Introduction
Esophageal cancer is one of the most aggressive
human cancers. It is currently the sixth leading cause of
cancer-related mortality worldwide (1,2).
Cancer of the esophagus is associated with a very poor survival
rate. Even in the most developed countries, the 5-year survival
rate ranges merely from 10–16% (3).
In China, the mortality rate of esophageal cancer is ranked fourth
among all cancer-related mortalities, and esophageal squamous cell
carcinoma (ESCC) is the major histological type (4,5).
Despite significant advances in screening, surgical care and
chemoradiotherapy techniques, the prognosis for patients with ESCC
remains poor (6,7). Thus, it is necessary to search for new
treatment strategies.
Numerous Chinese herbs have been discovered to be
potential sources of antitumor drugs (8,9).
Baicalein (5,6,7-trihydroxyflavone) is one of the key flavones
present in Scutellaria baicalensis Georgi, which has been
used for the treatment of inflammation, cardiovascular disease and
microbial infections (10–12). Accumulating evidence has
demonstrated the antitumor activity of this flavone in a variety of
human cancer cell lines (13–16).
The molecular mechanisms underlying these effects are speculated to
include the modulation of several classes of cyclin-dependent
kinases (CDKs) and CDK regulatory subunits (cyclins) to inhibit
cell cylce progression (16,17),
suppression of proliferation and induction of apoptosis (via
activation of the mitochondrial pathway and DNA fragmentation) in
malignant cells (14,16,18–20).
The PI3K/Akt pathway plays a critical role in
mammalian cell survival and resistance to apoptosis. Alterations in
the PI3K/Akt signaling pathway have also been implicated in the
occurrence and development of human cancer (21,22).
Activation of the PI3K/Akt pathway has been demonstrated to promote
survival of esophageal cancer cells in vitro, as well as
tumorigenicity and metastasis of human esophageal cancer in
vivo(23–25). In addition, it has been demonstrated
that the expression of cell proliferation and cell cycle-related
proteins (such as cyclin D1 and p27), as well as cell
apoptosis-related proteins (including Bcl-2 and Bax), as the
downstream targets of the PI3K/Akt pathway, were regulated by the
PI3K/Akt pathway in human ESCC cells (26).
Notably, baicalein-induced apoptosis and
proliferation retardation has been demonstrated to be mediated by
down-regulation of the PI3K/Akt pathway in human epidermoid
carcinoma (27) and bladder cancer
(17) cells. However, no studies
thus far have examined the effects of proliferation inhibition and
induced apoptosis of baicalein on esophageal carcinoma cells.
Therefore, we conducted an investigation to ascertain whether
baicalein was capable of downregulating the PI3K/Akt pathway in
ESCC EC-109 cells concurrently with induction of apoptotic cell
death. To our knowledge, the present study provides the first
direct evidence that baicalein induces apoptosis in ESCC cells, and
that the underlying mechanism may be activation of the PI3K/Akt
signaling pathway.
Materials and methods
Chemicals and reagents
RPMI-1640 medium, fetal bovine serum (FBS),
penicillin G and streptomycin were obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA). Dimethyl sulfoxide (DMSO),
ribonuclease A (RNase A), Annexin V-Fluorescein Isothiocyanate
(FITC) Apoptosis Detection kit,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and baicalein (C15H10O5, MW
270.24) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All
antibodies (mouse antibodies specific for β-actin, procaspase-9 and
-3, cleaved caspase-9 and -3, PARP, Bcl-2, Bax, Akt, p-Akt, NF-κB,
IκB, p-IκB, mTOR and p-mTOR) and horseradish peroxidase-conjugated
goat anti-mouse secondary antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Baicalein was dissolved
in DMSO. The final DMSO concentration was <1‰ (v/v) in all
experiments.
Cell culture
Human ESCC EC-109 cell line was obtained from the
China Center for Type Culture Collection (CCTCC; Wuhan, China).
Cultures were maintained in RPMI-1640 medium supplemented with 10%
FBS and antibiotics (100 U/ml penicillin and 100 μg/ml
streptomycin) at 37°C in a humidified atmosphere containing 5%
CO2. The study was approved by the Ethics Committee of
Zhengzhou University, Zhengzhou, China.
Examination of morphological changes by a
phase-contrast microscopic study
EC-109 cells (2×105 cells/well) were
maintained in 12-well plates for 24 h and treated with various
concentrations of baicalein (0, 10, 20 and 40 μM) for 24 h.
Morphological changes in cells due to each treatment procedure were
observed and photographed under a phase-contrast microscope.
Cell viability assay
Proliferation of cells was determined by an MTT
assay. Approximately 10,000 EC-109 cells/well were plated in
96-well plates. Following incubation overnight, cells were treated
with baicalein (0, 10, 20 and 40 μM). At various time points
(24–72 h) following baicalein treatment, the medium was removed and
MTT (20 μl of 5 mg/ml) was added to each well and incubated
at 37°C for 4 h. The plates were spun and the purple precipitates
of formazan were dissolved in 150 μl DMSO. Absorbance was
measured at 490 nm using an enzyme-linked immunosorbant asssay
(ELISA) plate reader. The viability of baicalein-treated EC-109
cells was expressed as a percentage relative to
non-baicalein-treated control cells. Control cells were considered
to be 100% viable.
Plate colony forming assay
Suspensions of EC-109 cells were inoculated in
6-well flat-bottomed plates with a density of 3×102
cells/well and 3 wells/group. Cells were dispersed evenly by
slightly shaking the plates and were then incubated with baicalein
at different concentrations in RPMI-1640 medium, with 10% FBS at
37°C and 5% CO2 for 14 days, until the visible clones
appeared. The medium was discarded and cells were carefully washed
twice with PBS. Following fixation with methanol for 15 min, cells
were stained with Giemsa’s solution for 15 min before washing with
tap water and air-drying. Clones with >50 cells were counted
with an ordinary optical microscope. All experiments were repeated
in triplicate and the average values are presented.
Hoechst 33258 staining
Following treatment with baicalein at various
concentrations for 48 h, cells were washed twice with PBS and fixed
in 1 ml of 4% paraformaldehyde for 10 min at 4°C. After washing
twice with PBS, cells were stained with 100 μl Hoechst 33258
in PBS for 15 min at room temperature in the dark, and then washed
with PBS. Cells were mounted and examined by fluorescence
microscopy (Olympus BX-51, Tokyo, Japan). Apoptotic cells were
identified by the condensation and fragmentation of their
nuclei.
