Introduction
Anatomical resection is the standard treatment for
early-stage lung cancer, yielding a locoregional control rate of
∼90% and a 5-year overall survival rate of 50–70% for stage I
non-small cell lung cancer (NSCLC) (1). However, a significant proportion of
NSCLC patients present with comorbidities and an advanced age,
causing them to be deemed medically inoperable. Chronic obstructive
pulmonary disease with emphysema and pronounced reduction of lung
capacity accounts for the majority of inoperable patients (2). Moreover, certain patients are
unwilling to undergo surgery. These patients are primarily referred
for radiation therapy (RT); with conventional RT, the rate of local
control has historically been poor (30–70%), with an overall 5-year
survival rate of only 15–30% (3,4).
Otherwise, patients refuse RT due to the long treatment period and
are observed without specific cancer therapy. The reason for poor
tumor control with conventional RT has been revealed to be an
insufficient total radiation dose, which is typically ≤60 Gy
(4).
Stereotactic body RT (SBRT), also referred to as
stereotactic ablative RT, is a form of high-precision RT for tumor
targets in extracranial sites, employing higher doses per fraction
and fewer fractions than conventional RT (5). SBRT delivers a much higher biological
effective dose (BED) compared with conventional RT and has reduced
local failure (<10%) comparable to the rates following surgery,
in patients with early-stage NSCLC (2,6–10).
In SBRT for lung cancer, a basic principle of RT, to
maximize the dose of radiation delivered to a tumor and to spare
normal tissue, becomes even more important. This is due to the fact
that rather than the differential radiosensitivities of normal and
target tissues, the geometry and/or intensity of the beams is the
predominant factor in sparing normal tissues (5). Studies have suggested that the use of
intensity-modulated RT (IMRT) in the process of radiosurgery or
SBRT has the potential to improve tumor coverage and spare normal
tissue (11,12). Volumetric-modulated arc therapy
(VMAT) is a novel extension of IMRT, reducing treatment times by
radiation delivery in a gantry rotation up to 360° with a dynamic
multi-leaf collimator motion, variable dose rates and gantry speed
modulation (13). However, few
clinical studies have been conducted that have adopted IMRT/VMAT
during SBRT for lung cancer.
SBRT is particularly challenging due to the added
complexities introduced by target motion during natural
physiological processes, such as respiration. Four-dimensional (4D)
computed tomography (CT) scans that correlate CT images with
respiratory phases have frequently been employed to take into
account respiration-related tumor motion (14). Integrated imaging devices in
treatment units presently allow CT scans (cone-beam CT) to be
performed immediately prior to treatment, while the patient is on
the treatment couch, thereby confirming that the patient and tumor
are positioned correctly (15).
In the present study, we report our clinical
investigation of SBRT in patients with stage I NSCLC. 4D CT
imaging, IMRT/VMAT for planning/delivery and image-guided RT with
cone-beam CT were employed. The clinical outcomes, including
treatment response rate, local disease control rate and toxicity,
were analyzed.
Patients and methods
Patients
Between December 2010 and March 2012, a total of 16
consecutive patients with primary NSCLC were treated using SBRT.
Patient- and treatment-related data were collected from a
prospectively registered database. Inclusion criteria for SBRT were
as follows: Pathologically confirmed NSCLC; clinical stage T1-2N0M0
according to the American Joint Committee on Cancer Staging Manual,
7th edition (16); longest tumor
diameter <5 cm; and Eastern Cooperative Oncology Group
performance scale score ≤2. Only patients who were considered to be
inoperable due to poor medical condition or refusal to undergo
surgery were included. The treated tumor was required to be further
than 2 cm in all directions from the proximal bronchial tree, which
was defined as the distal 2 cm of the trachea, carina and major
lobar bronchi up to their first bifurcation (7). Patients who had previously undergone
chemotherapy or RT for lung cancer were excluded. All patients
provided written informed consent, and the study was conducted in
compliance with the Declaration of Helsinki (17). The study was approved by the ethics
committee of Soonchunhyang University Hospital (Cheonan,
Korea).
