Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common cancers of the head and neck, particularly in Southern China
and Southeast Asia, where the annual incidence is >20 cases per
100,000 individuals (1,2). In Guangzhou province in China, the
annual incidence reaches 25 cases per 100,000 (3). A combination of radiotherapy and
adjuvant chemotherapy is now the standard treatment for NPC, and
these treatment modalities have been successful for certain
patients. However, the 5-year survival rate is only 50–60% due to
the frequency of distant metastasis and local recurrence, as well
as long-term secondary effects of radiotherapy and chemotherapy.
Additionally, there is almost no effective treatment for those who
are resistant to radiotherapy and have tumor recurrence. There is
an urgent need to develop new treatment strategies for this patient
population, which will be both better tolerated and more
effective.
Recently, much attention has been focused on the use
of dietary phytochemicals in cancer prevention and therapy due to
the pleiotropic effects of these agents on multiple
carcinogen-activated oncogenic pathways, and their equally
excellent safety profiles (4,5). Among
various natural products, indole-3-carbinol (I3C) has been reported
to inhibit cancer development by perturbing multiple cellular
signaling events (6). In our
previous research, we found that I3C induced mitochondria-mediated
apoptosis in the NPC cell line CNE-2 (7). In the stomach, I3C is rapidly
converted to a variety of condensation products, chiefly
3,3′-diindolyl-methane (DIM) (8).
Researchers have also found that DIM spontaneously formed from I3C
during cell culture experiments (9). Therefore DIM, rather than I3C, may be
the major compound available to cells following ingestion of I3C.
It has been shown that DIM inhibits the proliferation of a variety
of cancer cell types, including prostate (10,11),
breast (12,13) and colon (14) cancer cells, as well as certain
cervical cancer cell lines (15),
via induction of cell cycle arrest and apoptosis. Since
susceptibility to an agent may differ among different cancer cells,
it has remained unclear whether DIM has similar effects on human
NPC cells. In the present study, we investigated the effects of DIM
on CNE-2, a poorly differentiated human NPC cell line that is the
most common cell type in NPC. The possible pathways and molecular
mechanisms involved in its effects were also explored.
Materials and methods
Reagents and antibodies
DIM, dimethyl sulfoxide (DMSO), propidium iodide
(PI), bovine serum albumin (BSA) and Triton X-100 were purchased
from Sigma Company (St. Louis, MO, USA). The mitochondrial membrane
potential detection kit was purchased from Stratagene (Cedar Creek,
TX, USA). The respective antibodies against caspase-3, -8, -9, Bid,
Bax, Bak, Bcl-2, c-FLIP, cleaved caspase-3, -8, -9 and poly
(ADP-ribose) polymerase (PARP) were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Antibodies against NF-κB,
NF-κB p65 and p50, cyclin A, cyclin D1, cyclin E, cyclin-dependent
kinase (CDK)1, CDK2, p53, p21, p27, cytochrome c, Smac, Omi, and
GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA,
USA). The mouse monoclonal anti-X-linked apoptosis-inhibiting
protein (XIAP) antibody was purchased from BD Biosciences
Pharmingen, Inc. (San Jose, CA, USA). The anti-IκB-α (pS32)
phosphospecific antibody was purchased from Cell Signaling
Technology (Danvers, MA, USA). Anti-IKK-β and anti-p-IKK-β
antibodies were purchased from Imgenex (San Diego, CA, USA). The
study was approved by the ethics committee of Renmin Hospital of
Wuhan University, Wuhan, China.
Cell culture
CNE-2, a poorly differentiated human NPC cell line,
was obtained from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China) and cultured in RPMI-1640
medium (Gibco Laboratories, Grand Island, NY, USA), supplemented
with 10% fetal bovine serum (Sijiqing Biological Engineering
Company, Hangzhou, China) and 1% penicillin/streptomycin in a
humidified atmosphere of 5% CO2 at 37°C.
Cell viability assay
CNE-2 cells were treated with DMSO or different
concentrations of DIM (0, 15, 30, 50 and 100 μM) for 6, 12,
24 or 48 h. For the methylthiazolyldiphenyl-tetrazolium (MTT)
assay, 0.5 mg/ml MTT (pH 4.7; Sigma) was added 4 h before the end
of the culture time. After the supernatant was discarded, MTT
formazan precipitates were dissolved in 150 μl DMSO, shaken
mechanically for 10 min and then read immediately at 570 nm in a
plate reader. Wells without cells were used as blanks. Three
independent experiments were performed.
Cell proliferation assay
The inhibition of [3H]-TdR incorporation
into DNA was examined using the pulse-labeling method.
Proliferating CNE-2 cells were seeded into 96-well plates and
incubated for 16 h and then treated with DMSO or different
concentrations of DIM (0, 15, 30, 50 and 100 μM) for the
next 6, 12, 24 or 48 h. For the [3H]-TdR assay, CNE-2
cells were exposed to [3H]-TdR for 6 h. After the cells
were harvested onto glass fiber filters, the counts per minute
(cpm) were measured with a liquid scintillation counter (FJ-2107P,
Xi’an, China). Three independent experiments were performed.
Cell cycle analysis by flow
cytometry
Cells were grown in the absence or presence of DIM
(15, 30, 50 or 100 μM) or presence of DMSO for 48 h. For
cell cycle analysis, cells were collected, washed twice with 0.01 M
phosphate-buffered saline (PBS) and fixed in 70% ethanol overnight
at 4°C. The cells were subsequently washed in PBS, digested with
200 μl RNase (1 mg/ml) at 37°C for 30 min and stained with
800 μl PI (50 μg/ml) at room temperature for 30 min.
The cells were then washed with PBS and immediately analyzed using
flow cytometry. The percentages of nuclei in CNE-2 cells in each
phase of the cell cycle (G1, S and G2/M) were calculated using the
MultiCycle software program (Phoenix Flow Systems, San Diego, CA,
USA).
