Introduction
The formation of hepatocellular carcinoma (HCC) is
usually sporadic, but the increased incidence of HCC is associated
with familial disease syndromes such as familial adenomatous
polyposis (FAP) and Beckwith-Wiedemann syndrome (BWS) (1). High-intensity focused ultrasound
(HIFU) is, similar to laser treatment, a highly effective tool due
to its minimimally invasive properties and accurate location
targeting. Within a few seconds, sound waves access and thermally
coagulate the target tissue to a certain depth below the skin,
while sparing the surrounding tissue areas. HIFU therapy allows the
recording of an accurate definition of the target area and the
monitoring of therapy in real time. The aim of the HIFU therapy is
the complete denaturation of the localized tumor tissue. It is
believed that the cells are irreversibly damaged when heated to
above 60°C after a few seconds (<8 sec) regardless of the tissue
type. HIFU therapy, as an alternative treatment, has been used for
VX2 liver tumors in the rabbit, in which tumors have directly
invaded the local prostate tissue (2).
Computed tomography perfusion (CTP), as a tool to
obtain functional information about blood flow, efficiently locates
abnormal tissue perfusion which is difficult to detect accurately
with conventional CT and MRI (3).
CTP may be performed quickly and provide valuable data for
diagnosis. The present study aimed to investigate the changes of
the multislice CT (MSCT) perfusion parameters and histopathology of
the liver in rabbits with VX2 tumors before and after HIFU
therapy.
Materials and methods
Animal model
Eight New Zealand white rabbits weighing 4–5 kg were
used in this study. As the blood supply of the VX2 tumor is similar
to that of human HCC (4,5), VX2 carcinoma cells were used. VX2
cells were implanted into the hind limb of a donor rabbit and grew
in the hind limb. When the size of the tumors reached 7–8
mm3 in volume, the tumors were harvested and implanted
into the livers of eight rabbits 3 weeks prior to the HIFU
treatment. The VX2 liver tumors grew in all eight rabbits. All
animals received humane care in compliance with the Principles of
Laboratory Animal Care formulated by the National Society of
Medical Research and the Guide for the Care and Use of Laboratory
Animals, published by the US National Institutes of Health. The
protocol was approved by the Animal Care and Use Committee of
Binzhou Medical University.
HIFU therapy
The Haifu Model JC HIFU system (Chongqing HAIFU
Company, Chongqing, China) was used as described previously
(6). Briefly, the device is
equipped with a 12-cm diameter, single element piezo-ceramic
transducer fronted by acoustic lenses of varying focal lengths. An
AU3 US imaging device (Esaote, Genoa, Italy) is mounted coaxially
with the high-energy transducer allowing treatment to be guided in
real time. Each rabbit underwent two HIFU treatment sessions under
general anesthesia. Treatment consisted of a combination of single
and multiple overlapping ultrasonic pulses directed to the target
liver tumor. According to the protocols, a single tumor, or part of
a single tumor, was selected for ablation.
CTP imaging
CT scans were performed in the transverse plane
using a 64-channel multidetector CT scanner (Sensation Cardiac 64).
CTP consisted of a 60-sec series with 30 gantry rotations performed
in cine mode during the intravenous administration of iodinated
contrast material. Images were acquired and reconstructed at a
temporal sampling rate of 1 image per 2 sec, resulting in a series
of 20 images for each assessed section. Following unenhanced CT of
the whole liver, eight adjacent 7.2-mm-thick sections were selected
by starting at the level of the basal ganglia. A test bolus of 40
ml Ultravist 370 (Schering Health Care Ltd., Berlin, Germany) was
administered into a vein, and saline chaser bolus of 20 ml was
administered using a power injector at an injection rate of 5.0
ml/sec. At 4 sec after initiation of the contrast injection, a cine
scan was initiated with the gantry angle parallel to and above the
orbital roof to avoid radiation exposure to the lens. Dynamic CTP
scanning was performed on the four-layered region including the
circle of Willis for 40 sec with 7.2 mm thickness and 7.2 mm
coverage area.
