,
anti-cleavage caspase 3, 8 and 9 (Santa Cruz Biotechnology, Inc.).
After probing, the membranes were washed three times and then
incubated for 1 h at room temperature with the respective alkaline
phosphatase-conjugated secondary antibodies (Sigma, St. Louis, MO,
USA) before being visualized using a chemiluminescence detection
kit (Sigma).
Measurement of ROS generation
2’,7’-Dichlorofluorescein diacetate (DCFH-DA;
Beyotime Institute of Biotechnology, Shanghai, China) was used to
detect SPDT-mediated ROS production. DCFH-DA was added to the cell
suspension at a final concentration of 10 μmol/l and
incubated at 37°C in the dark for 30 min. Fluorescent
dichlorofluorescein generated from the oxidation of DCFH-DA was
measured by a fluorescence microplate reader (FLx800; BioTek,
Winooski, VT, USA).
Cell survival assay following addition of
different ROS scavengers
To confirm the involvement of ROS in SPDT, the above
procedures (SDT) were repeated in the presence of either 100
μg/ml final concentration of sodium azide (NaN3)
or 100 μg/ml final concentration of mannitol
(C6H14O6). Subsequently, cells
were subjected to an MTT assay.
Statistical analysis
Statistical evaluation was performed with a t-test
using analytical software tools from SPSS. Data are presented as
the mean values ± standard error of the mean (SE). P<0.05 was
considered to indicate a statistically significant result.
Results
Inhibition of cell growth with SPDT
compared with PDT or SDT
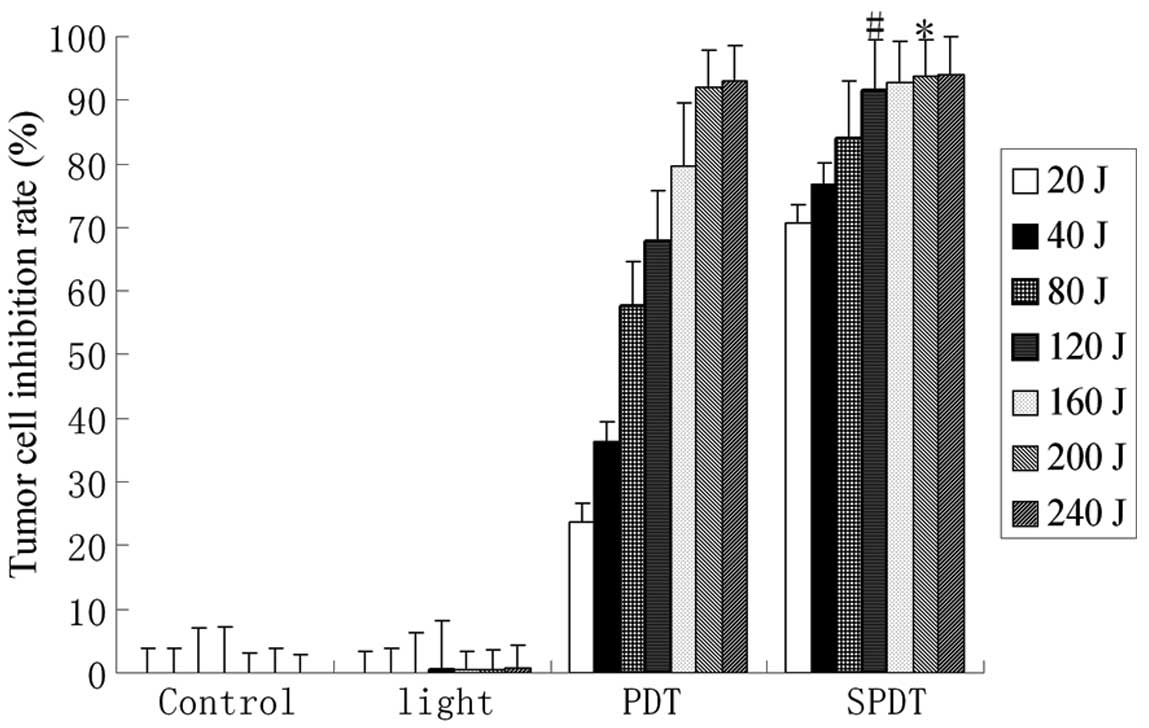
The growth inhibition rate of C6 glioma cells was
determined by MTT assay in SDT combined with different doses of
light irradiation (20–240 J/cm2). Although PDT or SDT
alone also inhibited cell growth, the inhibition rate in the SPDT
group was significantly higher than that in the SDT or PDT groups
at certain doses (light dose <200 J/cm2; P<0.05).