Before initiation of treatment, a complete history
was taken and patients underwent a physical examination,
contrast-enhanced CT imaging of the chest,
18F-fluorodeoxyglucose positron emission tomography
(PET)-CT scanning, a pulmonary function test and brain imaging
(contrast-enhanced CT or magnetic resonance imaging).
Treatment
The patients were treated using a Novalis Tx system
(Varian Medical Systems, Palo Alto, CA, USA and BrainLab,
Feldkirchen, Germany). During the simulation, patients were
immobilized in the supine position, with the arms above the head,
in a vacuum-bag restriction system (Vac-Lock, Civco Medical
Solutions, Kalona, IA, USA). Respiration-correlated 4D CT scans
were performed during uncoached quiet respiration using a Real-Time
Position Management (RPM) system (Varian Medical Systems) and a
16-slice CT scanner (Brilliance CT Big Bore, Philips Medical
Systems, Cleveland, OH, USA). Data were acquired for the duration
of a full respiratory cycle. Each reconstructed image was assigned
to a specific respiratory phase to collectively yield a set of 10
CT images, each of which reflected 10% of the respiratory cycle.
The gross tumor volume was delineated on the CT image for each
respiratory phase using the ‘lung window’ setting. No expansion was
made to account for microscopic disease extent, and the clinical
target volume was equivalent to the gross tumor volume. To
encompass the entire trajectory of the target, an internal target
volume was generated from the sum of the gross tumor volumes during
all 10 respiratory phases. The planning target volume (PTV) was
created by adding a 0.5-cm isotropic set-up margin around the
internal target volume. Critical structures, including the lungs,
spinal cord, esophagus, trachea, proximal bronchial tree, heart,
great vessels, ribs and skin, were outlined. Normal tissue dose
volume constraints were adapted from data in the Radiation Therapy
Oncology Group SBRT trial protocols (18).
All plans were created using the Eclipse treatment
planning system (Varian Medical Systems) and 6-MV photons, taking
into account inhomogeneity corrections. A fixed-field IMRT plan was
generated using 7–9 non-opposing coplanar beams. In the VMAT plan,
2–4 partial arcs were used. The same optimization objectives and
penalties were used for the IMRT and VMAT plans. The dose
fractionation schedules were 48 Gy/4 fractions or 55 Gy/5
fractions, delivered on consecutive days. Dosimetric criteria
mandated that 95% of the PTV was covered conformally by the
prescription dose and that 99% of the PTV received 90% of the
prescription dose. The cone-beam CT images of the tumor were
registered to the contours and images from the 4D CT planning data
sets and were used to guide patient localization. Pre-treatment
cone-beam CT and patient repositioning were repeated when the
set-up error was estimated to be ≥3 mm in any direction.
Evaluation and analysis
Patients were followed up every 3 months during the
first and second years, and every 6 months thereafter. Follow-up CT
scans were performed at each visit, but PET-CT scans were repeated
only in the event of suspected disease relapse. Tumor measurements
at each follow-up appointment were performed using the Response
Evaluation Criteria in Solid Tumors (19), in which a complete response (CR) is
total tumor disappearance and a partial response (PR) is a decrease
of ≥30% in the longest tumor diameter. Local control and survival
were measured from the time of diagnosis. Local failure was defined
as progressive and increasing CT scan abnormalities that were
confirmed by progressive and incremental increases in the
standardized uptake values of a lesion in serial PET-CT imaging,
with or without biopsy. Tumor progression in the hilar, mediastinal
or supraclavicular lymph nodes was considered regional failure. The
National Cancer Institute’s Common Toxicity Criteria (version 3.0)
were used to grade adverse events. Survival was estimated using the
Kaplan-Meier method and statistical analyses were performed using
the Statistical Package for the Social Sciences (SPSS) software,
version 14.0 (SPSS, Chicago, IL, USA).