Data analysis
Post-processing was performed using Siemens
perfusion CT software. The software relied on the central volume
principle to calculate perfusion parameters from the
time-concentration curve. The highest peak was selected as the
output vein and combined with time-density curves (TDCs) of
contrast agent through the tissue to obtain the hepatic blood flow
(HBF), hepatic blood volume (HBV), mean transit time (MTT) and
permeability-surface area product (PS). The abnormal perfusion
regions were observed to find the changes of distribution and
color. The abnormal perfusion regions were viewed as regions of
interest (ROI) if the surroundings demonstrated pathological
changes, these pathological regions were also included in the ROI.
When outlining ROI, it was important to avoid the great vessels.
Four consecutive absolute CTP data were obtained by the mirror
method.
Histology/immunohistology
The histopathological features of liver tissues were
assessed semi-quantitatively before and after the treatment. The
liver tumor samples were collected from rabbits before and after
the treatment. The samples were conserved in 10% buffered formalin,
and 5-μm-thick sample sections were prepared for hematoxylin and
eosin (H&E) staining to evaluate the basic histomorphology of
the specimens. Immunohistochemistry was performed using a rat
anti-CD3 antibody (pan-T-cell marker; Serotec, Oxford, UK), which
shows a wide range of species cross-reactivity (7), using the a streptavidin-biotin
detection system (Super Sensitive Immunodetection System; Biogenex,
San Ramon, CA, USA) as previously described (8).
Statistical analysis
Comparisons of CTP data between before and after
surgery were performed using a Student’s t-test. The data are
presented as mean ± SD. P<0.05 was considered to indicate
statistical significance. Data were analyzed with the SPSS 18.0
statistical software package (SPSS Inc., Chicago, IL, USA).
Results
The implanted VX2 liver tumors grew successfully in
eight rabbits. The size of the tumors ranged from 1.0 to 2.3 cm in
diameter. All tumors were successfully catheterized and a region of
hypervascular was visualized using digital subtraction angiography
(DSA). As expected, hypervascular phenomenon was generally higher
in the most viable, peripheral portion of the tumor. On ultrasound,
the liver VX2 tumors appeared iso- to hypoechoic. Livers of the
rabbits were biopsied successfully before and after HIFU treatment
with a needle biopsy under ultrasound guidance. There were no any
complications during the procedures and no signs of heavy bleeding
or arteriovenous fistula were observed in the harvested tumors
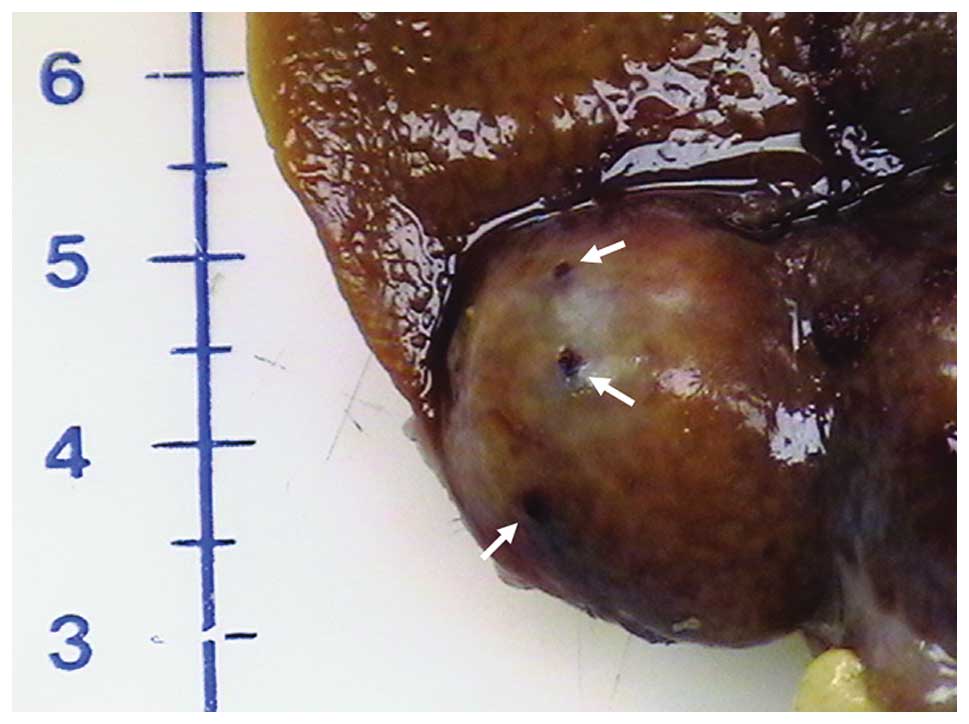
after HIFU treatment (Fig. 1).
