Introduction
Acinar cell carcinoma (ACC) of the pancreas is a
rare tumour, accounting for only 1% of cases of exocrine pancreatic
malignancies. For patients in whom surgery with curative intent is
not possible, there are no clear treatment guidelines (1). This tumour expresses molecular and
genetic alterations characteristic of colon cancer (2). ACC has none of the gene abnormalities
found in ductal pancreatic adenocarcinoma. In the current study we
present two cases, initially treated with the same chemotherapy.
The first patient responded to the initial treatment for some
months, but failed the second and third line of chemotherapy. The
second patient failed the first and second chemotherapy lines. Both
patients received panitumumab monotherapy following the
chemotherapy. The study was approved by the ethics committee of the
University Hospital Nuestra Señora de Candelaria, Santa Cruz de
Tenerife, Canary Islands, Spain. Written informed patient consent
was obtained from the patients.
Case reports
Case 1
A 42-year-old male patient, with a previous history
of asthma and type 2 diabetes mellitus (diagnosed 1 year before)
and who was a past smoker, was admitted in November 2009 due to
anorexia, weight loss (5 kg in the last 3 months), asthenia,
abdominal pain and constipation. The physical examination revealed
an oriented patient; no nodes were found; the lung and heart sounds
were clear; the liver was enlarged (4 cm below the right costal
margin); the spleen was felt 2 cm below the left costal margin; no
