Introduction
The incidence of malignant melanoma has increased
markedly over the past three decades, more rapidly than any other
solid malignancy. Standard of care chemotherapeutic agents, such as
dacarbazine and temozolomide, yield poor response rates of <20%
(1). Therefore, new strategies for
the treatment of advanced melanoma are urgently required.
Src tyrosine kinase family (SFK) members are known
to be overexpressed and/or activated in many primary types of human
cancer, typically through the mutational activation of upstream
growth factor receptor tyrosine kinases (2). Increased protein levels and kinase
activities of SFK have also been observed in melanoma (3,4).
Dasatinib is a small molecule tyrosine kinase inhibitor that was
initially isolated as a dual Src/ABL inhibitor (5), which has been approved by the Food and
Drug Administration (FDA) for imatinib-resistant chronic
myelogenous leukemia (CML) and Philadelphia chromosome-positive
(Ph+) acute lymphoblastic leukemia (ALL) treatment
(6,7). An abundance of studies support the
anti-tumor effects of dasatinib in cancer prevention and treatment,
including those concerned with triple-negative breast (8–10),
gastric (11), pancreatic (12), head and neck and lung cancer cell
lines (13), as well as with
myeloid leukemia (14).
However, preclinical studies have demonstrated
variable inhibition of melanoma cell growth by dasatinib in
vitro. Eustace et al identified an IC50 value
in the nanomolar range in only one out of five cell lines (15), Homsi et al demonstrated
variable sensitivity in three cell lines (4), Buettner et al revealed little
to no effect on viability (16) and
Kluger et al demonstrated that two out of eight melanoma
cell lines used in the study were growth inhibited by
concentrations <300 nM, whereas the other six were significantly
more resistant (17). Src may act
through different downstream signaling pathways. Hence, the
underlying regulatory mechanisms for the discrepancies in the
antiproliferative effects require investigation.
The RAS/RAF/MAPK pathway is deregulated in >90%
of malignant melanomas. MAPK activation is crucial for the
development of melanocytic neoplasia, and a constitutive activation
of this pathway has been associated with numerous types of cancer
(18,19). Notably, Maat et al
demonstrated a reduction in ERK1/2 activation in metastatic cell
lines compared with that of primary uveal melanoma (UM) cell lines,
and Src kinase was involved in the ERK1/2 activation (20). This suggests that Src may be
involved by regulating the ERK signaling pathway in melanoma
oncogenesis.
In the present study, we demonstrate that dasatinib
induces changes in cell morphology, characterized by an arborized
and contracted appearance, and accompanied by a reduction in cell
proliferation in primary melanoma cells. This morphological change
is associated with the restriction of ERK1/2 activity in the
cytoplasmic compartment.
Materials and methods
Antibodies and reagents
The following primary antibodies (Ab) were used:
Rabbit polyclonal antibody specific for GAPDH (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA); Src,
phospho-SrcTyr416,
phospho-ERK1/2Thr202/Tyr204 and ERK1/2 (Cell Signaling
Technology, Inc., Beverly, MA, USA). Dasatinib was a gift from Dr
Irwin Gelman (Roswell Park Cancer Institute, Buffalo, NY, USA). The
MEK1/2 inhibitor (U0126) was purchased from Calbiochem (San Diego,
CA, USA).
Cell culture
Melanoma cells were derived from primary melanoma
known as Mel-p. The metastatic melanoma cell line A375 was obtained
from the Typical Cell Culture Collection Committee of the Chinese
Academy of Sciences. Cells were maintained in Dulbecco’s modified
Eagle’s media (DMEM) supplemented with 10% fetal bovine serum
(FBS).
MTT assay
Cells (1,000 cells/well; 96-well plate) were
incubated overnight at 37°C in 5% CO2, in media with 10%
FBS. The following day, cells were treated with either a vehicle
control (dimethylsulfoxide, DMSO) or varying concentrations of
dasatinib/U0126, and allowed to grow for an additional 72 h. After
72 h, cell numbers were assessed by an MTT assay; 20 μl of 5
mg/ml MTT was added to each well. Subsequently, the plate was
incubated at 37°C and 5% CO2 for 4–5 h. The medium was
then removed and 150 μl of DMSO was added. The plate was
then incubated in the same conditions as previously for 5 min.