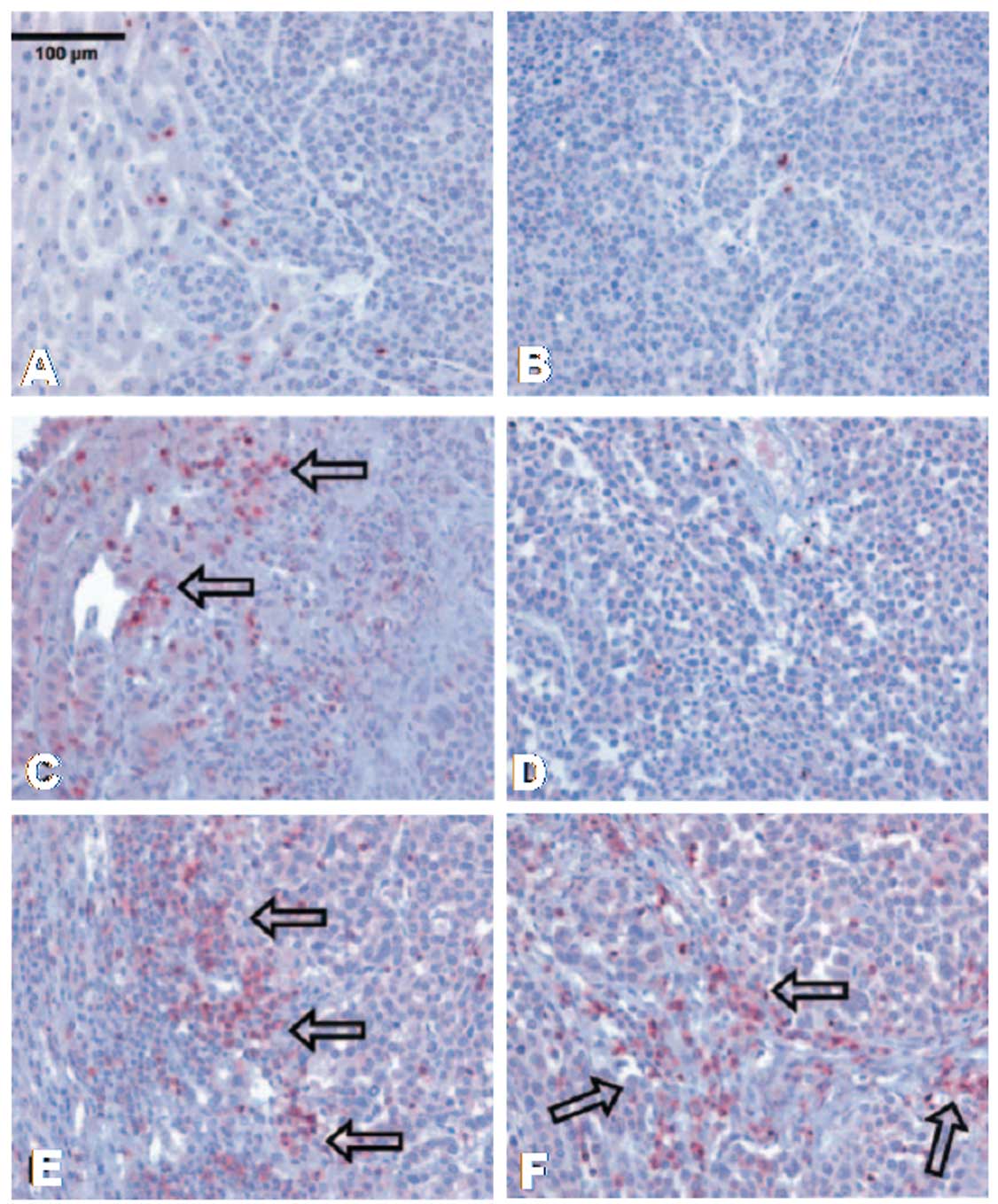
Results of immunohistology revealed that few
CD3+ lymphocytes were present in the liver tumor before
HIFU treatment (Fig. 2A and B). One
day after HIFU treatment, more CD3+ lymphocytes were
observed in the hemorrhagic margin around the tumor (Fig. 2C). Typical signs of cytoplasmic and
nuclear changes were also observed in tumor cells following HIFU
treatment. Here, infiltration of CD3+ lymphocytes was
rarely found, however, there were still more than prior to HIFU
treatment (Fig. 3D). Three weeks
after HIFU treatment, more CD3+ T cells were observed
not only in the margin between the normal tissue and the tumor
(Fig. 2E), but also in the middle
of the tumor (Fig. 2F).
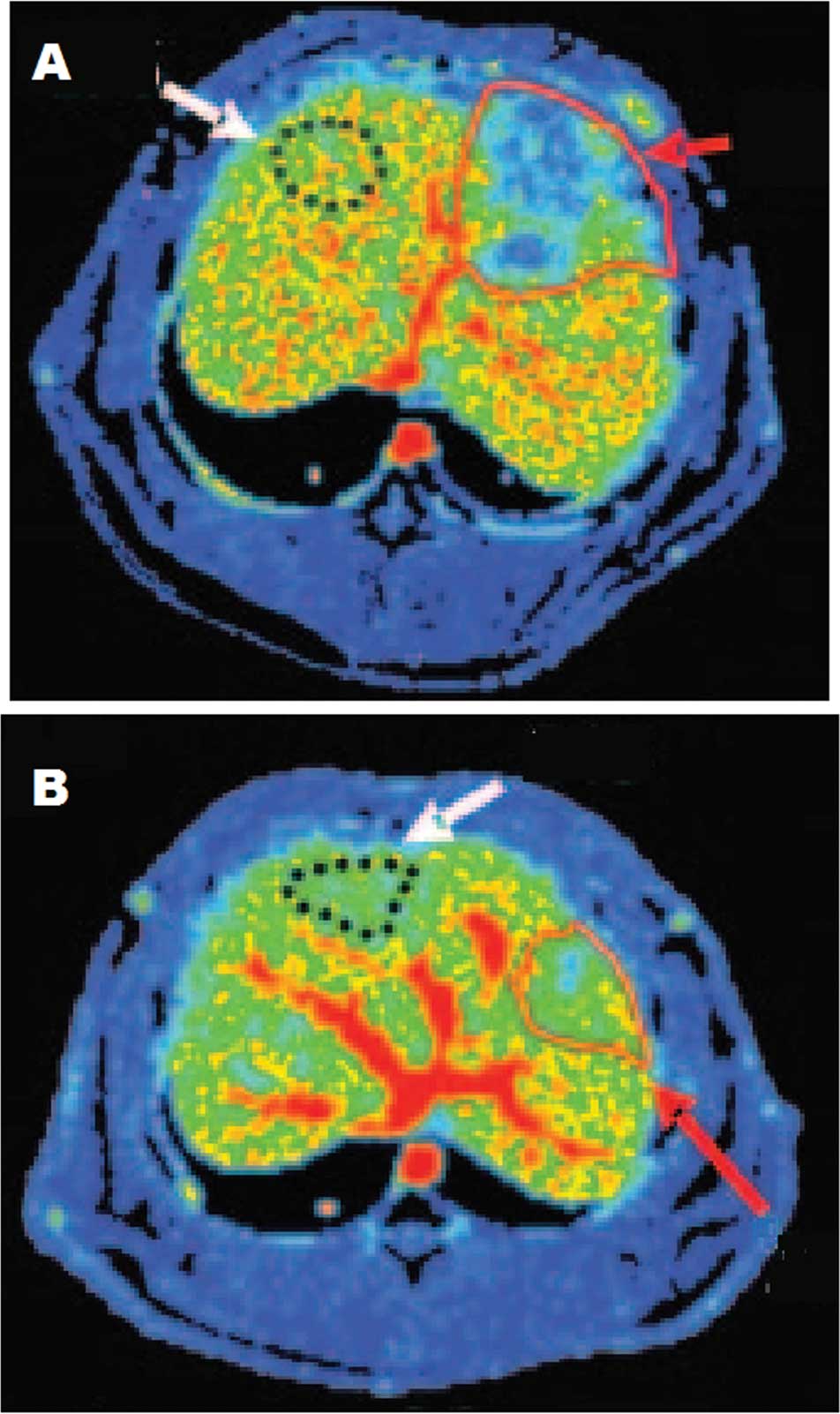
HBF maps obtained by CT perfusion were compared at 1
week before and 6 weeks after therapy to show the change of these
functional CT parameters during the growth of the tumor (Fig. 3).
Six weeks after HIFU therapy, MTT increased