Results
Patients
The patient, tumor and treatment characteristics are
summarized in Table I. The median
patient age was 76 years (range, 69–86) and 12 (75%) patients were
male. Nine of the patients’ tumors were clinically staged as
T1N0M0, while seven were T2N0M0. The histological subtypes were
squamous cell carcinoma in nine patients, adenocarcinoma in six and
large cell neuroendocrine carcinoma in one. The maximal tumor
diameter ranged from 1.5 to 5 cm (median, 2.8). Fifteen patients
were not appropriate candidates for surgery due to chronic
pulmonary disease, poor lung function, advanced age or other
chronic illnesses, and one patient refused to undergo surgery. Two
patients had a history of NSCLC or rectal cancer, diagnosed eight
and 10 years, respectively, prior to the current presentation. In
one patient with previous NSCLC (squamous), pneumonectomy of the
right lung was performed and novel NSCLC (squamous) developed in
the left lung. Conventional planning CT, as opposed to 4D CT, was
performed in one patient who suffered from severe kyphosis and
required treatment in a prone position.
 | Table IPatient, tumor and treatment
characteristics. |
Table I
Patient, tumor and treatment
characteristics.
| Characteristic | No. |
|---|
| Age (years) | |
| Median | 76 |
| Range | 69–86 |
| Gender | |
| Male | 12 |
| Female | 4 |
| Pathology | |
| Squamous | 9 |
| Adenocarcinoma | 6 |
| Large cell
neuroendocrine | 1 |
| cT
classification | |
| cT1a | 4 |
| cT1b | 5 |
| cT2a | 7 |
| Tumor size (cm) | |
| Median | 2.8 |
| Range | 1.5–5.0 |
| Tumor location
(lobe) | |
| Left
upper/lower | 6/3 |
| Right
upper/middle/lower | 2/1/4 |
| PTV volume (ml) | |
| Median | 89.3 |
| Range | 43.4–223.5 |
| Fractionation
scheme | |
| 48 Gy/4
fractions | 9 |
| 55 Gy/5