Proliferation was quantified by a plate reader at optical density
(OD) of 570 nm. The cell growth inhibition was calculated as
(T–T0)/(C–T0) × 100 (T, OD of the test well on exposure to the test
drug; C, OD of the vehicle control well; T0, OD at time zero). The
cell growth inhibition curve was generated by plotting cell growth
inhibition against drug concentration, and IG50 was
determined using GraphPad Prism 5 software (GraphPad Software,
Inc., La Jolla, CA, USA).
Cell morphology
Mel-p and A375 cells were plated overnight in 6-well
dishes in the presence or absence of dasatinib (30 nM) or U0126 (10
μM). The plates were photographed digitally using a
phase-contrast microscope.
Immunofluorescence analysis
Melanoma cells were plated on glass coverslips and
treated with DMSO or 30 nM dasatinib for 24 h, and then washed
twice with PBS. The cells were then fixed with 60% acetone/3.7%
formaldehyde at −20°C for 20 min, and blocked with 3% bovine serum
albumin (BSA) in PBS for 30 min at room temperature. Actin
filaments were stained with rhodamine-labeled phalloidin (1:500;
Sigma, St. Louis, MO, USA) and nuclei were stained with DAPI
(1:500; Invitrogen Life Technologies; Carlsbad, CA, USA) for 1 h.
Fluorescent images were captured using an Olympus inverted
microscope equipped with a Roper CoolSnap HQ CCD camera (Metronet
Technology Ltd. (Guangzhou, China). For p-ERK1/2 staining, melanoma
cells were plated on glass coverslips and treated with DMSO or 30
nM dasatinib for 24 h, and serum-starved overnight by incubation
with serum-free DMEM. The cells were stimulated with 10% FBS in
DMEM at the times indicated in the specific figure legends and were
immediately fixed with 60% acetone/3.7% formaldehyde at −20°C,
following the procedure described previously.
Western blot analysis
Cells grown in the presence or absence of dasatinib
or U0126 at the indicated concentration were plated in 10-cm dishes
and incubated with regular DMEM overnight, then lysed in RIPA
buffer. Proteins (40 μg per sample) were separated by
SDS-PAGE, blotted onto PVDF membranes that were blocked for 1 h
with 5% BSA in 1X Tris-buffered saline with 0.1% Tween-20 (TBST)
and then probed as described. Digital imaging and signal
quantification were performed using the Chemi-Genius2 Bio-Imager
(Syngene, Frederick, MD, USA) using GeneTools software.
Results and Discussion
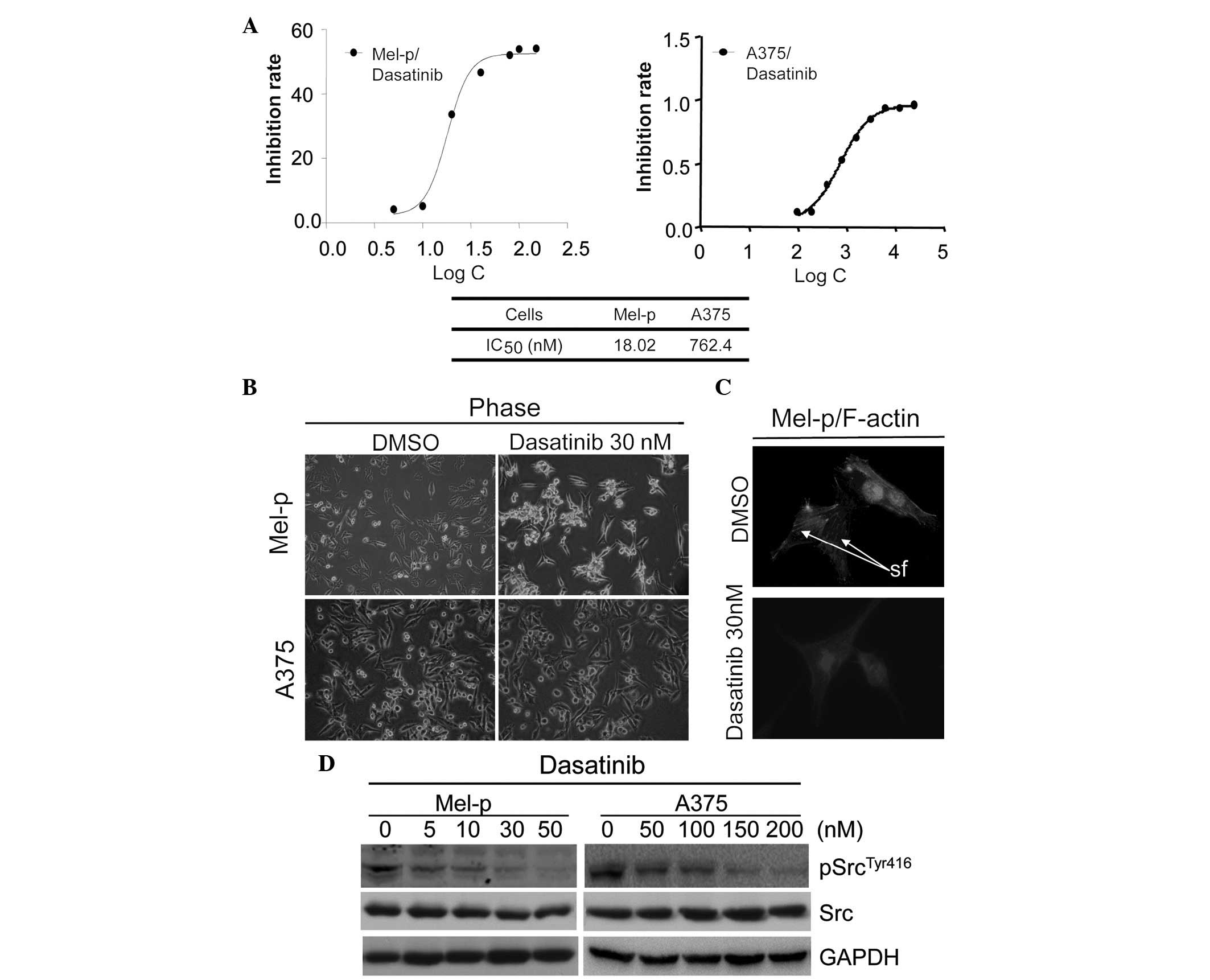
Dasatinib differentially inhibits cell
growth in melanoma cell lines
Previous studies have demonstrated variable
sensitivity to dasatinib in different melanoma cells. Recently,
Maat et al(20) demonstrated