DNA fragmentation assay
Following exposure to various concentrations of
baicalein for 48 h, EC-109 cells were collected by centrifugation
and washed twice with PBS. Cell pellets were resuspended in 40
μl of lysis buffer (0.1 M EDTA; 0.1 M Tris-HCl, pH 8.0 and
0.8% SDS) and subsequently treated with 10 μl RNase A (50
μg/ml) at 37°C for 1 h, and with 10 μl proteinase K
(20 μg/ml) at 50°C overnight. Extracted cellular DNA was
subjected to agarose gel (2.0%) chromatography at 35 V for 3 h.
Gels were photographed following staining with 0.5 μg/ml
ethidium bromide.
Measurements of cells in early and late
apoptosis
The ability of baicalein to induce apoptosis in
EC-109 cells was examined by Annexin V-FITC/propidium iodide (PI)
double-staining and flow cytometry. Preparations were treated with
baicalein at various concentrations for 48 h. Cells were then
harvested, resuspended to 5×105/ml in binding buffer (10
mM HEPES, pH 7.4; 150 mM NaCl; 5 mM KCl; 1 mM MgCl2 and
1.8 mM CaCl2), and doubly stained with Annexin V-FITC/PI
according to the manufacturer’s instructions. The percentages of
viable, early apoptotic, late apoptotic and necrotic cells were
determined using a FACSort flow cytometer (Becton Dickinson, San
José, CA, USA).
Western blot analysis
EC-109 cells were treated with 20 μM
baicalein for 0–72 h. Then, cells were harvested and lysed on ice
for 30 min in lysis buffer containing 50 mM Tris-HCl, pH 8.0; 150
mM NaCl; 20 mM EDTA; 50 mM NaF; 1% NP-40 and 0.02% NaN3.
The lysis buffer also contained protease inhibitors (1 mM PMSF and
1 μg/ml aprotinin) to prevent proteolysis and/or
dephosphorylation. Lysates were collected following centrifugation
at 12,000 rpm for 20 min at 4°C. Protein levels were quantified
using the Lowry method (28).
Equivalent weights of protein (50 μg/lane) were separated by
15% sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to polyvinylidine fluoride (PVDF)
membranes. Membranes were blocked with 5% non-fat milk in TBST
buffer for 1 h and then incubated with primary antibodies at 1:1000
dilution in 5% non-fat milk overnight at 4°C. Membranes were then
washed twice and incubated with secondary antibodies conjugated
with horseradish peroxidase at 1:1,000 dilution for 1 h at room
temperature. After extensive washing with TBST, protein bands were
visualized by the enhanced chemiluminescence reagent (Amersham
Pharmacia Biotech, Tokyo, Japan). The relative expression ratios of
experimentals and controls were calculated according to the
reference band of β-actin by the density using the software
Un-SCAN-IT gel Version 6.1 (Silk Scientific, Inc., Orem, UT, USA).
Experiments were repeated in triplicate.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Significance of the difference in means between groups was obtained
by analysis of variance (ANOVA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibition of cell growth by
baicalein
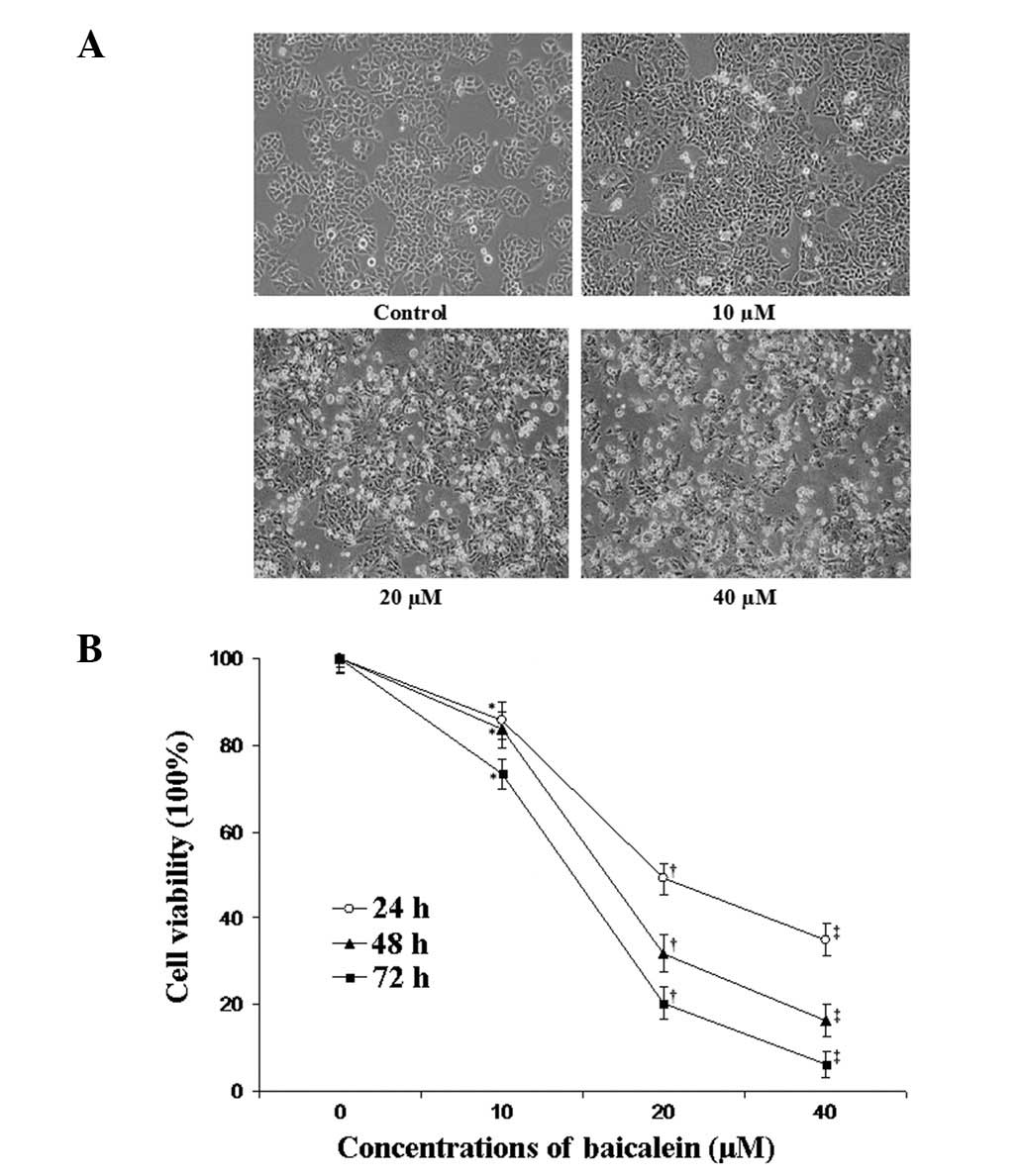
Cell morphology was examined using phase-contrast
microscopy. Microscopic observations revealed that EC-109 cells
exposed to various concentrations of baicalein underwent
significant morphological alterations (Fig. 1A). When exposed to baicalein at a
concentration of 10 μM, EC-109 cells began to shrink and
retract from the neighboring cells. At a concentration of 20
μM, floating cells began to appear in the culture medium.