fractions | 7 |
| SBRT technique | |
| Fixed-field
IMRT | 11 |
| VMAT | 5 |
Response and local control
The median follow-up period was 13 months (range,
4–20) for all patients and 14 months (range, 6–20) for surviving
patients. In the 14 evaluable patients, the response rate at 6
months, consisting of all patients with a CR (n=3; 21.4%) or a PR
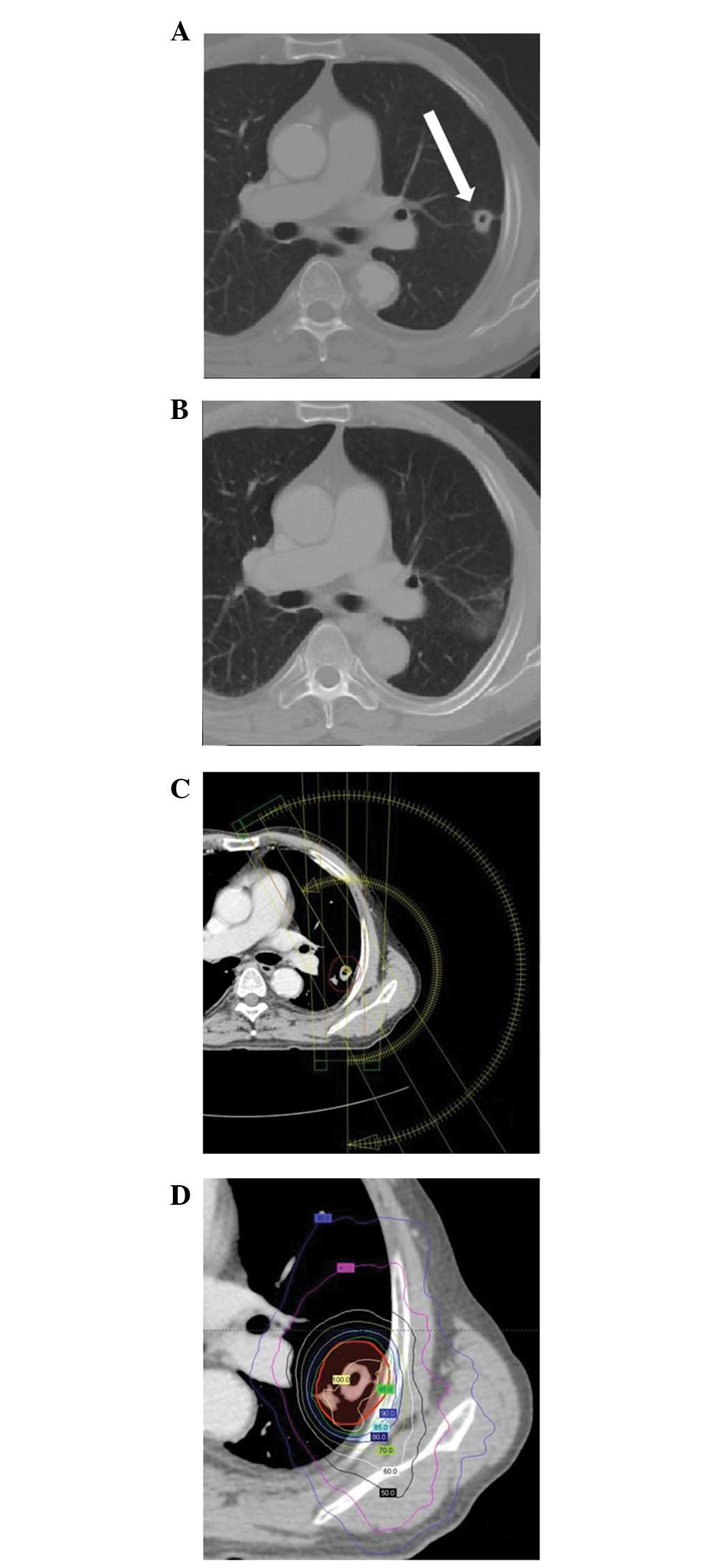
(n=8; 57.1%), was 78.6% (11/14). A typical case of a CR is
presented in Fig. 1. The remaining
three patients (21.4%) achieved a stable disease status. Two
non-evaluable patients, who did not survive the first 6 months,
demonstrated stable disease at 3 months.
One patient developed local failure 11 months after
SBRT. Another patient demonstrated regional failure in a subcarinal
lymph node at 4 months. All relapses were confirmed by a
combination of CT and PET-CT. Two patients did not survive; one of
whom developed subcarinal lymph node metastasis and died during
salvage chemotherapy at 5 months, while the other succumbed to a
cause unrelated to lung cancer (a cardiopulmonary event) at 4
months. The Kaplan-Meier estimates of local failure-free,
progression-free and overall survival rates at 18 months were 91.0,
85.2 and 87.5%, respectively.
Toxicity
All patients completed SBRT with no treatment
interruption. Despite the medical comorbidities and advanced age of
the patients, SBRT was well-tolerated. Five patients (31.3%)
reported no toxicities. Grade 1 toxicities included pulmonary
toxicity in seven patients (43.8%), transient mild erythema in
three patients (18.8%), fatigue in two patients (12.5%) and
dysphagia in one patient (6.3%). Grade 2 toxicities included
pulmonary toxicity in four patients (25.0%) and chest pain in two
patients (12.5%). No toxicity ≥ grade 3 was observed.