that inhibition of Src led to the growth reduction of primary uveal
melanoma cultures and cell lines, whereas metastatic cell line
growth was only slightly reduced. It was suggested that Src may be
involved in the initiation of melanoma oncogenesis. To test this
hypothesis, two melanoma cell lines (Mel-p, primary melanoma cells
and A375, metastatic melanoma cells) were examined for their
sensitivity to dasatinib in vitro using an MTT assay. The
IC50 values were calculated, following treatment with
dasatinib for 72 h. Mel-p cells demonstrated robust growth
inhibition with an IC50 value of 18.02 nM. Consistent
with a previous study (4), A375
cells were less responsive with an IC50 of 762.4 nM.
These results demonstrate that the inhibition of Src by dasatinib
leads to the growth inhibition of primary melanoma cells.
Dasatinib induces cell differentiation
and remodels the actin cytoskeleton in Mel-p cells
Notably, we observed that dasatinib treatment
induced changes in the morphology of Mel-p cells, which normally
present as flattened and extended cells. Upon dasatinib treatment
at a concentration of 30 nM, the cells displayed a markedly
different morphology that was characterized by an arborized and
contracted appearance (Fig. 1B),
which is recognized as a morphological indication of melanoma cell
differentiation (21). The
percentage of arborized cells following treatment with dasatinib
(30 nM) overnight was counted. The results revealed that 70.2% of
dasatinib-treated Mel-p cells were arborized in comparison to the
control cells (2%). By contrast, no morphological changes were
observed in the A375 cells treated with 30 nM of dasatinib
(Fig. 1B), while only minor
morphological changes were observed in the A375 cells treated with
a higher concentration of dasatinib (≥200 nM) that clearly
inhibited Src activation (Fig. 1D).
These results suggest that Src differentially regulates melanoma
cell morphology.
We further studied whether the remodeling of
cytoskeletal components, such as microfilaments, was involved in
the formation of dendrites in Mel-p cells. As demonstrated in
Fig. 1C, in untreated Mel-p cells,
actin was organized in stress fibers crossing the cytoplasm.
Following treatment with 30 nM dasatinib for 24 h, the actin
cytoskeletal structure was disrupted, creating a dense and compact
cell body. This suggests that inhibition of cell proliferation by
dasatinib is associated with changes in cell shape. Certain
fundamental cellular processes (cell growth and differentiation)
are profoundly influenced by cell shape and substrate adhesion/cell
spreading (22,23).