EC-109 cells incubated in concentrations of 40 μM of
baicalein lost their original morphological (flat, polygonal) shape
and additional floating cells appeared. An MTT assay was
implemented to examine the viability of EC-109 cells exposed to 0,
10, 20 and 40 μM of baicalein in the culture medium for 24,
48 and 72 h. It was found that baicalein significantly decreased
the cell viability of EC-109 cells in a concentration- and
time-dependent manner (Fig.
1B).
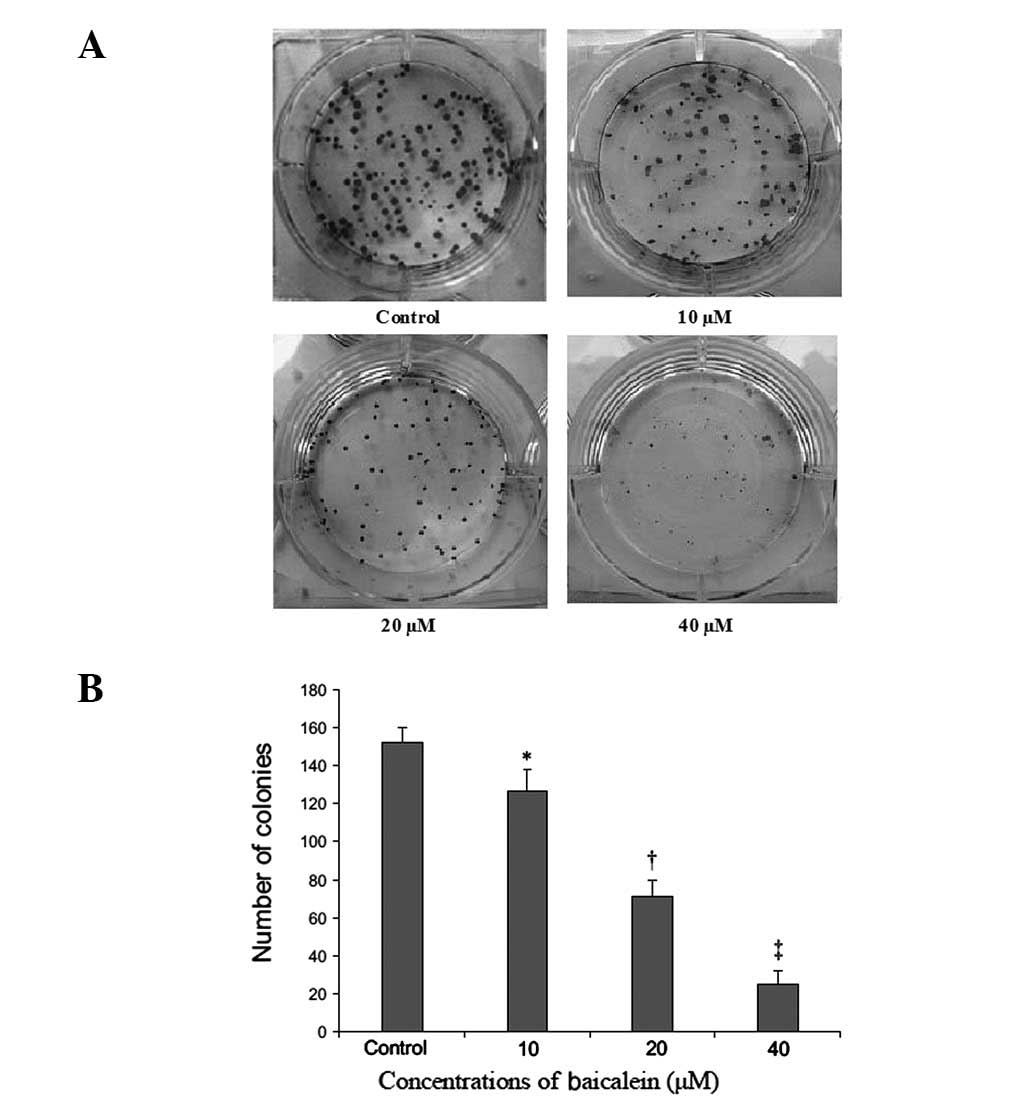
Inhibition of colony formation
Baicalein also suppressed plate colony formation of
EC-109 cells at 14 days post-seeding (Fig. 2A). The number of colonies for
control preparations was 152±5.1. By contrast, the number of
colonies that formed for preparations treated with baicalein at 10,
20 and 40 μM were 127±4.5, 71±9.2 and 25± 4.5, respectively
(P<0.05) (Fig. 2B).
Baicalein induces apoptosis in EC-109
cells
Hoechst 33258 stain is sensitive to DNA and is used
to assess changes in nuclear morphology. The Hoechst 33258 staining
assay revealed that cells demonstrated apoptotic features including
nuclear shrinkage and chromatin condensation or fragmentation,
following treatment with baicalein for 48 h. The rate of cells with
a profile of chromatin condensation and fragmented fluorescent
nuclei increased in a concentration-dependent manner (Fig. 3A). The ability of baicalein to
induce DNA fragmentation, a hallmark of apoptosis, was examined
after 48 h of culture. No significant fragmentation was observed in
preparations treated with either solvent or 10 μM baicalein.