Discussion
The present study analyzed patients with stage I
NSCLC receiving SBRT, in whom the 6-month response rate was 78.6%
(CR, 21.4%; PR, 57.1%). Response rates for lung SBRT have been
described by a number of authors, and treatment response rates
following SBRT for lung cancer have been found to improve until ∼1
year post-treatment (7–10,20).
Timmerman et al reported CR and PR rates of 51% and 38%,
respectively, following SBRT in 55 patients with early-stage NSCLC
(7). A CR occurred at 1.6 –42.6
months (median, 6.5) after the completion of SBRT. Mohammed et
al investigated the time course of radiographic tumor responses
following SBRT for primary or metastatic lung tumors (20). The CR and PR rates were 3 and 43% at
6 weeks, 15 and 38% at 4 months, and 27 and 64% at 1 year,
respectively. Taremi et al analyzed 108 patients with stage
I NSCLC receiving SBRT and the treatment response rate was greater
at 1 year (CR, 30.5%; PR, 37.5%) compared with at 3 months (CR, 7%;
PR, 68.4%) (9). Further follow-up
of patients in the present study may reveal greater responses than
those described here.
Local control and survival rates at 18 months (91.0
and 87.5%, respectively) were comparable to previously demonstrated
outcomes (2,6–10).
Disease relapse occurred in two patients and one patient showed
mediastinal lymph node metastasis at 4 months. As the metastasis
appeared soon after SBRT, we speculate that the patient harbored
occult tumors at diagnosis that went undetected during initial CT
and PET staging. Local disease progression developed in another
patient after 11 months. Local failure may be due to either a
geographic miss or radiation resistance, even with high BEDs. The
former appeared to have a greater influence as a growing mass
appeared within 1 cm of the PTV. Notably, the patient’s planning
process did not involve 4D CT due to severe kyphosis.
The 4D CT scans provide information on not only the
extent of tumor motion but also the different spatial tumor
positions (14). A target volume
that includes only areas with demonstrable tumor appearance is
smaller than the conventional PTV, which includes greater
non-specific and universal isotropic margins and covers areas that
do not harbor the tumor at any point during the respiration cycle.
In addition to this smaller volume, 4D-based target definition is
important in avoiding target misses (21). Normal treatment planning CT using
modern CT scanners that acquire scans in a short time (fast-CT)
only displays the tumor for a certain moment of the respiration
cycle and may contain motion-induced artifacts that cause
inadequate visualization of the tumor (22). Underberg et al evaluated the
differences between 4D CT-based target volumes and targets defined
in several consecutive fast-CT scans; the 4D scans captured motion
that was missed by fast-CT (23).
The present study supports the use of 4D CT in SBRT planning for
lung lesions.
Non-clinical planning studies have validated the
suitability of IMRT for the setting of radiosurgery or SBRT. These
studies have demonstrated significant dosimetric improvements for
small and irregularly shaped lesions compared with the results of
other techniques, with reductions in critical organ irradiation
(11,24). However, few published clinical
studies have adopted IMRT during SBRT for lung cancer (12). Although we did not compare
dosimetric parameters with those from conventional
three-dimensional planning in the present study, highly conformal
target coverage with homogenous dose distribution, and with
radiation exposure of normal tissue well below the recommended dose
volume constraints, was achieved with IMRT. None of the patients,
including the patient with previous pneumonectomy, experienced
severe (≥ grade 3) toxicity. In addition to the radiation dose
distribution, treatment time also requires consideration in SBRT
planning/delivery, as SBRT for lung tumors is mostly applied in
medically inoperable patients who are often elderly with other
medical problems. Decreases in the treatment time associated with
VMAT are capable of reducing the likelihood of patient movement as
a result of discomfort and minimizing the random error introduced
by intrafraction tumor motion (13,25).
Five patients in the present study, who were more fragile, were
treated using VMAT, which permitted a reduction in the beam-on
time. If dosimetric parameters are not inferior to those for
fixed-field IMRT, we plan to preferentially treat patients using
this technique. More detailed descriptions of the IMRT/VMAT methods
and dosimetric analysis will be presented in a further study.