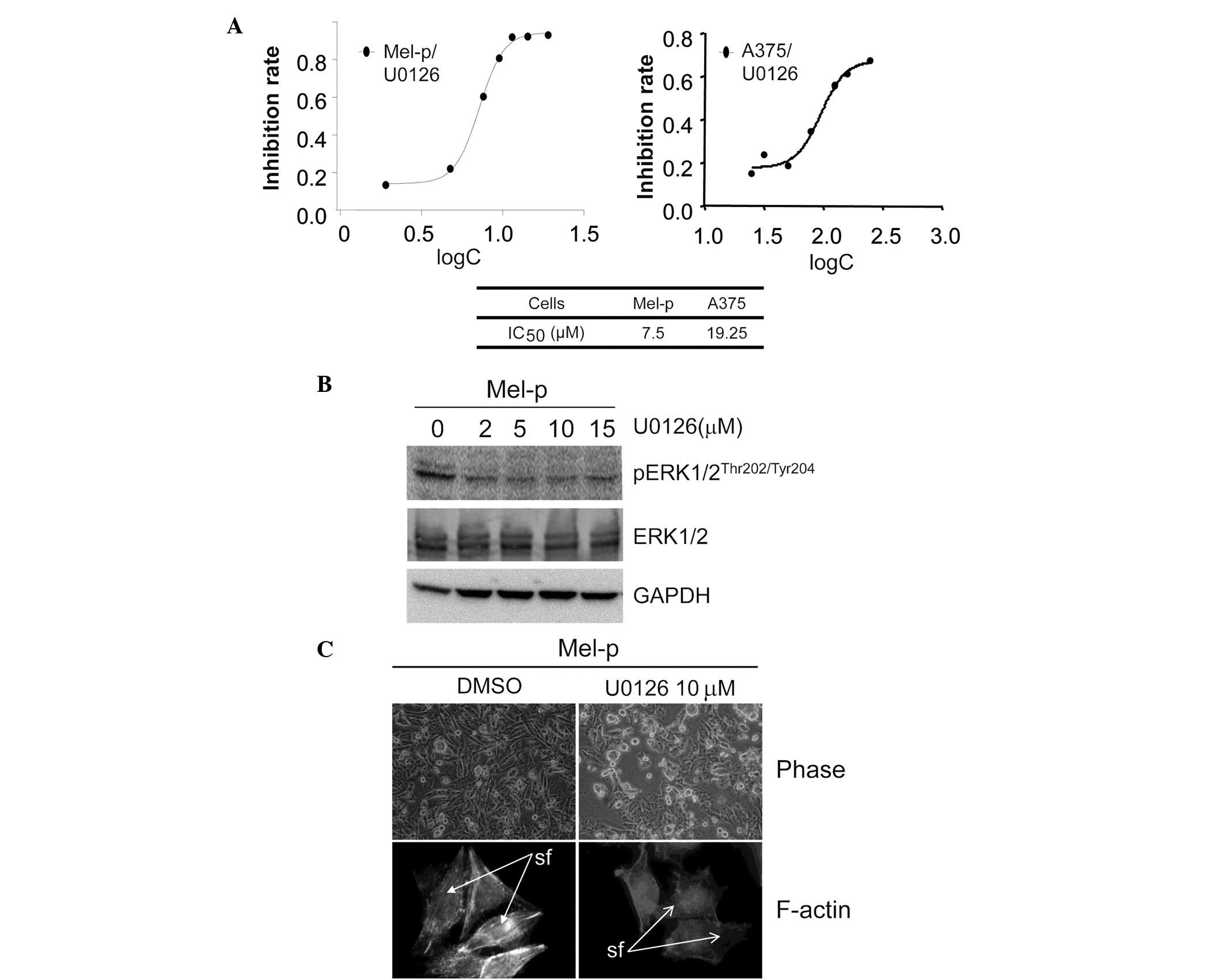
U0126 inhibits the proliferation of Mel-p
cells
Cell shape perturbation, particularly that induced
by cytoskeleton-disrupting drugs, alters the activity of specific
signaling intermediates (24).
Moreover, drug-initiated alterations in both the microfilament and
microtubule networks also mobilize intracellular signaling elements
and activate the ERK, JNK and p38 mitogen-activated protein kinases
(MAPKs) (25,26). In a number of mammalian cell types,
the Ras/MAPK cascade is the principal mitogenic signaling pathway
and MAPK activation is essential for cell growth (27). Alesiani et al demonstrated
that downregulation of the RAF/MEK/ERK pathway sensitizes melanoma
cells to 5,7-dimethoxycoumarin treatment, accompanied by
morphological changes including dendrite outgrowth (28).