noticeably from 5.45±0.27 to 10.38±2.22 ml/100 g/min (P<0.05)
and PS decreased significantly from 79.03±3.41 to 68.13±0.21 ml/100
g/min (P<0.05). HBF decreased from 265.53±5.26 to 256.97±8.07
ml/100 g/min, while HBV decreased from 21.91±1.38 to 20.85±1.27
ml/100 g. However, no significant differences in HBF and HBV were
found (Table I).
 | Table I.Comparison of HBF, HBV, MTT and PS
between 1 week before and 6 weeks after therapy. |
Table I.
Comparison of HBF, HBV, MTT and PS
between 1 week before and 6 weeks after therapy.
| CTP data | Preoperative 1
week | Postoperative 6
weeks | T-value | P-value |
|---|
| HBF, ml/100
g/min | 265.53±5.26 | 256.97±8.07 | 0.845 | 0.442 |
| HBV, ml/100 g | 21.91±1.38 | 20.85±1.27 | 0.631 | 0.131 |
| MTT, sec | 5.45±0.27 | 10.38±2.22 | −6.687 | 0.002a |
| PS, ml/100 g/min | 79.03±3.41 | 68.13±0.21 | 5.704 | 0.011a |
Discussion
Phenomenon has indicated that focused ultrasound may
activate tumor-specific immune response. However, the physical
properties of ultrasound limits the application of HIFU in the
air-filled organs (lung, stomach, intestines, gall bladder,
pancreas and parts of the esophagus) and those that are covered by
bone (thoracic organs). The absorption of these interfaces may also
lead to unwanted damage to adjacent organs (9,10). In
cases of insufficient coupling, a similar mechanism leads to
redness and burning of the outer skin (11,12).
Another problem is the shifting of abdominal organs by respiratory
motion. The organs may be shifted by up to 20 mm within a
respiratory cycle and this significantly limits the efficiency and
safety of the procedure. A solution to this problem can only be
provided by reliable real-time imaging, which provides direct
control of focus localization. Ideally, this would also be
integrated into an automatic readjustment to compensate for the
respiratory amplitude in the treatment system (13), although this technique has not yet
been fully developed.