Apoptosis assays by flow cytometry
To assay the effect of DIM on cell apoptosis, CNE-2
cells were exposed to DMSO or 0, 15, 30, 50 or 100 μM DIM
for 48 h. The cultured cells were trypsinized and 1×106
cells were collected by centrifugation. After washing with PBS and
centrifuging at 1,000 × g for 5 min, the cell pellet was
resuspended in Annexin V-FITC/PI and finally analyzed with a
FACSort flow cytometer (Becton-Dickinson, Mountain View, CA,
USA).
Analysis of mitochondrial membrane
potential
Mitochondrial membrane potential (Δψm) was measured
using the mitochondrial membrane potential detection kit. Briefly,
cells were stained with Rh123 for 15 min and then measured on a
FACScan flow cytometer according to the instruction manual.
Western blot analysis
Following treatment with 0, 15, 30, 50 or 100
μM DIM for 48 h, cells were washed with PBS, harvested and
lysed in RIPA buffer [50 mM Tris-HCl (pH 8.0) with 150 mM NaCl,
1.0% Nonidet P-40, 0.5% sodium deoxycholate and 0.1% sodium dodecyl
sulfate] containing protease inhibitor and phosphatase inhibitor
(Roche, Indianapolis, IN, USA). Protein concentration was
determined using the BCA reagent (Pierce, Rockford, IL, USA). Equal
amounts of protein were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a
polyvinylidene difluoride membrane. After blocking with 5% BSA in
TBS at room temperature for 1 h, membranes were incubated with an
appropriate dilution of primary antibody at 4°C overnight.
Membranes were washed extensively with 0.1% Tween-20 in TBS and
incubated with secondary antibody at room temperature for 1 h.
Bands corresponding to the antibodies were detected by enhanced
chemiluminescence (Pierce) and quantified using Quantity One
imaging software (Bio-Rad, Hercules, CA, USA).
NF-κB DNA binding activity
measurement
CNE-2 cells were plated in 100-mm dishes at a
density of 1×106 cells and cultured. After 24 h, the
cultures were treated with DMSO or DIM (0, 15, 30, 50 or 100
μM) for 6, 12, 24 and 48 h. Following treatment, cells were
resuspended in 10 mM Tris-HCl (pH 7.5)/5 mM MgCl2/0.05%
Triton X-100 and lysed with a homogenizer. The homogenate was
centrifuged at 3,000 × g for 15 min at 4°C. The nuclear pellet was
then resuspended in an equal volume of 10 mM Tris-HCl (pH 7.4)/5 mM
MgCl2 followed by the addition of one nuclear pellet
volume of 1 M NaCl/10 mM Tris-HCl (pH 7.4)/4 mM MgCl2.
The lysing nuclei were put on ice for 30 min before centrifugation
at 10,000 × g for 15 min at 4°C. The supernatant (nuclear extract)
was removed and the protein concentration was measured using the
BCA protein assay. To determine NF-κB DNA binding activation,
electrophoretic mobility shift assay (EMSA) was carried out.
Briefly, the NF-κB oligonucleotide 5′-AGTTGAGGGGACTTTCCCAGGC-3′
DNA-binding sequence was labeled by the Biotin 3′ end labeling kit,
and 10 μg nuclear proteins were incubated for 20 min at room
temperature with biotin-labeled DNA probes in the 20 μl
shift-binding buffer comprising 25 mM EDTA, 5 mM MgCl2,
0.05% NP-40, 2.5% glycerol, 50 ng/μl poly(dI·dC) and 0.2
μg BSA. Nucleo-protein complexes were loaded onto the
pre-electrophoresis 6% non-denaturing polyacrylamide gels in 0.5X
Tris-boric acid-EDTA buffer at 100 V for 2 h at room temperature.
The electrophoresed binding reactions were transferred to a nylon
membrane by capillary transfer system overnight at room
temperature. The signal was detected using the conjugated
streptavidin-horseradish peroxidase and chemiluminescent substrate
and quantified with Quantity One imaging software (Bio-Rad).
Statistical analysis
All experiments were repeated at least three times
independently, and values are expressed as the mean ± standard
deviation (SD). Analysis of variance (ANOVA) with subsequent
Bonferroni’s test was used to determine significant differences in
multiple comparisons. Values of P<0.01 were considered to
indicate a statistically significant result.
Results
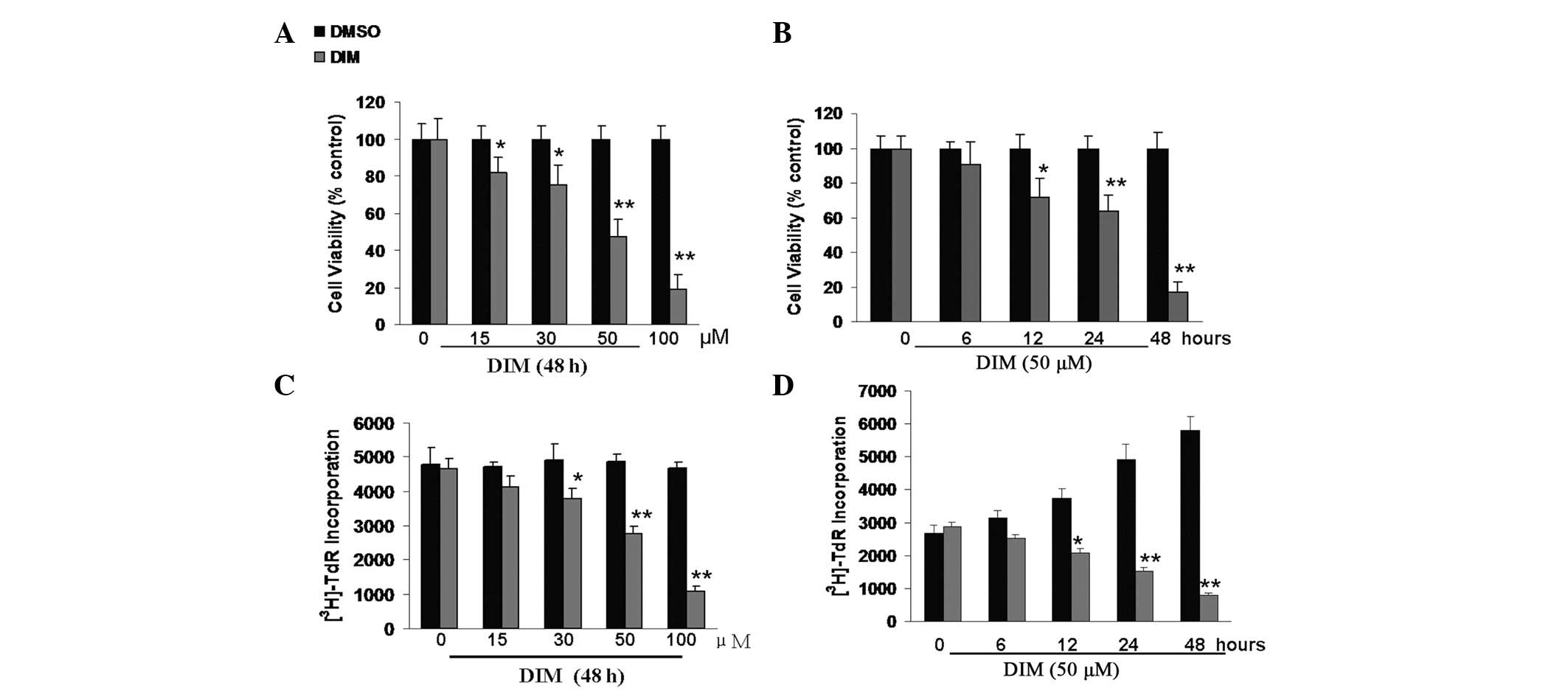
Effects of DIM on the viability and
proliferation of CNE-2 cells
CNE-2 cells were exposed to DMSO and various
concentrations of DIM for 48 h or 50 μM of DIM for 6–48 h.