To address whether there is an association between
Src, MAPK and the actin cytoskeleton, the effect of ERK on cell
proliferation and morphology was subsequently investigated.
Treatment with the MEK inhibitor, U0126, resulted in a significant
decrease in cell proliferation in Mel-p cells compared with vehicle
control-treated cells. The IC50 value following a 72-h
treatment was calculated (Fig. 2A).
However, 20 μM U0126 did not significantly decrease the
growth of A375 cells. This result indicates that inhibition of
primary melanoma cell growth by dasatinib may be associated with
the activation of ERK. We demonstrated that ERK activity was
significantly inhibited in Mel-p cell lines following treatment
with the MEK inhibitor, U0126 (Fig.
2B). By contrast, U0126 exhibited almost no effect on cell
morphology and the cytoskeleton. Notably, U0126 induced a level of
cell rounding in Mel-p cells similar to that induced by dasatinib
treatment (Fig. 2C). This suggests
that part of the cytoskeletal remodeling induced by dasatinib is
due to the inhibition of MEK activation.
Dasatinib inhibits nuclear translocation
of ERK signaling in Mel-p
Maat et al identified Src to be a crucial
upstream tyrosine kinase for ERK1/2 activation in primary uveal
melanoma (20), suggesting that
Src-ERK1/2 signaling may be important for primary melanoma growth.
A previous study confirmed the contribution of c-Src to cell
shape-dependent ERK1/2 activation (29). It is also well known that growth
stimulation by v-Src requires the activation of MEK/ERK signaling
(30). Elements of the Ras/Raf/MAPK
cascade associate with a microfilament-linked signaling ‘particle’,
suggesting a cell structural basis for MAPK activation (31,32).
v-Src-induced loss of stress fibers and morphological
transformation have been demonstrated previously (33).
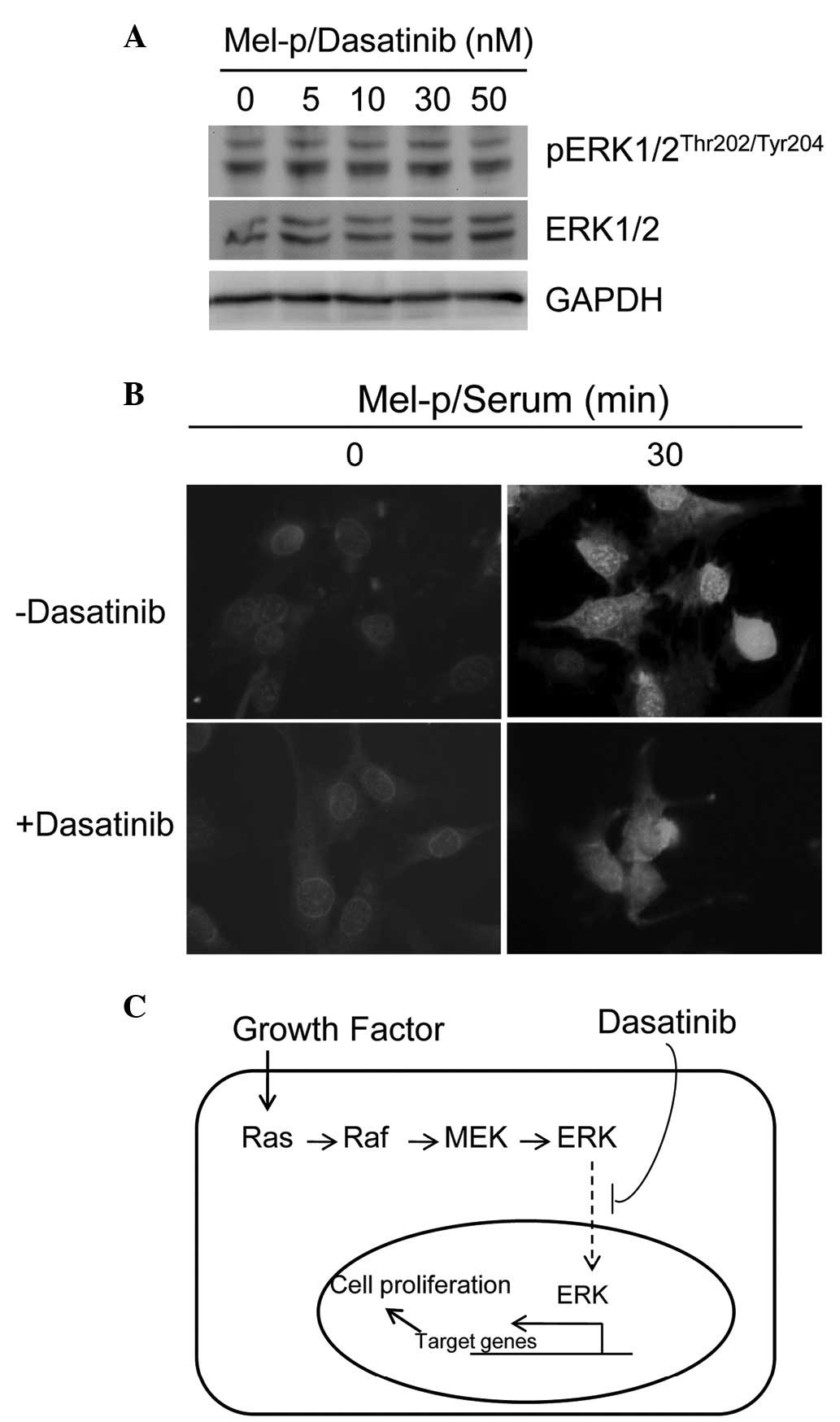
Furthermore, the effects of dasatinib on Src-ERK
signaling were evaluated in Mel-p cell lines in the present study.