The theoretical possibility has been discussed that
the spread of tumor cells could be inhibited through the mechanical
effects of focused ultrasound (9).
Studies of this issue, however, have shown that the metastatic rate
is not increased by treatment with HIFU (14–16).
In addition, several research groups have revealed an inhibitory
effect on the growth of existing metastases, a reduction in the
development of metastases or even regression of existing metastases
(16–19). These phenomena may be associated
with the activation of endogenous antitumor immunity. According to
this theory, tumor cells rupture and fragments of the cells act as
specific antigens. Wu et al showed a significant increase in
CD4+ lymphocyte population after the HIFU treatment of
osteosarcoma and renal cell carcinoma, and it may be associated
with an activated systemic cellular immune response (15). The first indications of this
phenomenon were reported by Wagai and Kaketa in 1970, who revealed
that tumor-bearing rats had significantly higher rates of
resistance against proliferating tumor cells following treatment
with HIFU (17). Wu et al
also showed that cancer cells exhibit malignant characteristics,
including invasiveness, unregulated growth, metastasis and
immortality, after HIFU treatment (18). This may prove to be a great
advantage over conventional surgery, in which excessive growth of
metastases following resection of the tumor itself is often
observed. The reason for this may be the release of growth factors,
an imbalance of pro- and antiangiogenic factors or the general
immunosuppression following surgical intervention (20,21).
CTP data processing that can typically be achieved
in 5 min is performed with postprocessing software using either
rate-of-upslope estimation of HBF or deconvolution analysis. Only
deconvolution analysis leads to quantitatively accurate results,
including in areas with low perfusion (22). Generally, absolute values of CTP
parameters can be calculated by CTP software, such as CT Perfusion
2 (23,24). However, Leenders et al found
that CTP parameters varied in a large range due to individual
differences and the experience of operational staff (25). Comparing HBF, HBV, MTT and PS values
between abnormal regions and mirror-image control regions is an
effective method of measuring the status of underperfusion present
in a given case or location.
In summary, our findings suggest that HIFU therapy
may be a simple and effective method for the treatment of liver
tumors. CTP, as a smart method to obtain functional information
about HBF, is able to quantify tumor vascularity and angiogenesis
in liver tumors.
References
|
1.
|
Buendia MA: Genetic alterations in
hepatoblastoma and hepatocellular carcinoma: common and distinctive
aspects. Med Pediatr Oncol. 39:530–535. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tofts PS, Lloyd D, Clark CA, et al: Test
liquids for quantitative MRI measurements of self-diffusion
coefficient in vivo. Magn Res Med. 43:368–374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Prat F, Centarti M, Sibille A, et al:
Extracorporeal high-intensity focused ultrasound for VX2 liver
tumors in the rabbit. Hepatology. 21:832–836. 1995.PubMed/NCBI
|
|
4.
|
Ramirez LH, Juliéron M, Bonnay M, et al:
Stimulation of tumor growth in vitro and in vivo by suramin on the
VX2 model. Invest New Drugs. 13:51–53. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Geschwind JF, Artemov D, Abraham S, et al:
Chemoembolization of liver tumor in a rabbit model: assessment of
tumor cell death with diffusion-weighted MR imaging and histologic
analysis. J Vasc Interv Radiol. 11:1245–1255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kennedy JE, Wu F, ter Haar GR, et al:
High-intensity focused ultrasound for the treatment of liver
tumours. Ultrasonics. 42:931–935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Beineke A, Siebert U, Wunschmann A, et al:
Immunohistochemical investigation of the cross-reactivity of
selected cell markers from various species for characterization of
lymphatic tissues in the harbour porpoise (Phocoena
phocoena). J Comp Pathol. 125:311–317. 2001. View Article : Google Scholar
|
|
8.