When the light dose was >200 J/cm2, the inhibition
rate was not significantly different from that in the PDT group
(P>0.05). However, in the SPDT groups, when the light dose was
>120 J/cm2, the inhibition rate was not significantly
increased with increasing light dose (P>0.05). Thus, our data
indicates that HMME-mediated SPDT induced a synergetic killing
effect on C6 cells at specific light doses (Figs. 1 and 2). A similar killing effect was achieved
by SPDT with lower doses of light compared with PDT alone with
higher doses of light.
SDT-induced apoptosis
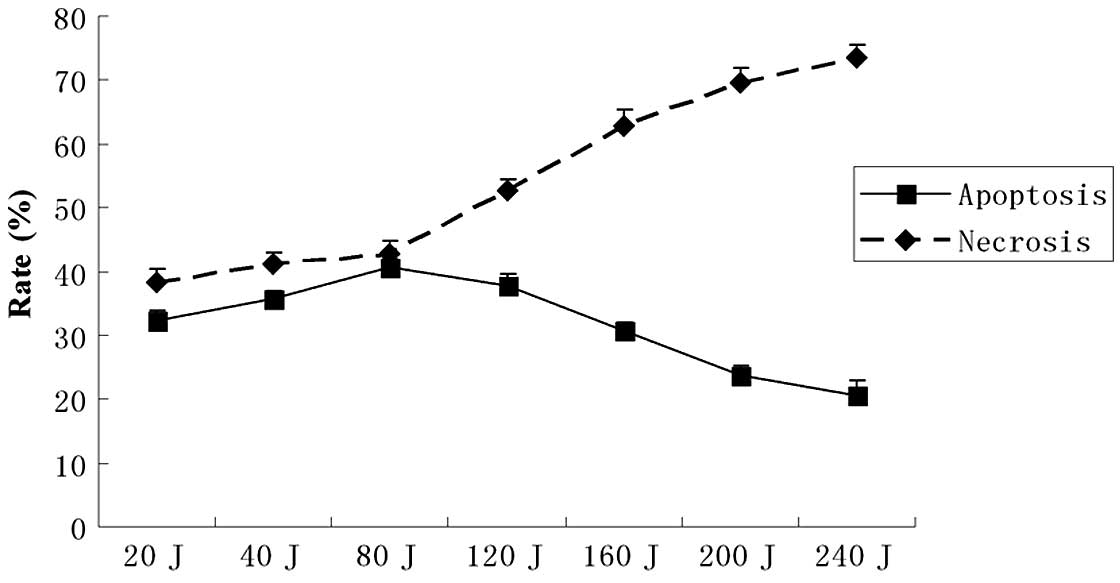
The flow cytometry assay showed marked changes in
the cell profile following SPDT. Using flow cytometry, we evaluated
the effect of combining SDT with different doses of light
irradiation (20–240 J/cm2) on apoptosis or necrosis in
C6 cells. In the SPDT group, the cell apoptosis rate rose with the