Dasatinib caused complete or near-complete inhibition of Src
activity, as measured by phosphorylation at Y416 in western blot
analysis following treatment overnight with concentrations ≥30 nM
(Fig. 1D). However, no significant
change in ERK phosphorylation was observed with dasatinib treatment
(Fig. 3A), suggesting that ERK
activation is not associated with Src inhibition.
Smith et al demonstrated that retinoic
acid-induced differentiation of F9 cells results in uncoupling of
MAPK activation and c-Fos expression (34). It was of interest to determine
whether a similar regulation of the MAPK pathway occurs in Mel-p
cells treated with dasatinib. To confirm that dasatinib-induced
differentiation alters MAPK nucleo-cytoplasmic localization,
activated pERK1/2 localization using indirect immunofluorescence
microscopy was examined in the current study. In untreated cells,
pERK1/2 was detectable in the nuclei within 5 min, reaching a
maximum by 30 min and remaining visible 30–60 min after serum
addition (Fig. 3B, upper panel). In
dasatinib-treated cells, activated ERK1/2 was readily detected
within 5 min (data not shown). However, the pattern of pERK1/2
cellular distribution was markedly different between untreated and
treated cells. In dasatinib-treated cells, pERK1/2 was mainly
distributed in the cytoplasm following serum addition (Fig. 3B, lower panel). Thus, nuclear
translocation of activated pERK1/2 is impaired in dasatinib-treated
cells, suggesting that dasatinib disrupts ERK1/2 signaling.
Conclusions
Dasatinib has been demonstrated to be a
differentiation-inducing compound in human multipotent mesenchymal
stromal cells (35) and
megakaryocytes (36). In the
present study, we have demonstrated that dasatinib induces
morphological (abored formation) differentiation in Mel-p cells.
Several mechanisms have been proposed to explain the reduction in
cell proliferation and impaired growth factor responsiveness that
accompany differentiation. This study indicates that dasatinib
induces differentiation and uncouples MAPK activation by
suppressing the nuclear translocation of activated MAPK.
Abbreviations:
|
SFK
|
Src tyrosine kinase family;
|
|
CML
|
chronic myelogenous leukemia;
|
|
ALL
|
acute lymphoblastic leukemia;
|
|
UM
|
uveal melanoma;
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium;
|
|
FBS
|
fetal bovine serum;
|
|
BSA
|
bovine serum albumin;
|
|
MAPKs
|
mitogen-activated protein kinases
|
Acknowledgements
This study was supported by the
Research Grants of Shenzhen Science and Technology Project
(ZYA201106080030A). The authors would like to thank Shenzhen
Biomedical Research Support Platform and Shenzhen Public Service
Platform for Molecular Diagnosis of Dermatology for their technical
assistance.
References
|
1.
|
Gogas HJ, Kirkwood JM and Sondak VK:
Chemotherapy for metastatic melanoma: time for a change? Cancer.
109:455–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Frame MC: Newest findings on the oldest
oncogene; how activated src does it. J Cell Sci. 117:989–998. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Homsi J, Cubitt C and Daud A: The Src
signaling pathway: a potential target in melanoma and other
malignancies. Expert Opin Ther Targets. 11:91–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Homsi J, Cubitt CL, Zhang S, Munster PN,
Yu H, Sullivan DM, Jove R, Messina JL and Daud AI: Src activation
in melanoma and Src inhibitors as therapeutic agents in melanoma.