The MTT assay was used to examine the effect of DIM on cell
viability. Results showed that DIM inhibited CNE-2 cell viability
in a time- and dose-dependent manner. When exposed for 48 h, the
viability of CNE-2 cells was significantly inhibited at
concentrations of 15–100 μM DIM (Fig. 1A). At a concentration of 50
μM, DIM caused a significant decrease in the viability of
CNE-2 cells at exposure times of 12 h or longer (Fig. 1B).
To examine the effect of DIM on the proliferation of
CNE-2 cells, we used the pulse-labeling method to detect
[3H]-TdR incorporation into DNA. Treatment with DIM also
induced growth inhibition of CNE-2 in a dose- and time-dependent
manner. Concentrations of 30–100 μM DIM were able to inhibit
the growth of CNE-2 at 48 h (Fig.
1C), and even a 12 h exposure to 50 μM DIM significantly
decreased the proliferation of CNE-2 cells (Fig. 1D).
DIM induces cell cycle arrest at the G1
phase and reduces the levels of CDKs, CDK inhibitors (CDKIs) and
cyclin proteins
It has been shown that I3C induces G1 cell cycle
arrest in breast cancer cells (13). To determine whether DIM regulates
cell cycle progression in CNE-2 cells, the DNA was stained with PI,
then FACS analysis was performed. The results showed that the
percentage of CNE-2 cells arrested in the G0/G1 phase was markedly
higher in the 15 μM DIM-treated group than in either the
DMSO or untreated groups (59.2±1.9 vs. 44.3±1.0 and 42.4±0.4%,
respectively, P<0.05). Treatment of cells with DIM for 48 h
resulted in a dose-dependent increase in the percentage of cells in
the G0/G1 phase, with a concomitant reduction in cell numbers in
the S phase (Fig. 2A).
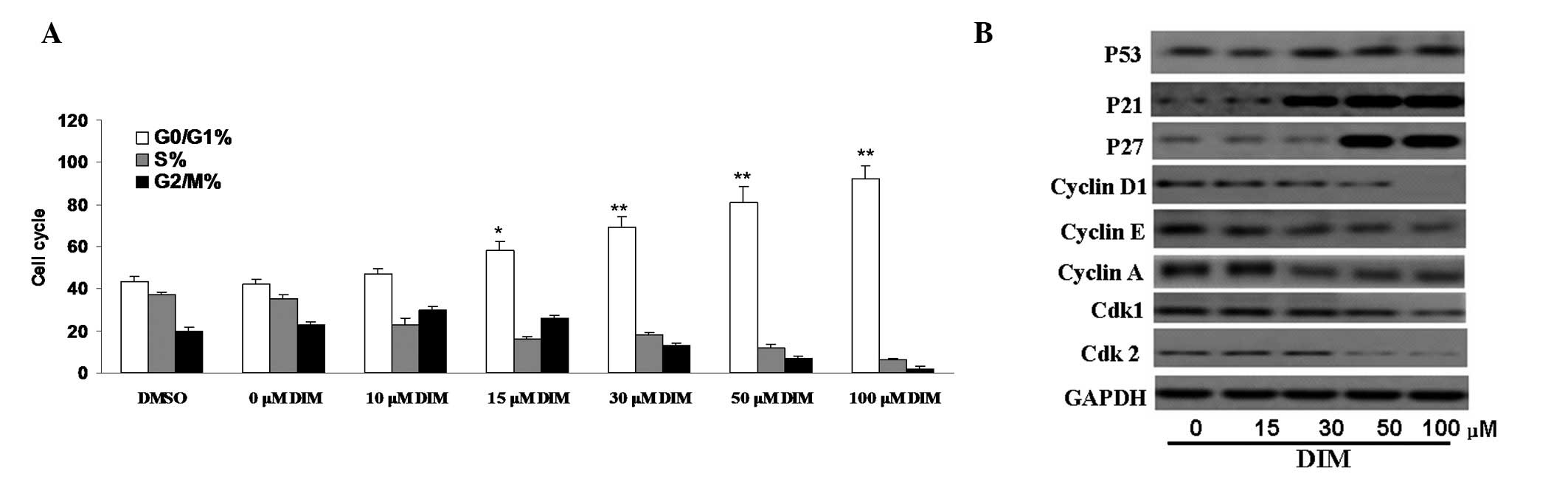 | Figure 2.Effect of 3,3′-diindolylmethane (DIM)
on the cell cycle of CNE-2 cells. (A) Treatment of cells with
various concentrations of DIM for 48 h resulted in dose-dependent
increases in the percentage of cells in G0/G1 phase. The percentage
of CNE-2 cells arrested in the G0/G1 phase was much higher in the
DIM-treated group than in the dimethyl sulfoxide (DMSO) or
untreated groups (*P<0.05, **P<0.01).