However, fragmentation was clearly observable in preparations
treated with 20 and 40 μM baicalein (Fig. 3B). An annexin V-FITC and PI
double-staining technique was implemented to investigate whether
baicalein induced apoptosis in these cells. The percentage of
EC-109 cells undergoing early apoptotic cell death was increased by
baicalein in a concentration-dependent manner (Fig. 3C). The percentage of cells
undergoing apoptosis was determined by the sum of cells in early
and late apoptosis. After 48 h of treatment, 2.5±0.35, 18.8±1.15,
25.5±1.99 and 30.8±2.25% of cells were apoptotic at concentrations
of 0, 10, 20 and 40 μM of baicalein, respectively (Fig. 3D).
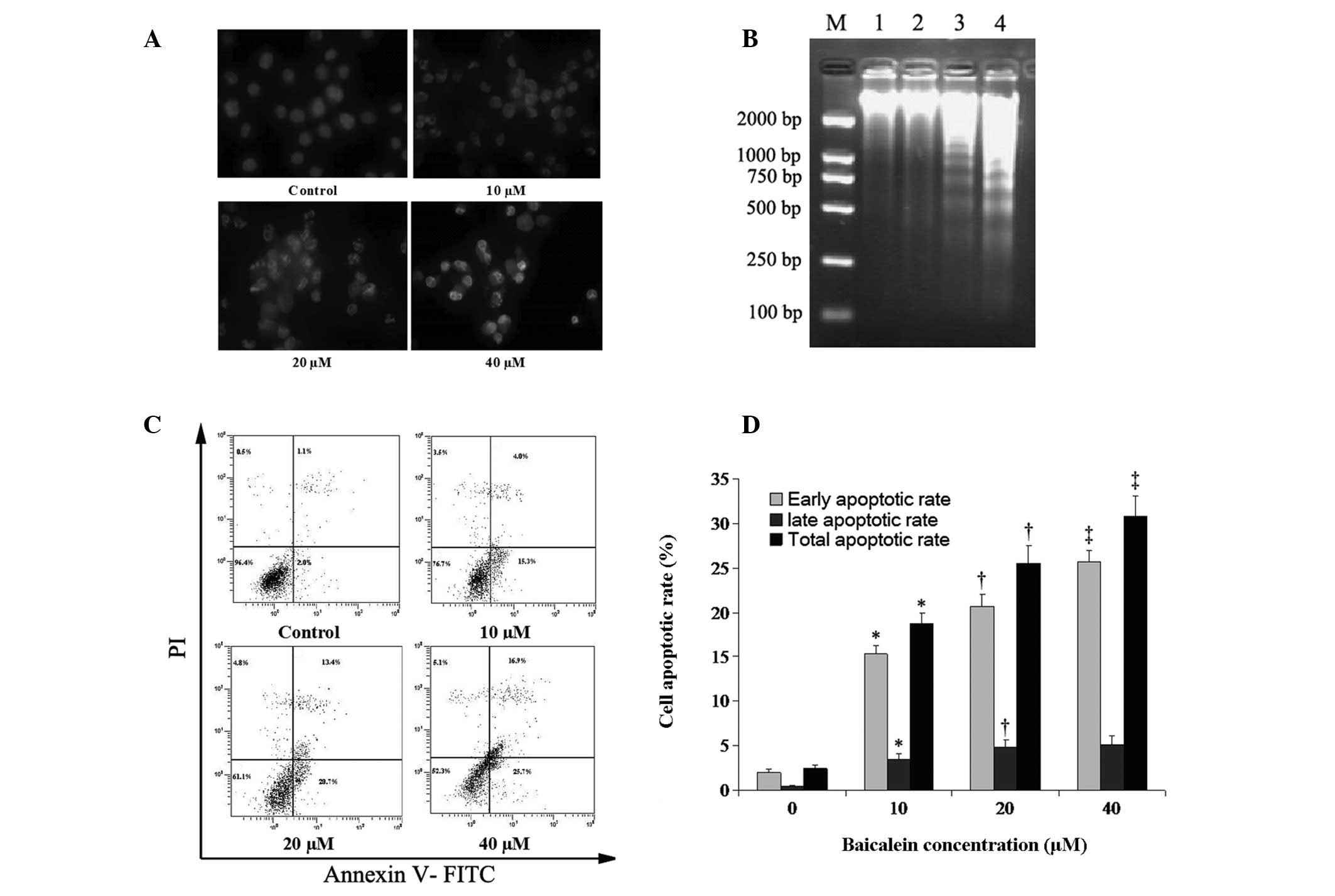 | Figure 3.Induction of apoptosis in EC-109
cells by baicalein. (A) Hoechst 33258 staining. The impact of cell
apoptosis of baicalein on cultured EC-109 cells without baicalein
or following treatment with baicalein at 10, 20 and 40 μM
for 48 h. (B) EC-109 cells were treated for 48 h with baicalein at
0 (lane 1), 10 (lane 2), 20 (lane 3), and 40 μM (lane 4).
Lane M shows the migration of D2000 markers (100, 250, 500, 750,
1,000 and 2,000 bp). (C) Annexin V-FITC/PI double staining and flow
cytometry were used to determine the percentages of cells in
apoptosis. Viable, early apoptotic, late apoptotic and necrotic
cells were determined following treatment for 48 h with baicalein
at various concentrations. Bottom left quadrants, viable cells;
bottom right quadrants, early apoptotic cells; top right quadrants,
late apoptotic cells; top left quadrants, necrotic cells. (D)
Percentages of cells in apoptosis at each baicalein concentration.
Cells in the bottom and top right quadrants were summed to obtain
the total number of apoptotic cells. Findings are presented as the
mean of three similar experiments ± standard deviation.