Melanoma Res. 19:167–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lombardo LJ, Lee FY, Chen P, Norris D,
Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM,
Fairchild C, et al: Discovery of N-(2-chloro-6-methyl-
phenyl)-2-(6-(4-(2-hydroxyethyl)-
piperazin-1-yl)-2-methylpyrimidin-4- ylamino)
thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase
inhibitor with potent antitumor activity in preclinical assays. J
Med Chem. 47:6658–6661. 2004. View Article : Google Scholar
|
|
6.
|
Steinberg M: Dasatinib: a tyrosine kinase
inhibitor for the treatment of chronic myelogenous leukemia and
philadelphia chromosome-positive acute lymphoblastic leukemia. Clin
Ther. 29:2289–2308. 2007. View Article : Google Scholar
|
|
7.
|
Talpaz M, Shah NP, Kantarjian H, Donato N,
Nicoll J, Paquette R, Cortes J, O’Brien S, Nicaise C, Bleickardt E,
Blackwood-Chirchir MA, Iyer V, Chen TT, Huang F, Decillis AP and
Sawyers CL: Dasatinib in imatinib-resistant Philadelphia
chromosome-positive leukemias. N Engl J Med. 354:2531–2541. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Finn RS, Dering J, Ginther C, Wilson CA,
Glaspy P, Tchekmedyian N and Slamon DJ: Dasatinib, an orally active
small molecule inhibitor of both the src and abl kinases,
selectively inhibits growth of basal-type/‘triple-negative’ breast
cancer cell lines growing in vitro. Breast Cancer Res Treat.
105:319–326. 2007.PubMed/NCBI
|
|
9.
|
Pichot CS, Hartig SM, Xia L, Arvanitis C,
Monisvais D, Lee FY, Frost JA and Corey SJ: Dasatinib synergizes
with doxorubicin to block growth, migration, and invasion of breast
cancer cells. Br J Cancer. 101:38–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Nautiyal J, Majumder P, Patel BB, Lee FY
and Majumdar AP: Src inhibitor dasatinib inhibits growth of breast
cancer cells by modulating EGFR signaling. Cancer Lett.
283:143–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Okamoto W, Okamoto I, Yoshida T, Okamoto
K, Takezawa K, Hatashita E, Yamada Y, Kuwata K, Arao T, Yanagihara
K, Fukuoka M, Nishio K and Nakagawa K: Identification of c-Src as a
potential therapeutic target for gastric cancer and of MET
activation as a cause of resistance to c-Src inhibition. Mol Cancer
Ther. 9:1188–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nagaraj NS, Smith JJ, Revetta F,
Washington MK and Merchant NB: Targeted inhibition of SRC kinase
signaling attenuates pancreatic tumorigenesis. Mol Cancer Ther.
9:2322–2332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Johnson FM, Saigal B, Talpaz M and Donato
NJ: Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses
invasion and induces cell cycle arrest and apoptosis of head and
neck squamous cell carcinoma and non-small cell lung cancer cells.
Clin Cancer Res. 11:6924–6932. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Guerrouahen BS, Futami M, Vaklavas C,
Kanerva J, Whichard ZL, Nwawka K, Blanchard EG, Lee FY, Robinson
LJ, Arceci R, Kornblau SM, Wieder E, Cayre YE and Corey SJ:
Dasatinib inhibits the growth of molecularly heterogeneous myeloid
leukemias. Clin Cancer Res. 16:1149–1158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Eustace AJ, Crown J, Clynes M and
O’Donovan N: Preclinical evaluation of dasatinib, a potent Src
kinase inhibitor, in melanoma cell lines. J Transl Med. 6:532008.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Buettner R, Mesa T, Vultur A, Lee F and
Jove R: Inhibition of Src family kinases with dasatinib blocks
migration and invasion of human melanoma cells. Mol Cancer Res.
6:1766–1774. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kluger HM, Dudek AZ, McCann C, Ritacco J,
Southard N, Jilaveanu LB, Molinaro A and Sznol M: A phase 2 trial
of dasatinib in advanced melanoma. Cancer. 117:2202–2208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Goding CR: Mitf from neural crest to
melanoma: signal transduction and transcription in the melanocyte
lineage. Genes Dev. 14:1712–1728. 2000.PubMed/NCBI
|
|
19.