(B) Proteins controlling the phase of the cell cycle, such as CDK1,
CDK2, cyclin D1, cyclin A and cyclin E, were found to be
downregulated by treatment with DIM using western blot analysis.
Meanwhile, expression levels of CDK1, as well as p53, p27 and p21,
were decreased in the presence of DIM. |
To confirm cell cycle arrest, we further examined
cell cycle regulatory molecules in DIM-treated CNE-2 cells. Our
results showed that the levels of CDK2 and CDK1 proteins in CNE-2
cells were reduced significantly following treatment with 50–100
μM DIM for 48 h. The levels of cyclin D1, cyclin A and
cyclin E were also significantly suppressed following treatment
with 50–100 μM DIM. The expression levels of CDKIs involved
in cell cycle arrest, such as p21 and p27, were increased in a
time-dependent manner in cells treated with DIM. Thus, DIM induces
cell cycle arrest by affecting the expression of multiple
cyclin-related cellular signaling proteins (Fig. 2B).
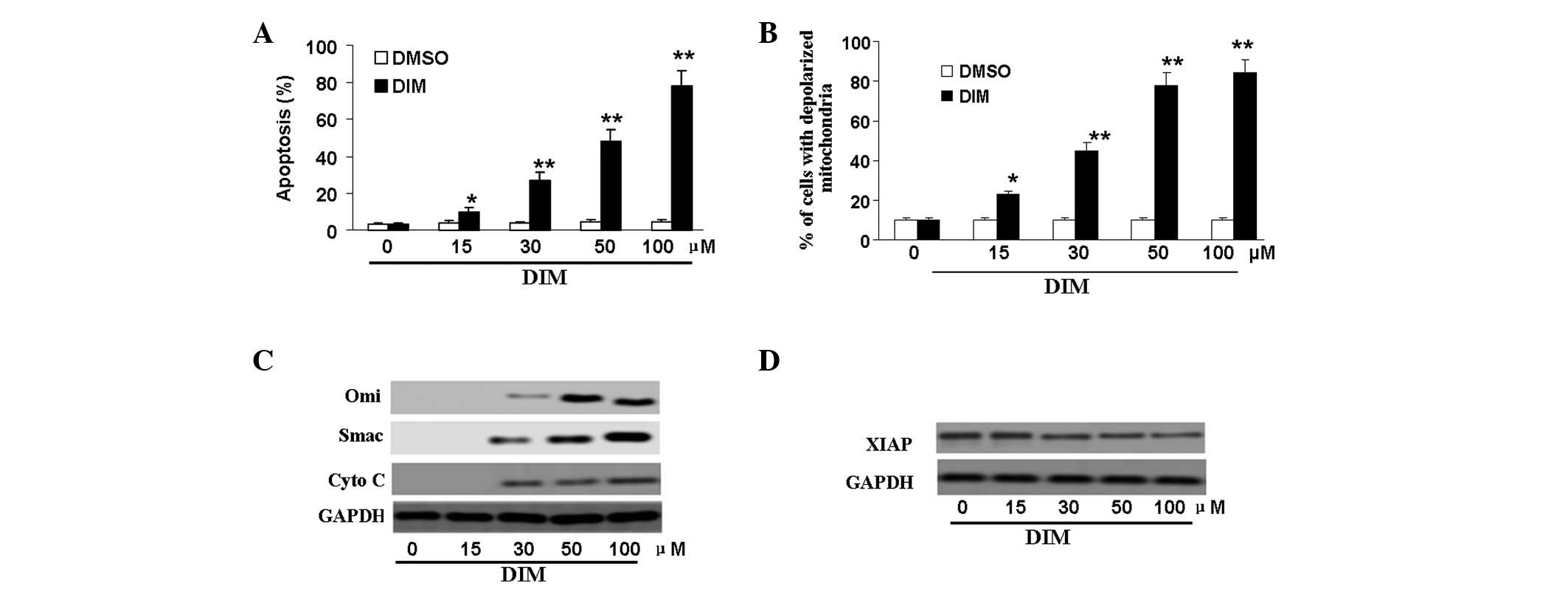
DIM-induced apoptosis is accompanied by
mitochondrial changes and mitochondria-related protein
alterations
To determine whether DIM inhibited CNE-2 cell
viability by inducing apoptosis, flow cytometric analysis was
carried out using double-staining with Annexin V-FITC and PI. CNE-2
cells were treated with DMSO or DIM at the indicated concentrations
for 48 h. The results showed that apoptotic cells were markedly
increased following treatment with DIM. DIM induced apoptosis in a
dose-dependent manner. A concentration of 15 μM DIM
significantly increased the percentage of apoptotic cells at 48 h
according to densitometry analysis (P<0.05); when the
concentration of DIM was 30 μM, the numbers of apoptotic
cells were increased almost 10-fold compared with those in
DMSO-treated cells (Fig 3A).
To explore the mechanisms by which DIM induces
apoptosis, we evaluated the role of mitochondria in DIM-treated
CNE-2 cells. Specifically, Δψm was examined by flow cytometry. As
shown in Fig. 3B, a dose-dependent
increase in the level of mitochondrial membrane depolarization was
observed.
Since changes in mitochondrial membrane potential
are usually associated with increased permeability of the outer
mitochondrial membrane, allowing efflux of apoptogenic proteins to
the cytosol (16), we measured the
release of cytochrome c, Smac and Omi from the mitochondria to the
cytosol by western blot. As expected, the expression of cytochrome
c, Smac and Omi proteins in the cytoplasm increased in a
dose-dependent manner (Fig. 3C);
when the concentration of DIM was 30 μM, the effects were
significant (P<0.01). A previous study had demonstrated that
cytosolic Smac could bind XIAP and neutralize its anti-apoptotic
activity (17). In this study, we
further demonstrated that treatment with DIM at 30–100 μM
decreased the expression of XIAP (P<0.05; Fig. 3D).