increasing light dose when the light irradiation dose was <80
J/cm2. Conversely, the cell apoptosis rate reduced as
the light dose increased when the light dose was >80
J/cm2; however, the necrosis rate increased notably when
the light dose was >80 J/cm2. The results suggested
that SPDT induced the highest level of cell apoptosis when SDT was
combined with a light irradiation dose of 80 J/cm2
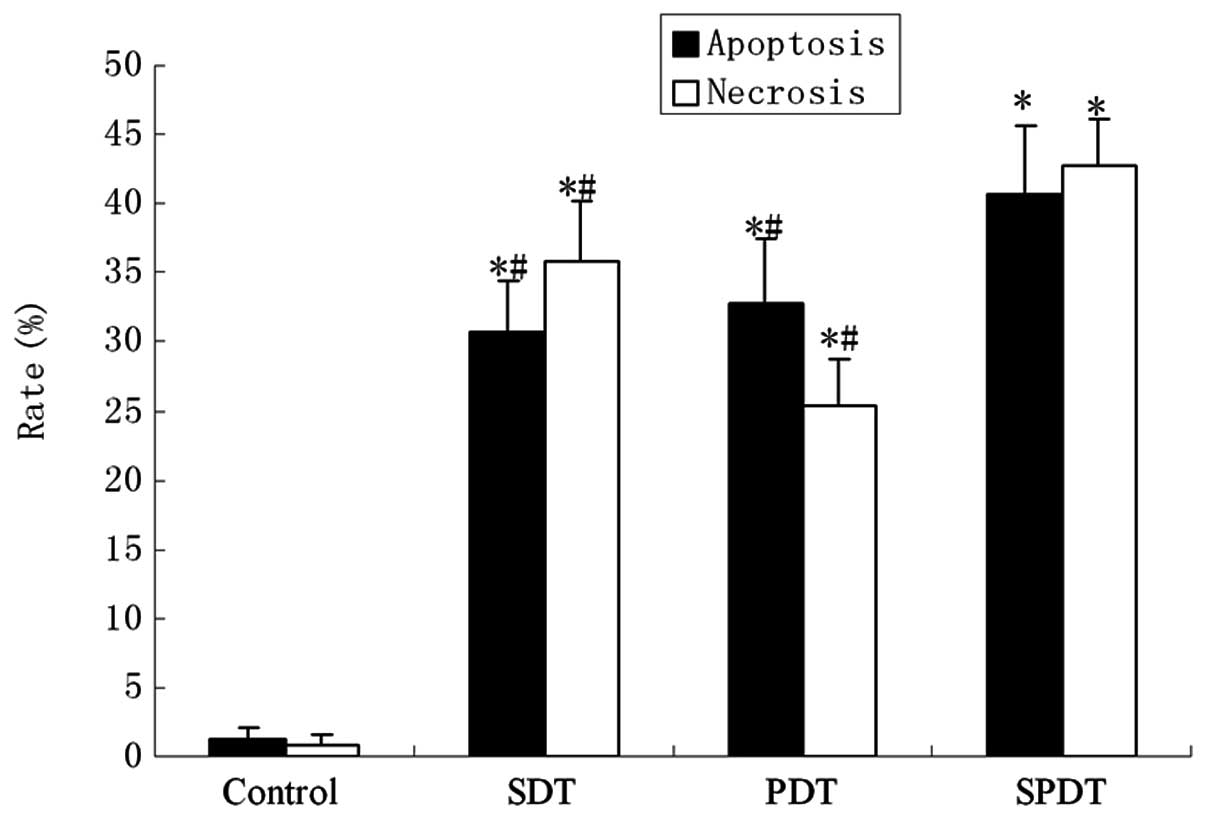
(Fig. 3). The apoptotic rate was
∼40.62±5.01% with a light irradiation dose of 80 J/cm2
in SPDT. The apoptosis rate of C6 glioma cells in the SPDT group
was the highest among all groups (P<0.05), while the SDT and PDT
groups were higher compared with the control (P<0.01), but lower
than the SPDT group (P<0.05). HMME-mediated SPDT also increased
the apoptotic rate of C6 cells in certain conditions (Fig. 4).