oedema was present and the neurological examination was normal. The
following tests were performed: i) Blood analysis and chemistry
tests. The most notable reults were Hb 12 g/dl; leukocytes
5,400/mm3; platelets 173,000/mm3; glucose 113
mg/l; calcium 15 mg/dl; gamma glutamyl transpeptidase 177 U/l;
alkaline phosphatase 149 U/l and α-fetoprotein (AFP) 16,7 ng/ml;
other tests were normal; ii) CT of thorax and abdomen revealed
bilateral pulmonary nodules of ∼1 cm in diameter and mediastinal
lymph nodes, ranging between 1.1 and 2.1 cm. Multiple
heterogeneous, ill-defined liver nodules of various sizes were
visible (the largest being 8.8 cm diameter in the right lobe).
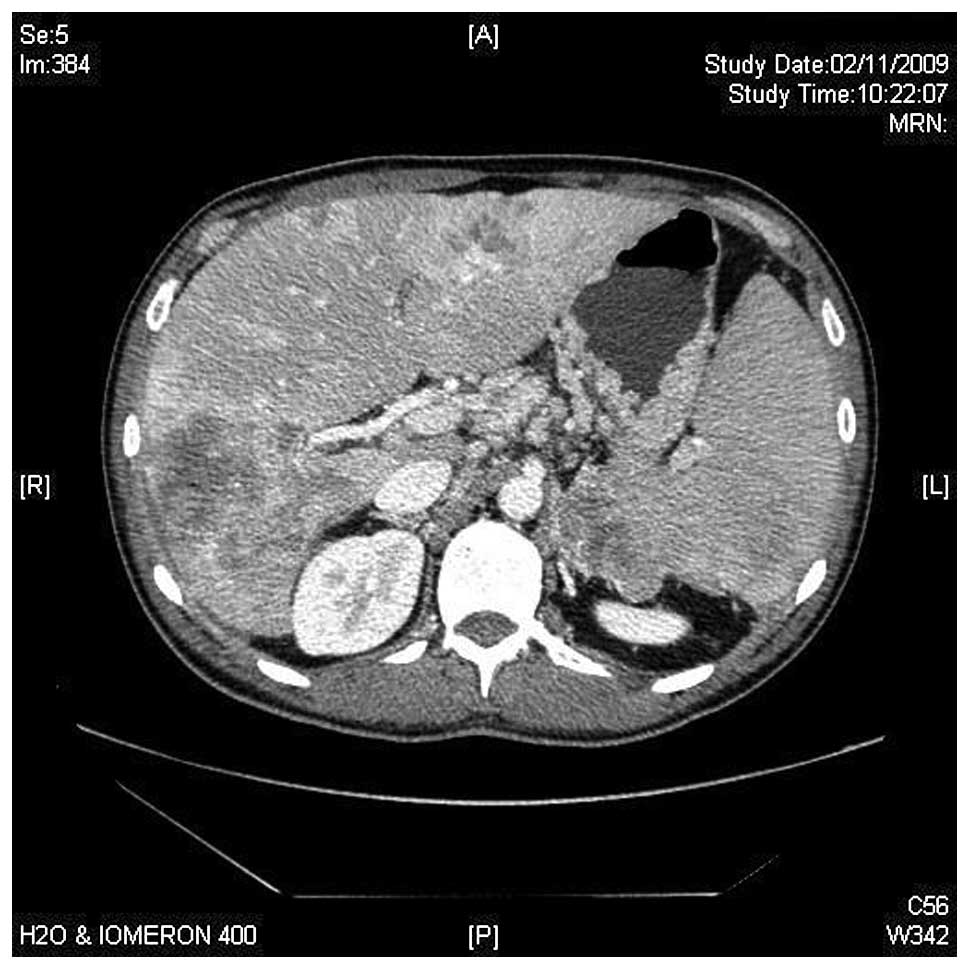
There was also a pancreatic mass involving corpus and tail of 8×7.2
cm, infiltrating the left adrenal gland and spleen vessels. Spleen
enlargement of 17.1 cm was noted (Fig.
1); iii) Percutaneous liver biopsy. The pathologic examination
revealed the presence of an acinar cell carcinoma of the pancreas.
The mutational state of KRAS was determined and tumour was
KRAS wild-type.
Calcium levels were controlled with i.v.
saline/furosemide and zoledronic acid. Chemotherapy
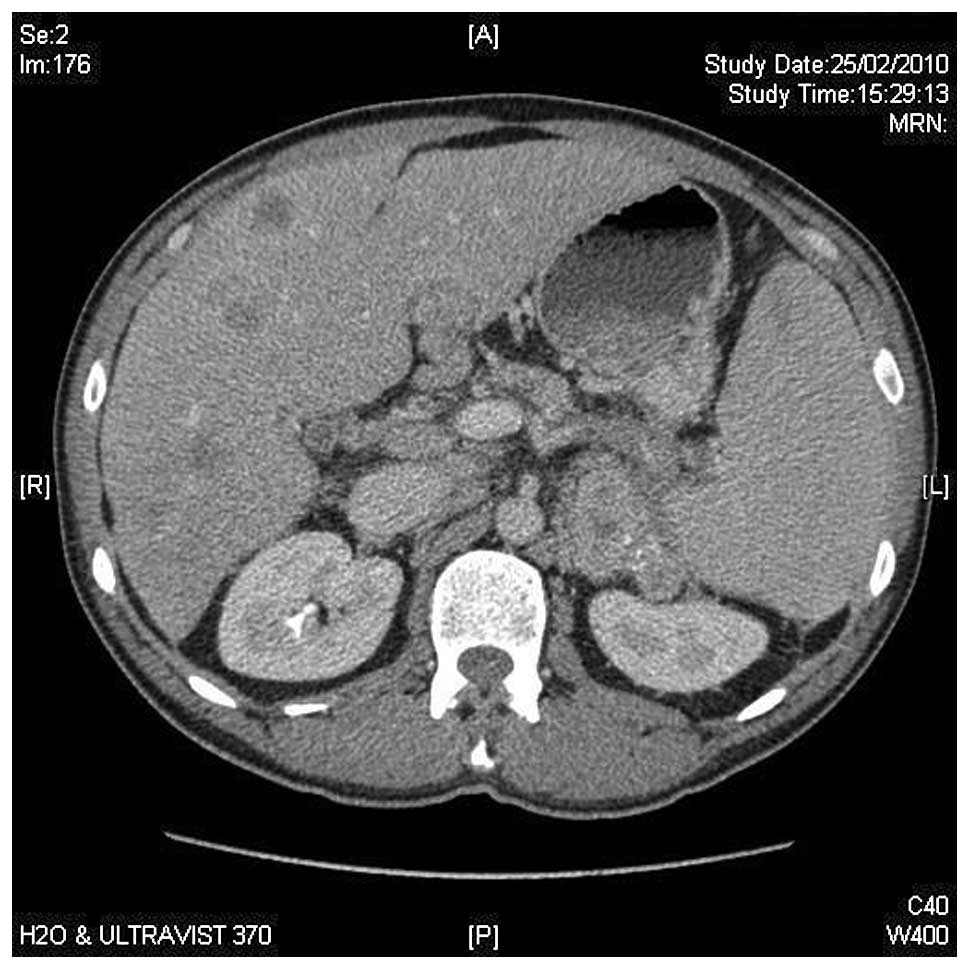
(capecitabine/oxaliplatin) was started. After three months of
treatment, clinical and radiological improvement was achieved
(Fig. 2). After the eighth cycle,
the patient developed an allergy against oxaliplatin, and
oxaliplatin was altered to irinotecan. In July 2010, progressive
disease with refractory hypercalcaemia was diagnosed. Elevated