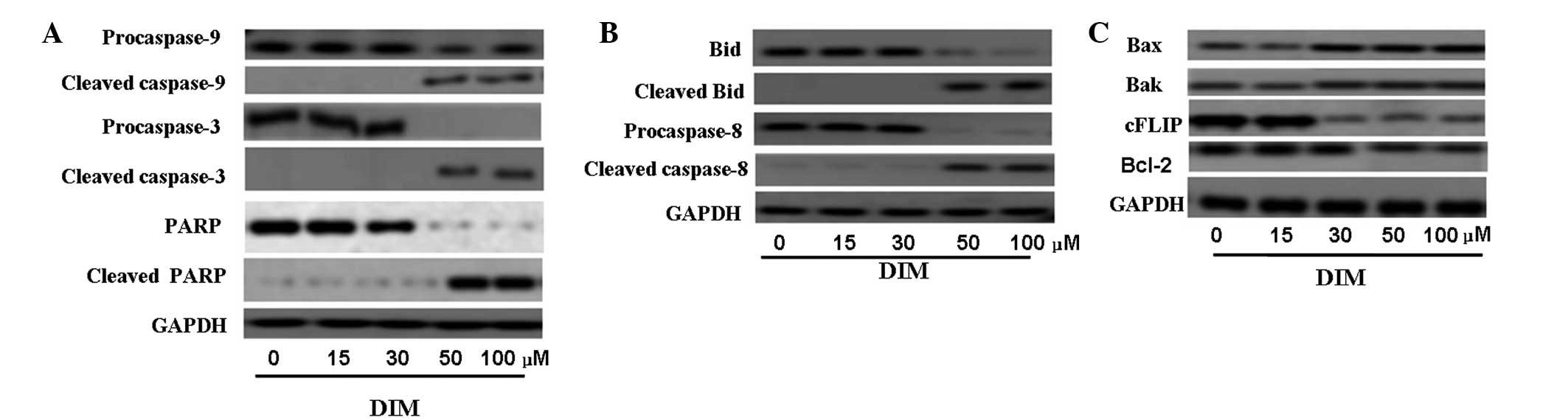
Effects of DIM on caspase protein and
apoptotic protein levels
Caspases are important regulators of cell apoptosis.
Caspases -8 and -9 are the initiator caspases, while caspase-3 is
the ‘executioner enzyme’. To better characterize the pathway
through which DIM exerts its apoptotic effects in CNE-2 cells, we
examined the effects of DIM on the levels and activities of
caspases. CNE-2 cells were treated with 0, 15, 30, 50 or 100
μM DIM for 48 h. The western blot results shown in Fig. 4 indicate that 50 μM DIM
significantly increased cleavage of caspase -9, -8 and -3 after 48
h of treatment and that this activation was strengthened by 100
μM DIM. The increases in cleaved caspase-9, -8 and -3 were
associated with decreases in procaspase-9, -8 and -3. As expected,
an increase in cleaved PARP, a substrate of caspase 3, was also
observed with 50 μM DIM treatment. Bid, well known as a
linker between the endogenous mitochondrial pathway and death
receptor mediated extrinsic apoptotic pathway, was also activated
by DIM.
Mitochondrial membrane depolarization can result
from the action of pro-apoptotic and/or anti-apoptotic members of
the Bcl-2 family (18). Thus, we
examined the effects of DIM on Bcl-2, Bak and Bax protein
expression as well as c-FLIP which is an inhibitor of apoptosis
mediated by the death receptors Fas, DR4 and DR5. The results of
western blot analysis showed that DIM suppressed the expression of
anti-apoptotic proteins such as Bcl-2 and cFLIP and increased the
levels of pro-apoptotic proteins such as Bax and Bak in a
dose-dependent manner (Fig.
4C).
DIM suppresses NF-κB activation in a
dose- and time-dependent manner
NF-κB is a key regulator and transcription factor of
genes that mediate apoptotic signaling pathways; it also plays
critical roles in cell proliferation. We therefore strongly
suspected that inactivation of NF-κB would be involved in the
apoptotic pathways of CNE-2 cells treated with DIM. Western blot
analysis for nuclear NF-κB and its p65 and p50 subunit proteins in
CNE-2 cells showed that DIM (30–100 μM) suppressed nuclear
NF-κB expression and also decreased expression of its p50 and p65
subunits, and these effects occurred in a dose-dependent manner
(Fig. 5A).