Two fractionation schedules, 48 Gy/4 fractions and
55 Gy/5 fractions, were implemented in the present study. BEDs
calculated using a linear-quadratic model (α/β assumed to be 10)
were 105.6 and 115.5 Gy10, respectively (26). These BEDs have been more widely
adopted in Japan and are lower than those with the 60 Gy/3 fraction
scheme (BED=180 Gy10) mainly employed in North America
(6,10). A BED >100 Gy10 is
generally accepted as an adequate cut-off dose; below this
threshold, local failure risk is higher (10,27).
However, Stephans et al demonstrated no difference in local
control or survival rates between 50 Gy/5 fractions and 60 Gy/3
fractions in SBRT for patients with medically inoperable stage I
NSCLC, and chest wall toxicity was more common with the latter
scheme (28). When the one episode
of local recurrence is regarded as being due to a geographical
miss, the 100% local control rate in the present study suggests
that there may be no large dose-response gain above these modest
BEDs. However, the present study requires further follow-up; the
optimum dose fractionation in SBRT for lung cancer may be
elucidated through prospective randomized studies.
In conclusion, the current study provides additive
evidence for establishing the favorable efficacy and safety of SBRT
for patients with stage I NSCLC. Novel techniques using IMRT/VMAT
were feasible in lung SBRT, and 4D CT was demonstrated to be
necessary for simulation and planning in order to precisely account
for tumor motion during respiration.
References
|
1
|
Chang MY and Sugarbaker DJ: Surgery for
early stage non-small cell lung cancer. Semin Surg Oncol. 21:74–84.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baumann P, Nyman J, Hoyer M, et al:
Outcome in a prospective phase II trial of medically inoperable
stage I non-small-cell lung cancer patients treated with
stereotactic body radiotherapy. J Clin Oncol. 27:3290–3296. 2009.
View Article : Google Scholar
|
|
3
|
Rowell NP and Williams CJ: Radical
radiotherapy for stage I/II non-small cell lung cancer in patients
not sufficiently fit for or declining surgery (medically
inoperable): a systematic review. Thorax. 56:628–638. 2001.
View Article : Google Scholar
|
|
4
|
Qiao X, Tullgren O, Lax I, Sirzen F and
Lewensohn R: The role of radiotherapy in treatment of stage I
non-small cell lung cancer. Lung Cancer. 41:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buyyounouski MK, Balter P, Lewis B, et al:
Stereotactic body radiotherapy for early-stage non-small-cell lung
cancer: report of the ASTRO Emerging Technology Committee. Int J
Radiat Oncol Biol Phys. 78:3–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagata Y, Takayama K, Matsuo Y, et al:
Clinical outcomes of a phase I/II study of 48 Gy of stereotactic
body radiotherapy in 4 fractions for primary lung cancer using a
stereotactic body frame. Int J Radiat Oncol Biol Phys.
63:1427–1431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Timmerman R, Paulus R, Galvin J, et al:
Stereotactic body radiation therapy for inoperable early stage lung
cancer. JAMA. 303:1070–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bral S, Gevaert T, Linthout N, et al:
Prospective, risk-adapted strategy of stereotactic body
radiotherapy for early-stage non-small-cell lung cancer: results of
a Phase II trial. Int J Radiat Oncol Biol Phys. 80:1343–1349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taremi M, Hope A, Dahele M, et al:
Stereotactic body radiotherapy for medically inoperable lung
cancer: prospective, single-center study of 108 consecutive
patients. Int J Radiat Oncol Biol Phys. 82:967–973. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Onishi H, Shirato H, Nagata Y, et al:
Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I
non-small cell lung cancer: updated results of 257 patients in a
Japanese multi-institutional study. J Thorac Oncol. 2(7 Suppl 3):
S94–S100. 2007. View Article : Google Scholar
|
|
11
|
Benedict SH, Cardinale RM, Wu Q, Zwicker
RD, Broaddus WC and Mohan R: Intensity-modulated stereotactic
radiosurgery using dynamic micro-multileaf collimation. Int J
Radiat Oncol Biol Phys. 50:751–758. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Videtic GM, Stephans K, Reddy C, et al:
Intensity-modulated radiotherapy-based stereotactic body
radiotherapy for medically inoperable early-stage lung cancer:
excellent local control. Int J Radiat Oncol Biol Phys. 77:344–349.