*P<0.05 compared with the solvent control;
†P<0.05 compared with 10 μM baicalein;
‡P<0.05 compared with 20 μM baicalein. |
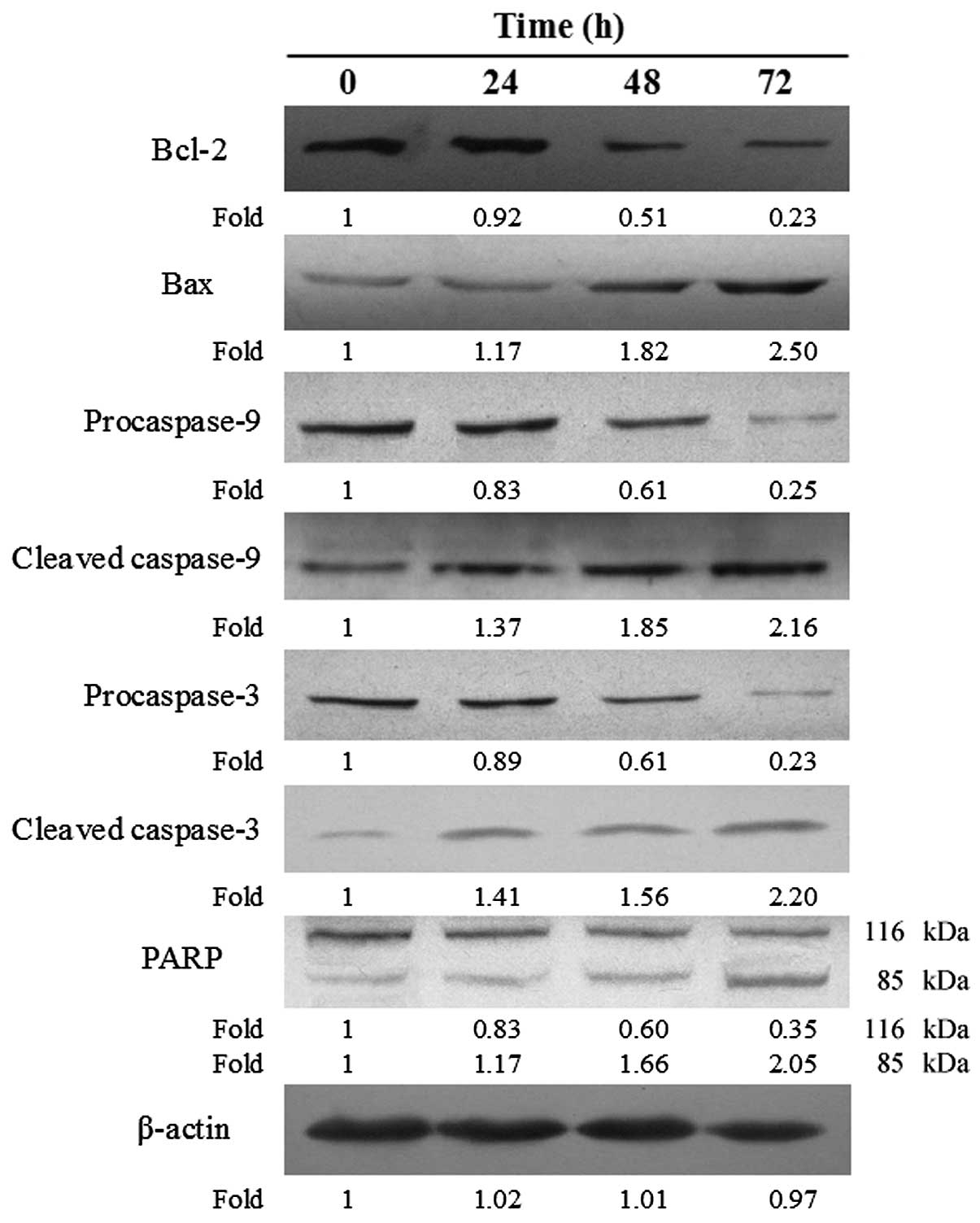
Activation of the intrinsic mitochondrial
apoptotic pathway
It was considered essential to ascertain whether
baicalein suppressed the viability of EC-109 cells and promoted DNA
fragmentation in such cells through activation of the intrinsic
(mitochondrial) apoptotic pathway. Therefore, expression of the
relevant apoptosis-related proteins was examined by western blot
analysis. Treatment with baicalein increased the expression of
pro-apoptotic proteins including Bax, activated (cleaved) caspase-3
and -9, and activated (cleaved) PARP. By contrast, expression of
the anti-apoptotic protein Bcl-2, procaspase-3 and -9, and of the
inactive form of PARP, was decreased following treatment with the
drug. The relative expression ratios of these proteins following
baicalein treatment was quantified by the density, and findings are
presented in Fig. 4.
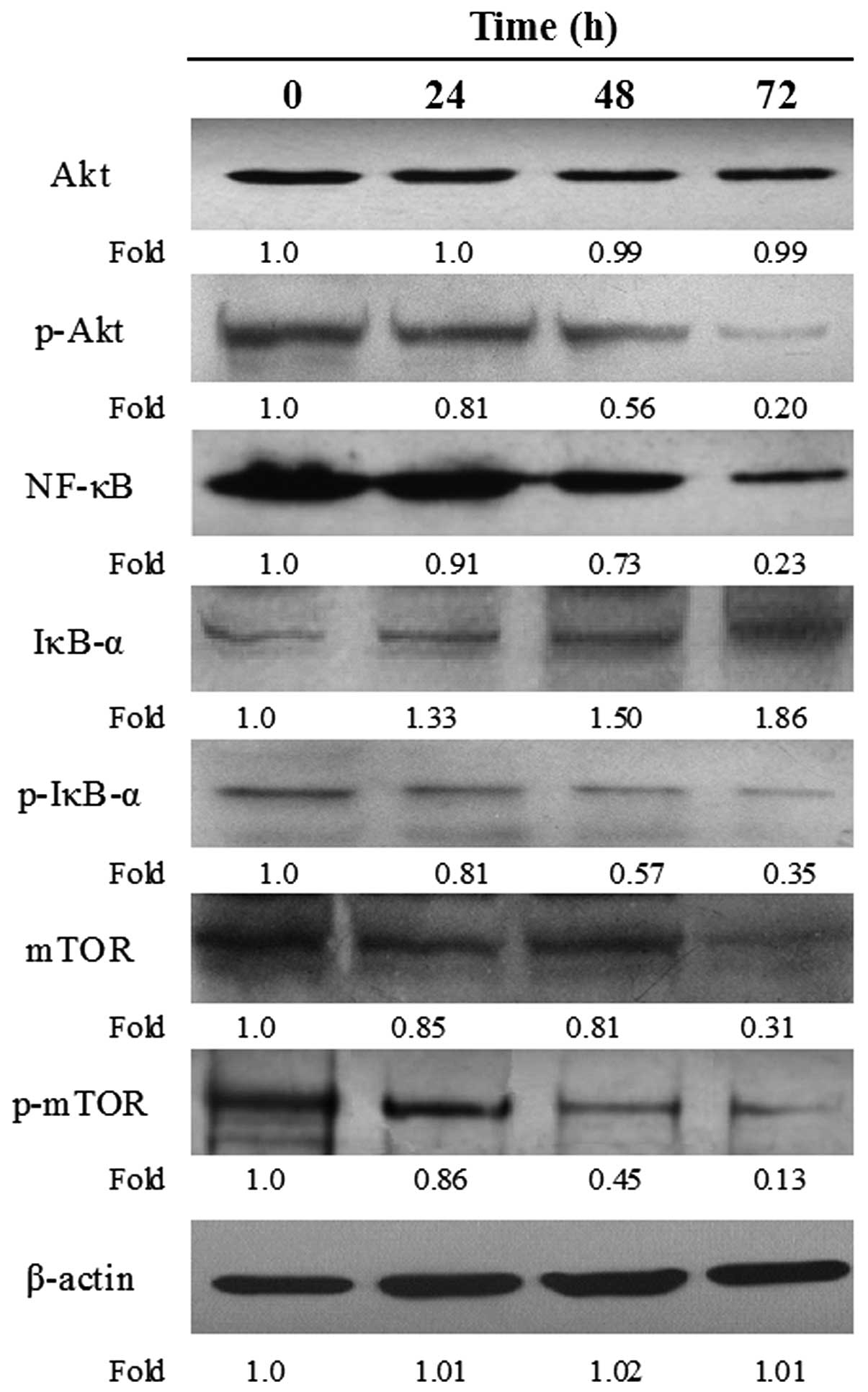
Suppression of the PI3K/Akt pathway
The effects of baicalein on suppression of the Akt
pathway in EC-109 cells were examined. Expression of the following
components in their various forms were measured: i) Akt (inactive)
and p-Akt (the activated form of Akt); ii) the transcription factor
NF-κB, the NF-κB inhibitor, IκB, and the degradable form of IκB,
p-IκB; iii) the cell cycle regulatory kinase mTOR (inactive) and
p-mTOR, the phosphorylated and active form of the kinase. A
decrease in p-Akt and p-mTOR was observed at 24 h of baicalein
treatment. Dramatic reductions in the expression of NF-κB and p-IκB
were observed in response to baicalein at 48 h of baicalein
treatment. These reductions were time- dependent. By contrast,
expression of IκB increased with the time of baicalein treatment.
The marked decreases in expression of total cellular NF-κB and
p-IκB, accompanied by significant increases in IκB expression, in
response to baicalein treatment, were interpreted to indicate a
condition wherein nuclear NF-κB signaling was dramatically impaired
(Fig. 5).
Discussion
The current study has demonstrated that baicalein is
toxic to esophageal carcinoma cells in culture. Treatment with this
flavone resulted in a marked decrease in the viability of cultured
EC-109 cells and in the number of colonies that these cells formed.
Baicalein treatment caused EC-109 cells to undergo apoptosis, as
demonstrated by changes in nuclear morphology, an increase in the
percentage of Annexin V-stainable cells and an increase in DNA
fragmentation.
The PI3K/Akt growth signaling pathway comprises a
series of serine/threonine kinase cascades that regulate a variety
of cellular processes including cell cycle progression, cell
survival and migration, and protein synthesis. Activated Akt acts