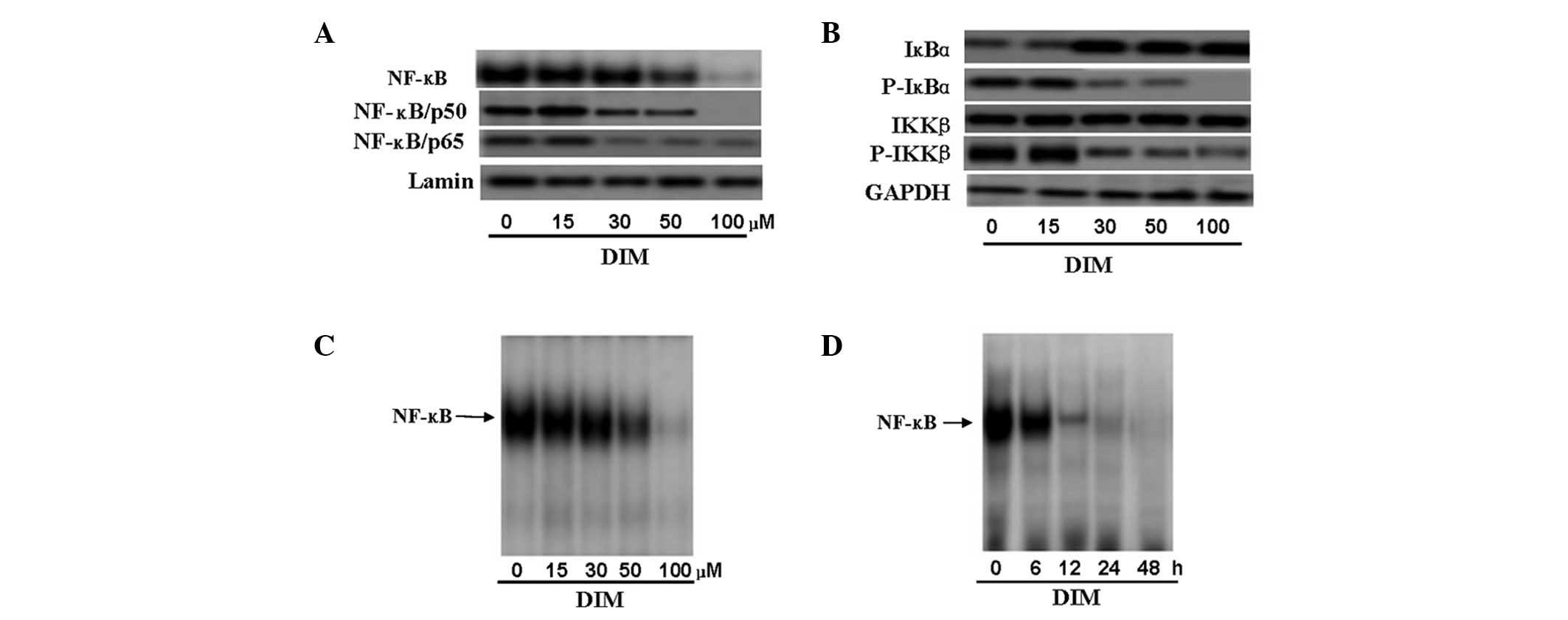 | Figure 5.Effect of 3,3′-diindolylmethane (DIM)
on NF-κB in CNE-2 cells. (A) Western blot analysis of nuclear NF-κB
and p65 and p50 subunit proteins in CNE-2 cells showed that DIM
(30–100 μM) suppressed nuclear NF-κB expression and also
decreased expression of the p50 and p65 subunits of NF-κB; these
effects occurred in a dose-dependent manner. (B) Cells were
incubated with different concentrations of DIM (0, 15, 30 50, and
100 μM) for 48 h, and cell extracts were prepared to check
the levels of IκB-α, p-IκB-α, IKK-β and p-IKK-β by western blot
analysis as described in Materials and methods. All samples were
probed with GAPDH to show equal protein loading. Phosphorylation of
IκB-α, which can activate NF-κB, was suppressed by DIM, while
decreased levels of p-IKK-β were found following treatment with
DIM. (C and D) To further confirm the effect of DIM on activation
of NF-κB in CNE-2 cells, nuclear proteins from cultured cancer
cells treated with DIM were subjected to analysis for NF-κB DNA
binding activity as measured by electrophoretic mobility shift
assay (EMSA). (C) For the dose-dependent analysis, cells were
incubated with different concentrations of DIM (0, 15, 30, 50 or
100 μM) for 48 h and then subjected to EMSA. The results
showed that 50 μM inhibited NF-κB DNA binding activity in
CNE-2 cells significantly (P<0.01) and DIM inhibited NF-κB DNA
binding activity in a dose-dependent manner. (D) For time-dependent
analysis, cells were incubated with 50 μM DIM for 0, 6, 12,
24 or 48 h. The results showed that NF-κB DNA binding activity was
significantly suppressed at 12 h (P<0.01) and DIM inhibited
NF-κB DNA binding activity in a time-dependent manner. |
To further confirm the effect of DIM on the
activation of NF-κB in CNE-2 cells, nuclear proteins from cultured
cancer cells treated with DIM were subjected to analysis for NF-κB
DNA binding activity as measured by EMSA. We found that DIM
significantly inhibited the NF-κB DNA binding activity of CNE-2
cells in a time- and dose-dependent manner (Fig. 5C and D). Following treatment with 50
μM DIM for 12 h, NF-κB DNA binding activity was
significantly suppressed (P<0.01).
DIM inhibited IKK activation and IκB-α
phosphorylation
To further investigate DIM-induced downregulation of
the activity of the NF-κB pathway, we analyzed the phosphorylation
of IκB-α by western blot analysis. CNE-2 cells exposed to DIM
exhibited a decrease in IκB-α phosphorylation (Fig. 5B). This finding suggested that DIM
could interfere with IκB-α phosphorylation and thereby prevent its
degradation, thus blocking the nuclear translocation of NF-κB.
We further tested the effects of DIM on the
expression levels of IKK proteins by western blot analysis using
anti-p-IKK-β and anti-IKK-β antibodies, which are required for
phosphorylation of IκB-α. The results showed that DIM had no effect
on the expression of IKK-β, but decreased the p-IKK-β protein level
in a dose-dependent manner (Fig.
5B).
Discussion
To obtain better clinical results in the treatment
of NPC, it is essential to identify novel therapeutic agents that
have less toxicity. Recently, dietary chemopreventive
phytochemicals have attracted interest from numerous researchers.
Epidemiological studies have shown that diets rich in fruits and
vegetables are associated with a lower risk of cancer (18). A number of studies have demonstrated
a decreased incidence of various types of cancer in individuals who
consume large amounts of cruciferous vegetables, such as broccoli,
cabbage and cauliflower (19).
These vegetables were found to contain glucobrassicin, which
undergoes hydrolysis by cooking (20). The main hydrolysis product of
glucobrassicin is I3C. I3C can be converted into a number of
polymeric products, of which DIM is the main one (21). It has been reported that DIM
inhibits the growth of many cancers in in vivo or in
vitro studies (10–14). However, an antitumor effect of DIM
in human nasopharyngeal carcinoma, one of the most common cancers
in Southern China, has not yet been thoroughly reported.
Dysregulation of proliferation and apoptosis are
linked to the development of most cancers. In this study, we have
demonstrated that DIM significantly decreased cell proliferation in
CNE-2 cells in a dose- and time-dependent manner. We found that the
inhibitory effect of DIM on the growth of CNE-2 cells may result
from G0/G1 cell cycle arrest. In recent research, Choi et al
found that DIM inhibited HT-29 human colon cancer cells and was
able to induce cell cycle arrest with 10–30 μM DIM, which is
consistent with our results (22).