levels of parathyroid hormone (PTH; 435.8 pg/ml) were detected and
primary hyperparathyrodism was suspected. As magnetic resonance
imaging (MRI) and sonography excluded the presence of parathyroid
gland hyperplasia or tumour, an ectopic PTH secretion was
suspected. Immunohistochemical study of the small amounts of tissue
remaining from the percutaneous liver biopsy showed no evidence of
PTH.
Thereafter, a third line of chemotherapy was started
with bevacizumab-FOLFIRI. After 2 months of treatment, control of
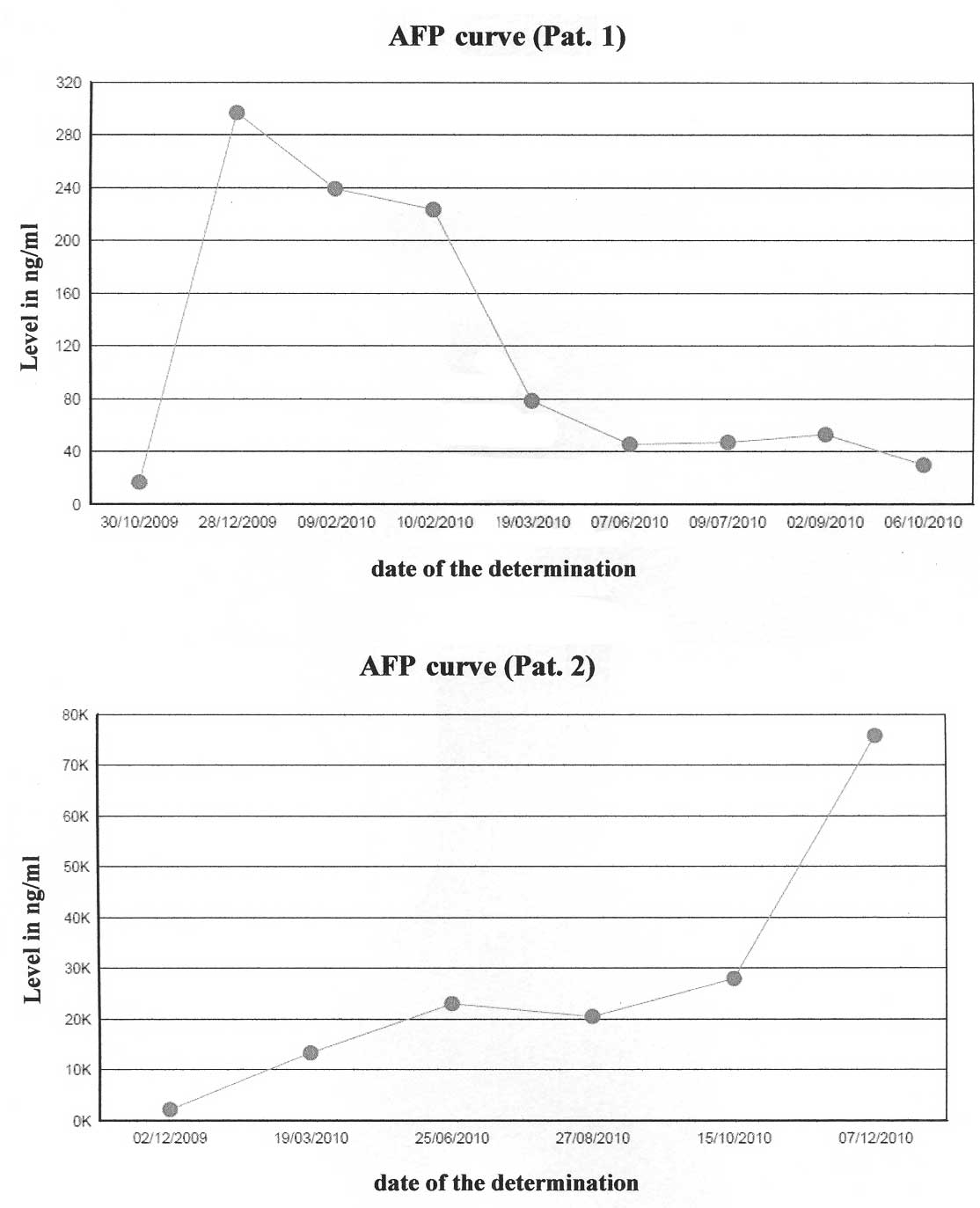
hypercalcaemia was achieved, with a drop in AFP levels.
Nevertheless, the patient developed grade III gastrointestinal
toxicity and persistent thrombopaenia. Monotherapy with panitumumab
was started. After 3 doses, despite an improvement in liver
function tests and AFP, the patient developed abdominal pain,
delirium, significant asthenia and uncontrolled hypercalcaemia.
Palliative treatment was started. The patient succumbed to the
disease two days later, 12 months after diagnosis.
Case 2
A 64-year-old male patient with a previous history
of amoxycillin allergy, heavy smoking, chronic obstructive lung
disease, alcohol intake (>40 g/day) and newly diagnosed type 2
diabetes mellitus, was admitted in December 2009 due to weight
loss, fever and jaundice. The physical examination showed an
oriented jaundiced patient, no nodes were felt, lung and heart
sounds were clear; the abdomen was normal and no oedema was
present. The following tests were performed: i) Blood analysis and