2010. View Article : Google Scholar
|
|
13
|
McGrath SD, Matuszak MM, Yan D, Kestin LL,
Martinez AA and Grills IS: Volumetric modulated arc therapy for
delivery of hypofractionated stereotactic lung radiotherapy: A
dosimetric and treatment efficiency analysis. Radiother Oncol.
95:153–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keall P: 4-dimensional computed tomography
imaging and treatment planning. Semin Radiat Oncol. 14:81–90. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grills IS, Hugo G, Kestin LL, et al:
Image-guided radiotherapy via daily online cone-beam CT
substantially reduces margin requirements for stereotactic lung
radiotherapy. Int J Radiat Oncol Biol Phys. 70:1045–1056. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York: 2010
|
|
17
|
No authors listed. World Medical
Association Declaration of Helsinki: ethical principles for medical
research involving human subjects. JAMA. 284:3043–3045. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
The Radiation Therapy Oncology Group
Clinical Trials (Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable.aspx).
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
20
|
Mohammed N, Grills IS, Wong CY, et al:
Radiographic and metabolic response rates following image-guided
stereotactic radiotherapy for lung tumors. Radiother Oncol.
99:18–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hof H, Rhein B, Haering P, Kopp-Schneider
A, Debus J and Herfarth K: 4D-CT-based target volume definition in
stereotactic radiotherapy of lung tumours: comparison with a
conventional technique using individual margins. Radiother Oncol.
93:419–423. 2009.
|
|
22
|
Balter JM, Ten Haken RK, Lawrence TS, Lam
KL and Robertson JM: Uncertainties in CT-based radiation therapy
treatment planning associated with patient breathing. Int J Radiat
Oncol Biol Phys. 36:167–174. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Underberg RW, Lagerwaard FJ, Cuijpers JP,
Slotman BJ, van Sörnsen de Koste JR and Senan S: Four-dimensional
CT scans for treatment planning in stereotactic radiotherapy for
stage I lung cancer. Int J Radiat Oncol Biol Phys. 60:1283–1290.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cardinale RM, Benedict SH, Wu Q, Zwicker
RD, Gaballa HE and Mohan R: A comparison of three stereotactic
radiotherapy techniques; ARCS vs. noncoplanar fixed fields vs
intensity modulation. Int J Radiat Oncol Biol Phys. 42:431–436.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holt A, van Vliet-Vroegindeweij C, Mans A,
Belderbos JS and Damen EM: Volumetric-modulated arc therapy for
stereotactic body radiotherapy of lung tumors: a comparison with
intensity-modulated radiotherapy techniques. Int J Radiat Oncol
Biol Phys. 81:1560–1567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yaes RJ, Patel P and Maruyama Y: On using
the linear-quadratic model in daily clinical practice. Int J Radiat
Oncol Biol Phys. 20:1353–1362. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palma D and Senan S: Stereotactic
radiation therapy: changing treatment paradigms for stage I
nonsmall cell lung cancer. Curr Opin Oncol. 23:133–139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stephans KL, Djemil T, Reddy CA, et al: A
comparison of two stereotactic body radiation fractionation
schedules for medically inoperable stage I non-small cell lung
cancer: the Cleveland Clinic experience. J Thorac Oncol. 4:976–982.
2009. View Article : Google Scholar : PubMed/NCBI
|