This result was strengthened by our examination of proteins
controlling the cell cycle phase transition. Using western blot
analysis, we found that DIM reduced the levels of the CDK1, CDK2,
cyclin A, cyclin D1 and cyclin E proteins at 48 h in a
dose-dependent manner. Meanwhile, the apoptotic effect of DIM in
CNE-2 cells was analyzed using a dual staining approach with PI and
Annexin V. Our findings revealed that apoptosis of CNE-2 cells was
increased in the DIM-treated groups. These findings were consistent
with those of previous research and provided further support for
the anticancer effect of DIM. Self-sufficiency in growth signals
and escaping from programmed cell death are the main changes in
cell physiology necessary to promote malignant growth (23). Therefore, a bioactive agent such as
DIM, which has the ability to inhibit cell cycle progression and
induce apoptosis in NPC cells, may potentially be utilized as a
chemopreventive agent for NPC.
In the present study, we also attempted to explore
the mechanism of DIM-induced apoptosis in CNE-2 cells. Apoptosis is
a programmed cell death caused by a group of cysteine proteases
known as caspases. There are two major pathways in caspase cascade
activation: the extrinsic (death receptor) and the intrinsic
(mitochondrial) pathways. In the extrinsic (death receptor)
pathway, caspase-8 and -10 are activated following the recruitment
of Fas-associated death domain (FADD) protein and death domain (DD)
binding. In the intrinsic pathway, cytochrome c is released from
mitochondria in response to a variety of apoptotic stimuli. The
release of cytochrome c induces the cleavage of caspase-9, which
contributes to the activation of effector caspases such as
caspase-3 (24). The effector
caspases cleave a set of vital proteins such as PARP and eventually
lead to apoptosis (25).
Mitochondrial dysfunction is an important
characteristic of apoptotic cell death (26,27),
particularly in the intrinsic pathway. In the present study, we
examined perturbations in mitochondrial membrane potential under
DIM treatment. We showed that changes in CNE-2 cells associated
with apoptosis were accompanied by a loss of mitochondrial membrane
potential. We also found that DIM treatment resulted in the release
of cytochrome c, Smac and Omi into the cytosol and activation of
caspase-9 and -3 in a dose-dependent manner. From these results, we
can conclude that the intrinsic pathway is involved in DIM-induced
apoptosis of CNE-2 cells.
Bcl-2 has been shown to form membrane pores involved
in the homeostasis of cell organelles, inhibiting the mitochondrial
permeability transition and cytochrome c release, thereby
functioning to block apoptosis (28,29).
The ratio of pro- to anti-apoptotic molecules such as Bcl-2 and Bax
is considered to be a determinant for mitochondria-related
apoptosis. In the present study, we found that DIM downregulated
Bcl-2 and upregulated Bax in CNE-2 cells.
In this study, we found that DIM also increased the
levels of cleaved caspase-8 and Bid. Bid, a BH3 domain-containing
pro-apoptotic Bcl-2 family member, is a specific substrate of
caspase-8 in the extrinsic apoptotic signaling pathway. It is well
known as a linker between the endogenous mitochondrial pathway and
the death receptor-mediated extrinsic apoptotic pathway.
Full-length Bid is inactive and localized in the cytosol, while
cleaved Bid translocates to the mitochondria and transduces
apoptotic signals from the cytoplasm to the mitochondria,
increasing mitochondrial membrane permeability and the release of
apoptosis-associated mitochondrial proteins. FLIP is an important
antiapoptotic protein of the FAS-related apoptotic pathway that
blocks the activation of caspase-8. In the present stud, FLIP was
also found to be decreased in DIM-treated CNE-2 cells. Therefore,
mitochondria-dependent apoptosis may be one of the major mechanisms
by which DIM induces apoptosis in CNE-2 cells. It is possible that
both the extrinsic and intrinsic pathways are involved in
DIM-mediated apoptosis of CNE-2 cells.
NF-κB is one of the transcription factors regulating
the expression of numerous genes critical for cell survival. It
plays critical roles in the control of cell proliferation and
apoptosis, as well as tumor invasion, metastasis, drug resistance
and the stress response, by regulating the cell cycle, inhibiting
caspase activation, removing harmful oxygen radicals and defending
mitochondrial function (30). It
has been reported that inactivation of NF-κB makes cells more
sensitive to apoptosis-inducing agents (31). Under non-stimulating conditions,
NF-κB is sequestered in the cytoplasm through tight association
with the NF-κB inhibitory-protein IκB-α. In this study, we found by
western blot analysis that nuclear NF-κB expression in CNE-2 cells
was inhibited by DIM (50–100 μM) in a dose-dependent manner.
Using EMSA, we further demonstrated that the DNA binding activity
of NF-κB in CNE-2 cells was significantly suppressed by DIM in a
time- and dose-dependent manner. Expression of the p50 and p65
subunits of NF-κB was also suppressed in DIM-treated CNE-2 cells.
Thus, DIM inhibited NF-κB activation, resulting in the
downregulation of transcription of genes downstream of NF-κB such
as Bcl-2 and FLIP that counteract the action of pro-apoptotic
proteins including Bid, Bad and Bax, thus leading to the promotion
of cell apoptosis and inhibition of cell growth. The findings
suggest that inhibition of NF-κB may be an important mechanism for
the anti-proliferative and pro-apoptotic effects of DIM.
The IKK/IκB-α/NF-κB pathway is the major molecular
mechanism for NF-κB activation. The IKK kinase complex induces
phosphorylation of IκB-α at Ser32/Ser36, leading to degradation of
IκB-α proteins and resulting in the release of NF-κB; active NF-κB
(p65-p50 subunits) is thereby enabled to translocate into the
nucleus, where it binds NF-κB-specific DNA binding sites, allowing
transcription of downstream survival genes. Our present data show
that DIM decreased IκB-α degradation in a dose-dependent manner.
Unphosphorylated IκB-α remained bound to the p50-p65 complex,
preventing nuclear translocation and activation of NF-κB. We
further measured IKKs in the experimental cancer cells. p-IKK-β
expression was found to be decreased following DIM treatment,
indicating that DIM may inhibit the activation of IKK-β and thus
reduce IκB-α phosphorylation and degradation in CNE-2 cells.