chemistries. Total bilirubin 5.57 mg/dl; direct bilirubin 5.11
mg/dl; ASAT 274 U/l; ALAT 265 U/l; GGT 1,306 U/l; AP 730 U/l;
CA19.9, 267.9 ng/ml; ii) CT of abdomen. Dilatation of extra-hepatic
bile ducts, with a diameter of 1.5 cm, produced by an heterogeneous
enlargement of the head of pancreas. Liver cysts in the left lobe
and solitary metastasis (3.2 cm in diameter) in the right lobe.
Palliative derivative surgery was performed in November 2009 and a
mass protruding the duodenum was removed. The pathological
examination of the mass revealed an ACC of the pancreas. The level
of blood lipase was 410 U/l and that of AFP was 2,225.8 ng/ml. The
KRAS was wild-type.
Chemotherapy with capecitabine/oxaliplatin was
started. After 3 cycles of chemotherapy (March 2010), an increase
in AFP (13.356 ng/ml) was observed and liver and regional node
progression was diagnosed. Second-line therapy with weekly
irinotecan was performed. In June 2010, disease progression (AFP,
23.062 ng/ml) was diagnosed. Panitumumab therapy was started and
the patient remained clinically stable with a initial drop in AFP
level, until December 2010. Then, weight loss and asthenia
developed. Complementary tests showed an increase of AFP (75.871
ng/ml), progression in the primary lesion and liver metastases. The
patient was transferred to the palliative care unit, and succumbed
to the disease in January 2011.
Discussion
ACC is defined as a carcinoma exhibiting evidence of
pancreatic enzyme production by the neoplastic cells and may
secrete AFP, endocrine products or lipase into the circulation.