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Maat W, el Filali M, Dirks-Mulder A,
Luyten GP, Gruis NA, Desjardins L, Boender P, Jager MJ and van der
Velden PA: Episodic Src activation in uveal melanoma revealed by
kinase activity profiling. Br J Cancer. 101:312–319. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Busca R, Bertolotto C, Abbe P, Englaro W,
Ishizaki T, Narumiya S, Boquet P, Ortonne JP and Ballotti R:
Inhibition of Rho is required for cAMP-induced melanoma cell
differentiation. Mol Biol Cell. 9:1367–1378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Folkman J and Moscona A: Role of cell
shape in growth control. Nature. 273:345–349. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Aplin AE and Juliano RL: Integrin and
cytoskeletal regulation of growth factor signaling to the MAP
kinase pathway. J Cell Sci. 112:695–706. 1999.PubMed/NCBI
|
|
24.
|
Schmid-Alliana A, Menou L, Manie S,
Schmid-Antomarchi H, Millet MA, Giuriato S, Ferrua B and Rossi B:
Microtubule integrity regulates src-like and extracellular
signal-regulated kinase activities in human pro-monocytic cells.
Importance for interleukin-1 production. J Biol Chem.
273:3394–3400. 1998. View Article : Google Scholar
|
|
25.
|
Irigoyen JP, Besser D and Nagamine Y:
Cytoskeleton reorganization induces the urokinase-type plasminogen
activator gene via the Ras/extracellular signal-regulated kinase
(ERK) signaling pathway. J Biol Chem. 272:1904–1909. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Rijken PJ, van Hal GJ, van der Heyden MA,
Verkleij AJ and Boonstra J: Actin polymerization is required for
negative feedback regulation of epidermal growth factor-induced
signal transduction. Exp Cell Res. 243:254–262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Brunet A, Roux D, Lenormand P, Dowd S,
Keyse S and Pouyssegur J: Nuclear translocation of p42/p44
mitogen-activated protein kinase is required for growth
factor-induced gene expression and cell cycle entry. EMBO J.
18:664–674. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Alesiani D, Cicconi R, Mattei M, Bei R and
Canini A: Inhibition of Mek 1/2 kinase activity and stimulation of
melanogenesis by 5,7-dimethoxycoumarin treatment of melanoma cells.
Int J Oncol. 34:1727–1735. 2009.PubMed/NCBI
|
|
29.
|
Samarakoon R and Higgins PJ: Pp60c-src
mediates ERK activation/nuclear localization and PAI-1 gene
expression in response to cellular deformation. J Cell Physiol.
195:411–420. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Riley D, Carragher NO, Frame MC and Wyke
JA: The mechanism of cell cycle regulation by v-Src. Oncogene.
20:5941–5950. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Carothers Carraway CA, Fang H, Ye XH,
Juang SH, Liu YC, Carvajal ME and Carraway KL:
Membrane-microfilament interactions in ascites tumor cell
microvilli. Identification and isolation of a large
microfilament-associated membrane glycoprotein complex. J Biol
Chem. 266:16238–16246. 1991.
|
|
32.
|
Carraway CA, Carvajal ME and Carraway KL:
Association of the Ras to mitogen-activated protein kinase signal
transduction pathway with microfilaments. Evidence for a
p185(neu)-containing cell surface signal transduction particle
linking the mitogenic pathway to a membrane-microfilament
association site. J Biol Chem. 274:25659–25667. 1999.
|
|
33.
|
Fincham VJ, Chudleigh A and Frame MC:
Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal
disruption during transformation. J Cell Sci. 112:947–956.
1999.PubMed/NCBI
|
|
34.
|
Smith ER, Smedberg JL, Rula ME, Hamilton
TC and Xu XX: Disassociation of MAPK activation and c-Fos
expression in F9 embryonic carcinoma cells following retinoic
acid-induced endoderm differentiation. J Biol Chem.
276:32094–32100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Borriello A, Caldarelli I, Basile MA,
Bencivenga D, Tramontano A, Perrotta S, Della RF and Oliva A: The
tyrosine kinase inhibitor dasatinib induces a marked adipogenic
differentiation of human multipotent mesenchymal stromal cells.
PLoS One. 6:e285552011. View Article : Google Scholar
|
|
36.
|
Mazharian A, Ghevaert C, Zhang L, Massberg
S and Watson SP: Dasatinib enhances megakaryocyte differentiation
but inhibits platelet formation. Blood. 117:5198–5206. 2011.
View Article : Google Scholar : PubMed/NCBI
|