to phosphorylate Bad, Bax and caspase-9 or activate the NF-κB
pathway to promote the resistance of cancer cells to apoptosis
(29–32). In the present study, inhibition of
Akt phosphorylation, as opposed to downregulation of Akt
expression, was observed during treatment of EC-109 cells with
baicalein. As Akt acts early on in the PI3K/Akt signaling pathway,
it is conceivable that the growth-suppressive effects of baicalein
in EC-109 cells are attributable to an interaction of the drug with
the kinase. Due to the fact that PI3K expression/activity was not
measured in the present study, the involvement of this kinase in
the observed effects of baicalein remains unclear. Future studies
with various esophageal carcinoma cells lines have been planned to
explore the possibility that PI3K is targeted by baicalein.
NF-κB is a critical transcription factor in a
variety of physiological and pathological processes. One particular
function of NF-κB is to promote cell survival through the induction
of target genes, whose products inhibit components of the apoptotic
machinery in normal and cancerous cells (33). It has been demonstrated that NF-κB
plays a key role in progression, apoptosis and lymph node
metastasis in ESCC (34–36). As a downstream component of the
PI3K/Akt pathway (37), NF-κB
primarily resides in the cytosol in an inactive form; a heterodimer
composed of p50 and p65 through an interaction with the inhibitory
protein IκB (38). Agonist-induced
stimulation resulted in phosphorylation and subsequent proteosomal
degradation of IκB, thereby inducing nuclear translocation of NF-κB
to promote the transcription of its target genes (38). Activated Akt indirectly signals IκB
phosphorylation, thereby promoting transcription of anti-apoptotic
genes, whereas inactivation of Akt promotes apoptosis. The present
study demonstrated that treatment of EC-109 cells with baicalein
induced apoptosis by suppressing components of the PI3K/Akt/NF-κB
signaling pathway; the expression of p-Akt, NF-κB, and p-IκB. These
decreases were observed concurrently with increased expression of
non-phosphorylated IκB.
mTOR, a major downstream target of the PI3K/Akt
pathway, is an essential regulator of cell proliferation, protein
synthesis and the modulation of signals in various signaling
pathways (39,40). The mTOR pathway has a central role
in cell growth, as well as in invasion and metastasis of tumors
(41–43). It has been demonstrated that the
mTOR/p70S6K pathway was activated in ESCC, and rapamycin or siRNA
against mTOR rapidly inhibited expression of mTOR, arrested cells
in the G0/G1 phase and induced apoptosis of ESCC cells (44). In the present study, the results
revealed that a decrease in the expression of mTOR and p-mTOR along
with downregulated expression of p-Akt upon baicalein treatement,
corroborated well with the growth inhibition and induction of
apoptosis in EC-109 cells.