SPDT induces caspase 3, 8 and 9
activation
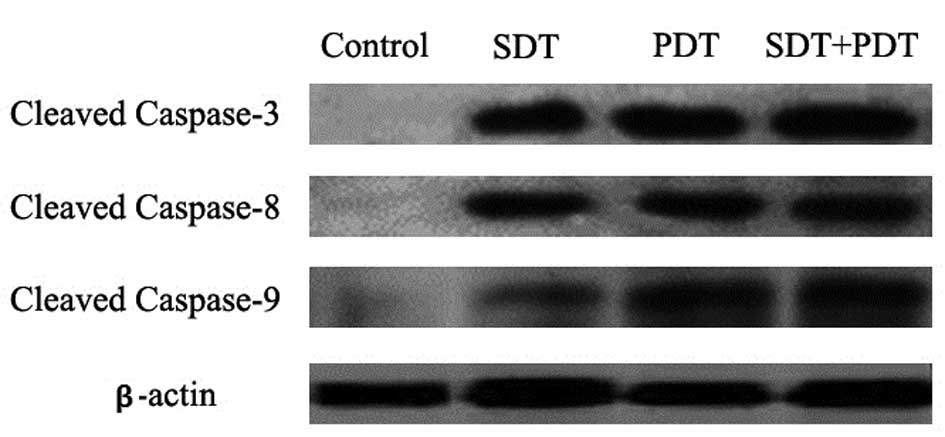
As shown by the western blot analysis of cytosolic
extracts, HMME-SPDT induced caspase 3, 8 and 9 cleavage with a
light irradiation dose of 80 J/cm2 in SPDT. Band
intensity quantitation measurements showed that the activation rate
of caspase 3, 8 and 9 was markedly higher than that in the SDT or
PDT groups alone (Fig. 5).
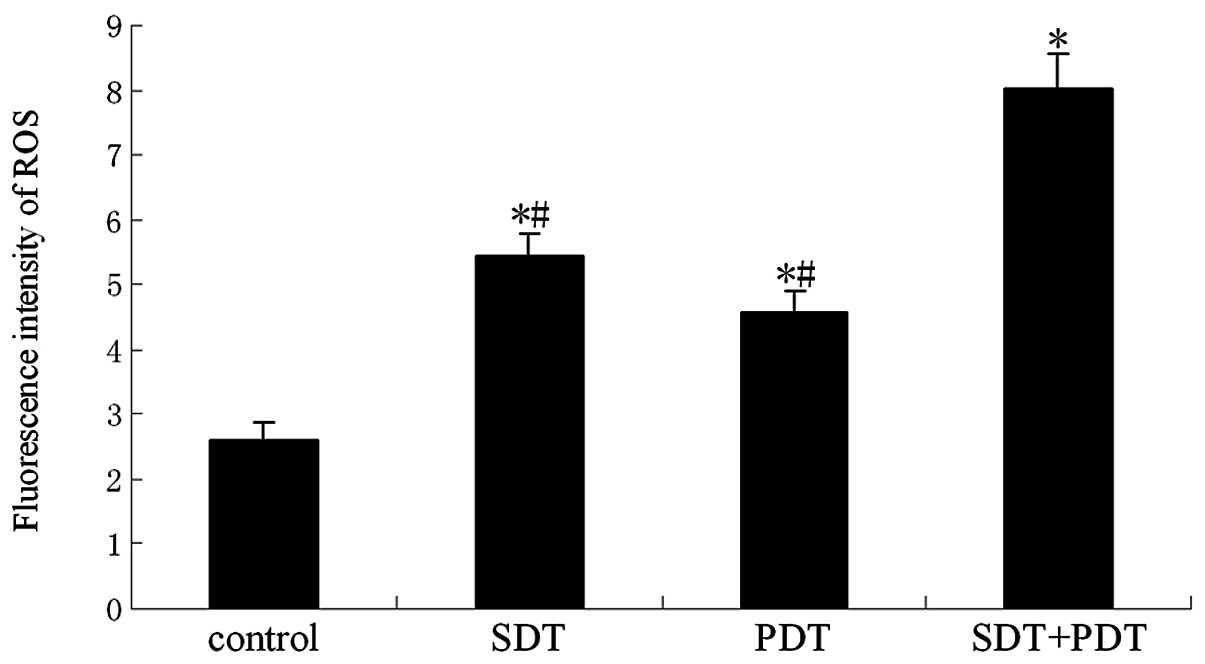
Effect of ROS on SDT-induced cell
killing
To demonstrate the effect of ROS in SPDT-induced
cell killing, ROS production was confirmed by the oxidation of
DCFH-DA. The results showed that the synergistic effect of SDT and
PDT generated more ROS than SDT or PDT alone at a light irradiation
dose of 80 J/cm2 in SPDT (P<0.01; Fig. 6). HMME-mediated SPDT killing of
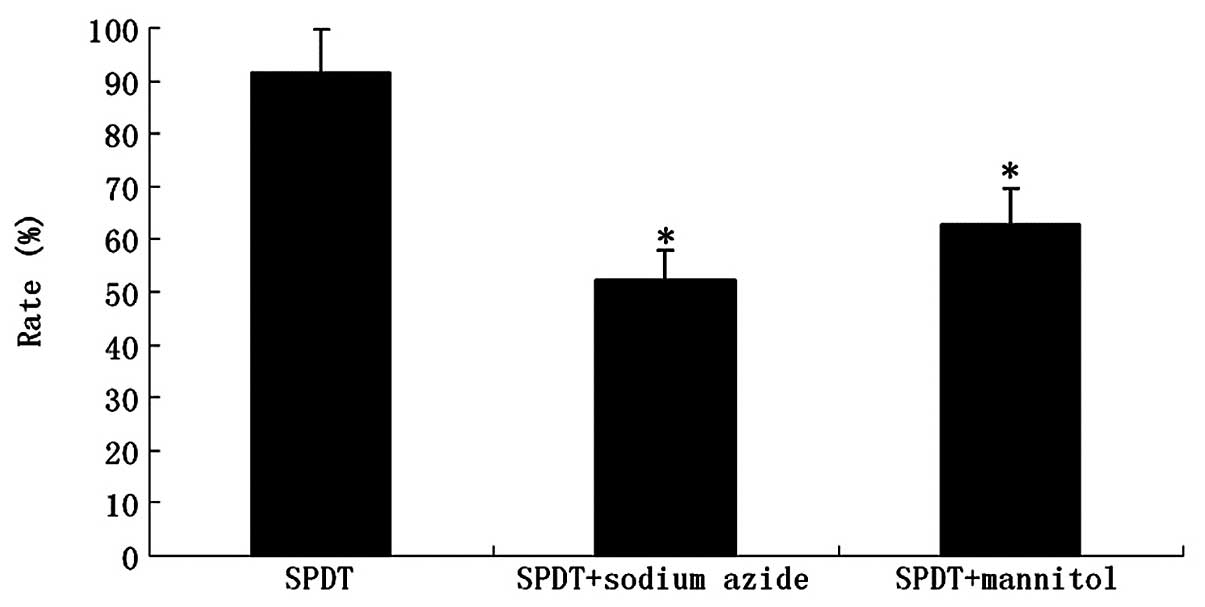
glioma cells was studied in the absence or presence of various ROS
scavengers (e.g., NaN3 and mannitol). The presence of
NaN3 significantly reduced the inhibition rate of
HMME-mediated SPDT. At the level of 100 μg/ml, the singlet
oxygen scavenger caused a reduction of almost 39% (P<0.01). The
presence of mannitol also reduced HMME-mediated SPDT killing by
∼29% (P<0.05; Fig. 7).
Discussion
SPDT is a new, combined therapy for treating cancer.
The basis of the therapy is to administer a small amount of