|
Neureiter D, Böhmer J, Kirchner T and
Aigner T: Pleomorphic adenomas of the parotid express different
mesenchymal phenotypes: demonstration of matrix gene expression
products characteristic of the fibroblastic and chondrocytic cell
lineages. Histopathology. 35:373–379. 1999. View Article : Google Scholar
|
|
9.
|
Fry FJ and Johnson LK: Tumor irradiation
with intense ultrasound. Ultrasound Med Biol. 4:337–341. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yang R, Reilly CR, Rescorla FJ, et al:
High-intensity focused ultrasound in the treatment of experimental
liver cancer. Arch Surg. 126:1002–1009. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Chapelon JY, Margonari J, Theillère Y, et
al: Effects of high-energy focused ultrasound on kidney tissue in
the rat and the dog. Eur Urol. 22:147–152. 1992.PubMed/NCBI
|
|
12.
|
Chapelon JY, Margonari J, Vernier F, et
al: In vivo effects of high-intensity ultrasound on prostatic
adenocarcinoma Dunning R3327. Cancer Res. 52:6353–6357.
1992.PubMed/NCBI
|
|
13.
|
Oosterhof GO, Cornel EB, Smits GA, et al:
Influence of high-intensity focused ultrasound on the development
of metastases. Eur Urol. 32:91–95. 1997.PubMed/NCBI
|
|
14.
|
Kramer G, Steiner GE, Gröbl M, et al:
Response to sublethal heat treatment of prostatic tumor cells and
of prostatic tumor infiltrating T-cells. Prostate. 58:109–120.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wu F, Wang ZB, Lu P, et al: Activated
anti-tumor immunity in cancer patients after high intensity focused
ultrasound ablation. Ultrasound Med Biol. 30:1217–1222. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kennedy JE: High-intensity focused
ultrasound in the treatment of solid tumours. Nat Rev Cancer.
5:321–327. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wagai T and Kaketa KI: Medical application
of intense ultrasound. Destruction of malignant tumor by intense
focused ultrasound. Annual Report of the Medical Ultrasound
Research Centre; pp. 35–37. 1970
|
|
18.
|
Wu F, Wang ZB, Cao YD, et al: Changes in
biologic characteristics of breast cancer treated with
high-intensity focused ultrasound. Ultrasound Med Biol.
29:1487–1492. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Vallejo R, Hord ED, Barna SA, et al:
Perioperative immunosuppression in cancer patients. J Environ
Pathol Toxicol Oncol. 22:139–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Vallancien G, Chartier-Kastler E, Harouni
M, et al: Focused extracorporeal pyrotherapy: experimental study
and feasibility in man. Semin Urol. 11:7–9. 1993.PubMed/NCBI
|
|
21.
|
Pernot M, Tanter M and Fink M: 3-D
real-time motion correction in high- intensity focused ultrasound
therapy. Ultrasound Med Biol. 30:1239–1249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wintermark M, Maeder P, Thiran JP, et al:
Quantitative assessment of regional cerebral blood flows by
perfusion CT studies at low injection rates: a critical review of
the underlying theoretical models. Eur Radiol. 11:1220–1230. 2001.
View Article : Google Scholar
|
|
23.
|
Eastwood JD, Lev MH, Wintermark M, et al:
Correlation of early dynamic CT perfusion imaging with whole-brain
MR diffusion and perfusion imaging in acute hemispheric stroke.
AJNR Am J Neuroradiol. 24:1869–1875. 2003.PubMed/NCBI
|
|
24.
|
Eastwood JD, Lev MH, Azhari T, et al: CT
perfusion scanning with deconvolution analysis: pilot study in
patients with acute middle cerebral artery stroke. Radiology.
222:227–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Leenders KL, Perani D, Lammertsma AA, et
al: Cerebral blood flow, blood volume and oxygen utilization.
Normal values and effect of age. Brain. 113:27–47. 1990.PubMed/NCBI
|