Suppression of Akt in cancer cells is associated
with activation of the mitochondrial apoptotic pathway involving
the caspase-9-dependent caspase cascade (45). Caspase-3, a downstream caspase, is
activated by caspase-9. Caspase-3 activation leads to cleavage and
inactivation of key cellular proteins such as PARP and the DNA
fragmentation factor (46). In our
study, treatment of EC-109 cells with baicalein was revealed to
increase the level of cleaved caspase-9 concurrently with a
decrease in procaspase-9 protein, to increase level of cleaved
caspase-3 concurrently with a decrease in procaspase-3 protein, to
increase expression of cleaved PARP concurrently with decreased
expression of uncleaved PARP, and to promote DNA degradation. These
findings support the hypothesis that apoptotic death in
baicalein-treated EC-109 cells is mediated by the following events
in sequence: Cleavage of procaspase-9, cleavage of procaspase-3,
cleavage of PARP and ultimately degradation of DNA. To further
delineate the signaling events involved in baicalein induced
apoptosis, the potential role of baicalein in regulating Bax and
Bcl-2 proteins, which regulate the essential change in
mitochondrial membrane permeability for apoptosis, was examined
(47). As a result, baicalein
markedly decreased anti-apoptotic Bcl-2 protein levels, while the
levels of pro- apoptotic Bax protein concomitantly increased. From
these data, it may be concluded that baicalein induced EC-109 cell
apoptosis by activating the intrinsic mitochondrial pathway.
In summary, the results of the present study
indicate that baicalein induced apoptosis in human ESCC EC-109
cells. This is mediated through suppression of the PI3K/Akt/NF-κB
and PI3K/Akt/mTOR signaling pathways which involves activation of
the mitochondrial death pathway components (including Bcl-2 family
proteins), as well as activation of caspase-3 and PARP. The results
of the present study suggest that baicalein may be an effective
chemopreventive agent for ESCC. However, further studies are
required to determine the upstream signaling that is involved in
the inhibition of this signaling pathway by baicalein in ESCC
cells. Furthermore, to delineate the potential of this agent in
esophageal cancer treatment, we intend to examine the antitumor
effects of baicalein in an animal model.
Acknowledgements
This study was supported by a research
grant from Zhengzhou University for Talent Recruitment. The authors
would like to thank Dr Hongli Zhang (Institute of Hematology and
Blood Diseases, Chinese Academy of Medical Sciences and Peking
Union Medical College, China) for his English correction and useful
discussion regarding this work.
References
|
1.
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2.
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer.
37:S4–66. 2001.PubMed/NCBI
|
|
3.
|
Sant M, Allemani C, Santaquilani M, Knijn
A, Marchesi F and Capocaccia R; EUROCARE Working Group: EUROCARE-4.
Survival of cancer patients diagnosed in 1995–1999 Results and
commentary. Eur J Cancer. 45:931–991. 2009.
|
|
4.
|
Xing D, Tan W and Lin D: Genetic
polymorphisms and susceptibility to esophageal cancer among Chinese
population (review). Oncol Rep. 10:1615–1623. 2003.PubMed/NCBI
|
|
5.
|
Stoner GD and Gupta A: Etiology and
chemoprevention of esophageal squamous cell carcinoma.
Carcinogenesis. 22:1737–1746. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Besharat S, Jabbari A, Semnani S, Keshtkar
A and Marjani J: Inoperable esophageal cancer and outcome of
palliative care. World J Gastroenterol. 14:3725–3728. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Alibakhshi A, Aminian A, Mirsharifi R,
Jahangiri Y, Dashti H and Karimian F: The effect of age on the
outcome of esophageal cancer surgery. Ann Thorac Med. 4:71–74.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wei WT, Chen H, Ni ZL, Liu HB, Tong HF,
Fan L, Liu A, Qiu MX, Liu DL, Guo HC, Wang ZH and Lin SZ: Antitumor
and apoptosis-promoting properties of emodin, an anthraquinone
derivative from Rheum officinale Baill, against pancreatic
cancer in mice via inhibition of Akt activation. Int J Oncol.
39:1381–1390. 2011.PubMed/NCBI
|
|
9.
|
Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH,
Yang JS, Lai KC, Lin JP, Tang NY, Lin JG and Chung JG: Antitumor
effects of emodin on LS1034 human colon cancer cells in
vitro and in vivo: roles of apoptotic cell death and
LS1034 tumor xenografts model. Food Chem Toxicol. 50:1271–1278.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kimura Y, Kubo M, Tani T, Arichi S,
Ohminami H and Okuda H: Studies on Scutellariae radix. III
Effects on lipid metabolism in serum, liver and fat cells of rats.
Chem Pharm Bull. 29:2308–2312. 1981.
|
|
11.
|
Lin CC and Shieh DE: The anti-inflammatory
activity of Scutellaria rivularis extracts and its active
components, baicalin, baicalein and wogonin. Am J Chin Med.
24:31–36. 1996.
|
|
12.
|
Lu Y, Joerger R and Wu C: Study of the
chemical composition and antimicrobial activities of ethanolic
extracts from roots of Scutellaria baicalensis Georgi. J
Agric Food Chem. 59:10934–10942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Miocinovic R, McCabe NP, Keck RW, Jankun
J, Hampton JA and Selman SH: In vivo and in vitro
effect of baicalein on human prostate cancer cells. Int J Oncol.
26:241–246. 2005.
|
|
14.
|
Ma Z, Otsuyama K, Liu S, Abroun S,
Ishikawa H, Tsuyama N, Obata M, Li FJ, Zheng X, Maki Y, Miyamoto K
and Kawano MM: Baicalein, a component of Scutellariae radix
from Huang-Lian- Jie-Du-Tang (HLJDT), leads to suppression of
proliferation and induction of apoptosis in human myeloma cells.
Blood. 105:3312–3318. 2005.PubMed/NCBI
|
|
15.
|
Chen CH, Huang LL, Huang CC, Lin CC, Lee Y
and Lu FJ: Baicalein, a novel apoptotic agent for hepatoma cell
lines: a potential medicine for hepatoma. Nutr Cancer. 38:287–295.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lee HZ, Leung HW, Lai MY and Wu CH:
Baicalein induced cell cycle arrest and apoptosis in human lung
squamous carcinoma CH27 cells. Anticancer Res. 25:959–964.