sensitizer, which is selectively taken up by cancer cells, and then
expose the body to light and sound to activate these sensitizers
(17–19). These techniques are known to have a
cancer-killing effect and are applicable to a wide range of
cancers, but the effect of killing malignant glioma cells is
unknown. We therefore used light and sound to activate the
photosensitizer, a hematoporphyrin derivative (HMME), and examined
its effectiveness and mechanisms of killing glioma cells.
For evaluating the effects of HMME-mediated SPDT, we
first determined the growth inhibition rate of C6 glioma cells in
the SPDT group compared with other groups. Our results indicated
that HMME-mediated SPDT induced a synergetic killing effect on C6
cells when the light dose was <200 J/cm2 (Figs. 1 and 2). SPDT at a lower light dose achieved a
better synergetic killing effect than the monotherapies. In the
SPDT groups, when the light dose was greater than 120
J/cm2, the inhibition rate was not significantly
increased with increasing light dose (P>0.05). This indicated
that SPDT had a sufficient killing effect when ultrasound generated
1 MHz frequency, 0.5 W/cm2 intensity and light
irradiation up to 120 J/cm2. SPDT effectively killed C6
cells with lower doses of light compared with PDT alone. Hence, we
determined that HMME-mediated SPDT was a good method for killing C6
gliomas.