In this study, we found that biological effects of
DIM occurred at 15–30 μM, but most effects on signaling
events required much higher (50 μM) concentrations,
suggesting that upregulation of pro-apoptotic molecules and
downregulation of anti-apoptotic molecules may partially contribute
to DIM-induced apoptosis in cancer cells. Other pathways may also
mediate the biological effects of DIM in CNE-2 cells.
In conclusion, DIM may inhibit cell proliferation
and induce the apoptosis of CNE-2 cells by regulating multiple
molecules in a mitochondria-dependent pathway. Although all of the
experiments here were performed in only one cell line and further
studies are needed, our results open a new avenue and challenge the
current paradigm for the prevention and/or treatment of
nasopharyngeal carcinoma. Following up on these points, the
effectiveness of DIM in the treatment of NPC is worth further
study.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Grant No.
30973280).
References
|
1.
|
Cho WC: Nasopharyngeal carcinoma:
molecular biomarker discovery and progress. Mol Cancer. 6:12007.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Marks JE, Phillips JL and Menck HR: The
National Cancer Data Base report on the relationship of race and
national origin to the histology of nasopharyngeal carcinoma.
Cancer. 83:582–588. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Busson P, Keryer C, Ooka T and Corbex M:
EBV-associated nasopharyngeal carcinomas: from epidemiology to
virus-targeting strategies. Trends Microbiol. 12:356–360. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Scott EN, Gescher AJ, Steward WP and Brown
K: Development of dietary phytochemical chemopreventive agents:
biomarkers and choice of dose for early clinical trials. Cancer
Prev Res (Phila). 2:525–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tan AC, Konczak I, Sze DM and Ramzan I:
Molecular pathways for cancer chemoprevention by dietary
phytochemicals. Nutr Cancer. 63:495–505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sarkar FH, Li Y, Wang Z and Kong D:
Cellular signaling perturbation by natural products. Cell Signal.
21:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Xu Y, Zhang J and Dong WG:
Indole-3-carbinol (I3C)-induced apoptosis in nasopharyngeal cancer
cells through Fas/FasL and MAPK pathway. Med Oncol. 28:1343–1348.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Grose KR and Bjeldanes LF: Oligomerization
of indole-3-carbinol in aqueous acid. Chem Res Toxicol. 5:188–193.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bradlow HL and Zeligs MA: Diindolylmethane
(DIM) spontaneously forms from indole-3-carbinol (I3C) during cell
culture experiments. In Vivo. 24:387–391. 2010.PubMed/NCBI
|
|
10.
|
Garikapaty VP, Ashok BT, Tadi K, Mittelman
A and Tiwari RK: 3,3′-Diindolylmethane downregulates pro-survival
pathway in hormone independent prostate cancer. Biochem Biophys Res
Commun. 340:718–725. 2006.
|
|
11.
|
Nachshon-Kedmi M, Yannai S and Fares FA:
Induction of apoptosis in human prostate cancer cell line, PC3, by
3,3′-diindolylmethane through the mitochondrial pathway. Br J
Cancer. 91:1358–1363. 2004.
|
|
12.
|
Rahman KW and Sarkar FH: Inhibition of
nuclear translocation of nuclear factor-{kappa}B contributes to
3,3′-diindolylmethane-induced apoptosis in breast cancer cells.
Cancer Res. 65:364–371. 2005.
|
|
13.
|
Hong C, Kim HA, Firestone GL and Bjeldanes
LF: 3,3′-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in
human breast cancer cells that is accompanied by Sp1-mediated
activation of p21 (WAF1/CIP1) expression. Carcinogenesis.
23:1297–1305. 2002.
|
|
14.
|
Kim EJ, Park SY, Shin HK, Kwon DY, Surh YJ
and Park JH: Activation of caspase-8 contributes to
3,3′-Diindolylmethane-induced apoptosis in colon cancer cells. J
Nutr. 137:31–36. 2007.
|
|
15.
|
Weng JR, Bai LY, Chiu CF, Wang YC and Tsai
MH: The dietary phytochemical 3,3′-diindolylmethane induces G2/M
arrest and apoptosis in oral squamous cell carcinoma by modulating
Akt-NF-κB, MAPK, and p53 signaling. Chem Biol Interact.
195:224–230. 2012.
|
|
16.
|
Adams JM: Ways of dying: multiple pathways
to apoptosis. Genes Dev. 17:2481–2495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tavani A and La Vecchia C: Fruit and
vegetable consumption and cancer risk in a Mediterranean
population. Am J Clin Nutr. 61(6 Suppl): 1374S–1377S.
1995.PubMed/NCBI
|
|
19.
|
Wang ZB, Liu YQ and Cui YF: Pathways to
caspase activation. Cell Biol Int. 29:489–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cohen JH, Kristal AR and Stanford JL:
Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer
Inst. 92:61–68. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bradfield CA and Bjeldanes LF:
Structure-activity relationships of dietary indoles: a proposed
mechanism of action as modifiers of xenobiotic metabolism. J
Toxicol Environ Health. 21:311–323. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Choi HJ, Lim do Y and Park JH: Induction
of G1 and G2/M cell cycle arrests by the dietary compound
3,3′-diindolylmethane in HT-29 human colon cancer cells. BMC
Gastroenterol. 9:392009.PubMed/NCBI
|
|
23.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Thorburn A: Death receptor-induced cell
killing. Cell Signal. 16:139–144. 2004. View Article : Google Scholar
|
|
25.
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Von Ahsen O, Waterhouse NJ, Kuwana T,
Newmeyer DD and Green DR: The ‘harmless’ release of cytochrome c.
Cell Death Differ. 7:1192–1199. 2000.
|
|
27.
|
Zamzami N, Marchetti P, Castedo M, et al:
Reduction in mitochondrial potential constitutes an early
irreversible step of programmed lymphocyte death in vivo. J Exp
Med. 181:1661–1672. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Chao DT and Korsmeyer SJ: BCL-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar
|
|
29.
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: from innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-κB antiapoptosis: induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998.
|