2005.PubMed/NCBI
|
|
17.
|
Chao JI, Su WC and Liu HF: Baicalein
induces cancer cell death and proliferation retardation by the
inhibition of CDC2 kinase and survivin associated with opposite
role of p38 mitogen-activated protein kinase and AKT. Mol Cancer
Ther. 6:3039–3048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Shieh DE, Cheng HY, Yen MH, Chiang LC and
Lin CC: Baicalin-induced apoptosis is mediated by Bcl-2-dependent,
but not p53-dependent, pathway in human leukemia cell lines. Am J
Chin Med. 34:245–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lu HF, Hsueh SC, Ho YT, Kao MC, Yang JS,
Chiu TH, Huamg SY, Lin CC and Chung JG: ROS mediates
baicalin-induced apoptosis in human promyelocytic leukemia HL-60
cells through the expression of the Gadd153 and
mitochondrial-dependent pathway. Anticancer Res. 27:117–125.
2007.
|
|
20.
|
Kuo HM, Tsai HC, Lin YL, Yang JS, Huang
AC, Yang MD, Hsu SC, Chung MC, Gibson Wood W and Chung JG:
Mitochondrial-dependent caspase activation pathway is involved in
baicalein-induced apoptosis in human hepatoma J5 cells. Int J
Oncol. 35:717–724. 2009.PubMed/NCBI
|
|
21.
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tokunaga E, Oki E, Egashira A, Sadanaga N,
Morita M, Kakeji Y and Maehara Y: Deregulation of the Akt pathway
in human cancer. Curr Cancer Drug Targets. 8:27–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Li H, Gao Q, Guo L and Lu SH: The
PTEN/PI3K/Akt pathway regulates stem-like cells in primary
esophageal carcinoma cells. Cancer Biol Ther. 11:950–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Li B, Cheung PY, Wang X, Tsao SW, Ling MT,
Wong YC and Cheung AL: Id-1 activation of PI3K/Akt/NFkappaB
signaling pathway and its significance in promoting survival of
esophageal cancer cells. Carcinogenesis. 28:2313–2320. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Li B, Tsao SW, Li YY, Wang X, Ling MT,
Wong YC, He QY and Cheung AL: Id-1 promotes tumorigenicity and
metastasis of human esophageal cancer cells through activation of
PI3K/AKT signaling pathway. Int J Cancer. 125:2576–2585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zhao H, Yang J, Fan T, Li S and Ren X:
RhoE functions as a tumor suppressor in esophageal squamous cell
carcinoma and modulates the PTEN/PI3K/Akt signaling pathway. Tumour
Biol. 33:1363–1374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Agarwal S, Achari C, Praveen D, Roy KR,
Reddy GV and Reddanna P: Inhibition of 12-LOX and COX-2 reduces the
proliferation of human epidermoid carcinoma cells (A431) by
modulating the ERK and PI3K-Akt signalling pathways. Exp Dermatol.
18:939–946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
29.
|
Song L, Xiong H, Li J, Liao W, Wang L, Wu
J and Li M: Sphingosine kinase-1 enhances resistance to apoptosis
through activation of PI3K/Akt/NF-κB pathway in human non-small
cell lung cancer. Clin Cancer Res. 17:1839–1849. 2011.PubMed/NCBI
|
|
30.
|
Tsuruta F, Masuyama N and Gotoh Y: The
phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax
translocation to mitochondria. J Biol Chem. 277:14040–14047. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Shen HM and Tergaonkar V: NFκB signaling
in carcinogenesis and as a potential molecular target for cancer
therapy. Apoptosis. 14:348–363. 2009.
|
|
34.
|
Tian F, Fan T, Jiang Y, Zhang X and Wang
X: A small interfering RNA targeting NF-κB p65 alone or combined
with 5-FU inhibits growth of esophageal squamous cell carcinoma in
nude mice. Pathol Res Pract. 208:32–38. 2012.
|
|
35.
|
Tian F, Zang WD, Hou WH, Liu HT and Xue
LX: Nuclear factor-kB signaling pathway constitutively activated in
esophageal squamous cell carcinoma cell lines and inhibition of
growth of cells by small interfering RNA. Acta Biochim Biophys Sin.
38:318–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Su C, Chen Z, Luo H, Su Y, Liu W, Cai L,
Wang T, Lei Y and Zhong B: Different patterns of NF-κB and Notch1
signaling contribute to tumor-induced lymphangiogenesis of
esophageal squamous cell carcinoma. J Exp Clin Cancer Res.
30:852011.
|
|
37.
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
Subunit of NF-kappa B through utilization of the Ikappa B kinase
and activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Vermeulen L, Vanden Berghe W and Haegeman
G: Regulation of NF-κB transcriptional activity. Cancer Treat Res.
130:89–102. 2006.
|
|
39.
|
Fingar DC, Richardson CJ, Tee AR, Cheatham
L, Tsou C and Blenis J: mTOR controls cell cycle progression
through its cell growth effectors S6K1 and 4E-BP1/eukaryotic
translation initiation factor 4E. Mol Cell Biol. 24:200–216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Fingar DC, Salama S, Tsou C, Harlow E and
Blenis J: Mammalian cell size is controlled by mTOR and its
downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16:1472–1487.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Bjornsti MA and Houghton PJ: The TOR
pathway: a target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Xu G, Zhang W, Bertram P, Zheng XF and
McLeod H: Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR
pathway in common human tumors. Int J Oncol. 24:893–900.
2004.PubMed/NCBI
|
|
43.
|
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue
P, Fu H and Khuri FR: Activation of Akt and eIF4E survival pathways
by rapamycin-mediated mammalian target of rapamycin inhibition.
Cancer Res. 65:7052–7058. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Hou G, Xue L, Lu Z, Fan T, Tian F and Xue
Y: An activated mTOR/p70S6K signaling pathway in esophageal
squamous cell carcinoma cell lines and inhibition of the pathway by
rapamycin and siRNA against mTOR. Cancer Lett. 253:236–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: a target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Orrenius S: Mitochondrial regulation of
apoptotic cell death. Toxicol Lett. 149:19–23. 2004. View Article : Google Scholar : PubMed/NCBI
|