Apoptosis is one of major modes of tumor cell death
(20). In this experiment, we used
light and sound to activate the photosensitizer HMME and examined
its effectiveness and the mechanisms by which it induces C6 glioma
cell apoptosis. Cell apoptosis was observed in HMME-mediated SPDT
in vitro. In our experiment, we revealed that the apoptosis
rate of C6 cells significantly increased when the light irradiation
dose was lower than 80 J/cm2; however, when the light
irradiation dose was above 80 J/cm2, the necrosis rate
of C6 cells significantly increased instead of apoptosis.
Experimental results indicated that HMME-mediated SPDT induced the
highest rate of C6 glioma cell apoptosis at a light dose of 80
J/cm2. These results indicated that SDT and PDT had a
synergic effect on inducing tumor cell apoptosis at a certain light
dose. It suggests that SPDT with low doses of light may induce
major C6 cell apoptosis; but in contrast it mainly results necrosis
with high doses of light.
As we know, apoptosis is often initiated by either
an extrinsic (activated caspase 8) or an intrinsic pathway
(activated caspase 9). The extrinsic pathway functions can directly
activate caspase 8 through the death receptors on the cell surface;
however, the intrinsic pathway regulates the activation of caspase
9, and subsequently the activation of caspase 3. In the present
study, we attempted to indentify the intrinsic or extrinsic
apoptosis pathways using an SPDT in vitro model. Our
experiment also demonstrated that HMME-mediated SPDT magnified the
release of cleaved caspase 3, 8 and 9 in cytoplasm. The changes in
the SDT or PDT group were not as apparent as those in the SPDT
group. These results showed that both the mitochondrial and death
receptor pathway may be two channels by which SPDT induces C6
glioma cell apoptosis.
Certain studies have demonstrated that ROS were
generated following by SDT or PDT (11,21).
Oxidative stress has been reported to initiate the killing effect
in SDT or PDT (22–24). In our experiment, we also detected
that SPDT increased ROS levels in vitro (Fig. 6). Although SDT or PDT alone
increased the generation of ROS, ROS generation in these groups was
much lower than that in the SPDT group. The presence of
NaN3 or mannitol significantly reduced the inhibition
rate of HMME-mediated SPDT. It suggested that increased ROS
generation was the key factor to killing C6 cells. These results
are similar to those found in other reports (12,13).
In summary, these results suggest that HMME-mediated
SPDT may be a promising method for the treatment of glioma.
Combined SDT with PDT at various conditions may cause different
biological effects.
Acknowledgements
This study was supported by the
National Natural Science Foundation (81072079), the Technological
Key Research Projects of Heilongjiang Province (GC10C304-1), and
Bureau of Health Foundation of Heilongjiang Province, China
Province (2010-129).
References
|
1.
|
Calzavara-Pinton PG, Venturini M and Sala
R: Photochemistry and photobiology. J Eur Acad Dermatol Venereol.
21:293–302. 2007. View Article : Google Scholar
|
|
2.
|
Kessel D, Jeffers R, Fowlkes JB and Cain
C: Effects of sonodynamic and photodynamic treatment on cellular
thiol levels. J Photochem Photobiol B. 32:103–106. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Worthington AE, Thompson J, Rauth AM and
Hunt JW: Mechanism of ultrasound enhanced porphyrin cytotoxicity.
Part I: a search for free radical effects. Ultrasound Med Biol.
23:1095–1105. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Yumita N and Umemura S: Sonodynamic
therapy with photofrin II on AH130 solid tumor. Pharmacokinetics,
tissue distribution and sonodynamic antitumoral efficacy of
photofrin II. Cancer Chemother Pharmacol. 51:174–178. 2003.
|
|
5.
|
Rosenthal I, Sostaric JZ and Riesz P:
Sonodynamic therapy - a review of the synergistic effects of drugs
and ultrasound. Ultrason Sonochem. 11:349–363. 2004.PubMed/NCBI
|
|
6.
|
Yumita N, Nishigaki R, Umemura K and
Umemura S: Hematoporphyrin as a sensitizer of cell-damaging effect
of ultrasound. Jpn J Cancer Res. 80:219–222. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kessel D, Jeffers R, Fowlkes JB and Cain
C: Porphyrin-induced enhancement of ultrasound cytotoxicity. Int J
Rad Biol. 66:2211994. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Miyoshi N, Misik V and Riesz P:
Sonodynamic toxicity of gallium-porphyrin analogue ATX-70 in human
leukemia cell. Radiat Res. 148:43–47. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Umemura S, Yumita N, Umemura K and
Nishigaki R: Sonodynamically induced effect of rose bengal on
isolated sarcoma 180 cells. Cancer Chemother Pharmacol. 43:389–393.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yumita N, Kawabata K, Sasaki K and Umemura
S: Sonodynamic effect of erythrosin B on sarcoma 180 cells in
vitro. Ultrason Sonochem. 9:259–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tachibana K, Uchida T, Ogawa K, Yamashita
N and Tamura K: Induction of cell-membrane porosity by ultrasound.
Lancet. 353:14091999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kessel D, Lo J, Jeffers R, Fowlkes JB and
Cain C: Modes of photodynamic vs. sonodynamic cytotoxicity. J
Photochem Photobiol B. 28:219–221. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jin ZH, Miyoshi N and Ishiguro K:
Combination effect of photodynamic and sonodynamic therapy on
experimental skin squamous cell carcinoma in C3H/HeN mice.
Dermatol. 27:294–306. 2000.PubMed/NCBI
|
|
14.
|
Kolarova H, Tomankova K, Bajgar R, Kolar P
and Kubinek R: Photodynamic and sonodynamic treatment by
phthalocyanine on cancer cell lines. Ultrasound Med Biol.
35:1397–1404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Dunn F, Averbuch AJ and O’Brien WD Jr: A
primary method for the determination of ultrasonic intensity with
the elastic sphere radiometer. Acoustica. 38:58–61. 1977.
|
|
16.
|
Li JH, Song DY, Xu YG, Huang Zheng and Wu
Yue: In vitro study of haematoporphyrin monomethyl ether-mediated
sonodynamic effects on C6 glioma cells. Neurol Sci. 29:229–235.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Liu QH, Wang P, Li M, et al: Apopotosis of
Ehrlich ascites tumor cells by sonochemical activated
hematoporphyrin. Acta Zool Sinica. 49:6202003.
|
|
18.
|
Tang W, Liu Q, Wang X, et al: Involvement
of caspase 8 in apoptosis induced by ultrasound-activated
hematoporphyrin in sarcoma 180 cells in vitro. J Ultrasound Med.
27:645–656. 2008.PubMed/NCBI
|
|
19.
|
Wang XB, Liu QH, Wang P, et al:
Enhancement of apoptosis by sonodynamic therapy with protoporphyrin
IX in isolate sarcoma 180 cells. Cancer Biother Radiopharm.
23:238–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Rieger L, Weller M, Bornemann M, et al:
BCL-2 family protein expression in human malignant glioma: a
clinical-pathological correlative study. J Neurol Sci. 155:68–75.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yumita N, Nishigaki R, Umemura K, et al:
Sonochemical activation of hematoporphyrin: an ESR study. Radiat
Res. 138:171–176. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Rogalska A, Koceva-Chyła A and Jóźwiak Z:
Aclarubicin-induced ROS generation and collapse of mitochondrial
membrane potential in human cancer cell lines. Chem Biol Interact.
176:58–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Das R, Roy A, Dutta N and Majumder HK:
Reactive oxygen species and imbalance of calcium homeostasis
contributes to curcumin induced programmed cell death in
Leishmania donovani. Apoptosis. 13:867–882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yang J, Wu LJ, Tashino S, Onodera S and
Ikejima T: Critical roles of reactive oxygen species in
mitochondrial permeability transition in mediating
evodiamine-induced human melanoma A375-S2 cell apoptosis. Free
Radic Res. 41:99–108. 2007. View Article : Google Scholar
|