Patients experience disorders of the parathyroid glands, diffuse
subcutaneous nodules and sclerotic lesions in cancellous bone; the
latter two findings are due to fat necrosis in the subcutaneous
tissue and bone (2).These tumours
have a mutation in the APC gene/β-catenin pathway, with a
genetic progression similar to colon cancer. The molecular changes
found in ACCs are the loss of heterozygosity at 1p, 5q25 at the
APC locus, 9p21 at the p16 locus and 17p13 at the p53
locus. K-ras mutations are not identified in ACC, by
contrast, activating mutations in the K-ras proto-oncogene
are found in almost all cases of pancreatic ductal adenocarcinoma,
as well as in early precursor lesions termed pancreatic
intraepithelial neoplasia (3,4). ACC
has none of the gene abnormalities that are commonly found in
ductal pancreatic adenocarcinomas, pancreatic endocrine tumours and
pancreatoblastoma (3,5,6).
Patients who are able to undergo surgical resection
have a median survival of 36 months. Surgical management is not
curative in the majority of patients. There is a 72% rate of
recurrent disease among patients who undergo a surgical resection,
a number of whom experienced distant metastases as opposed to local
recurrences (2). Patients with
stage IV disease have a 5-year survival rate of 17.2% (7). On multivariate analysis, age <65
years, well-differentiated tumours and negative resection margins
are independent prognostic factors for ACC (7).
For locally advanced or metastatic disease,
chemotherapeutic agents used in the treatment of colorectal cancer
may be effective in ACC of the pancreas due to the genetic
alteration in the APC/β-catenin pathway noted in acinar
cells of the pancreas. 5-Fluorouracil (5-FU) is the most commonly
used agent. Other agents that have been used are gemcitabine,
cisplatin, doxorubicin, irinotecan, oxaliplatin, docetaxel,
capecitabine, 5-FU/leucovorin, erlotinb, sunitinb and sirolimus
(1,8).
Our two patients received chemotherapy combinations
for colorectal cancers and their survival was similar, with a short
objective response in patient 1. In a study of genetic
abnormalities using fluorescence in situ hybridation (FISH)
in cells of five patients with ACC, one patient had a survival
period of 3 years after diagnosis and treatment with capecitabine
followed by FOLFOX (folinic acid/5-FU/oxaliplatin). This patient
had normal results in thymidine phosphorilase (TYMP) and
thymidylate synthetase (TYMS), perhaps partly explaining why
the patient responded to 5-FU and capecitabine (9).
Following the treatment guidelines for metastatic
colorectal cancers expressing wild-type K-ras gene,
anti-EGFR therapy with panitumumab was administered (10). With panitumumab, disease
stabilisation for four months was achieved in patient 2, with a
slight decrease in AFP levels. In patient 1, the three infususions
of panitumumab produced an improvement in the liver function tests
and AFP levels (Fig. 3). According
to their molecular and genetic profiles, ACC may be tumours which
benefit from targeted therapies.
References
|
1
|
Distler M, Rückert F, Dittert DD, et al:
Curative resection of a primarily unresectable acinar cell
carcinoma of the pancreas after chemotherapy. World J Surg Oncol.
7:222009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohori NP, Khalid A, Etemad B and
Finkelstein SD: Multiple loss of heterozygosity without K-ras
mutation identified by molecular analysis on fine-needle aspiration
cytology specimen of acinar cell carcinoma of pancreas. Diagn
Cytopathol. 27:42–46. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
De la O JP and Murtaugh LC: Notch and Kras
in pancreatic cancer: at the crossroads of mutation,
differentiation and signalling. Cell Cycle. 8:1860–1864.
2009.PubMed/NCBI
|
|
4
|
Royal RE, Wolff RA and Crane CH:
Pancreatic cancer. CANCER: Principles & Practice of Oncology.
DeVita V, Lawrence TS and Rosenberg SA: Wolter Kluver/Lippincott
Williams & Wilkins; Philadelphia: pp. 1086–1123. 2008
|
|
5
|
Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo
CJ, Conlon K, et al: Genetic and immunohistochemical analysis of
pancreatic acinar cell carcinoma. Frequent allelic loss on
chromosome 11p and alterations in the APC/beta catenin pathway. Am
J Pathol. 160:953–962. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holen KD, Klimstra DS, Hummer A, et al:
Clinical characteristics and outcomes from an institutional series
of acinar cell carcinoma of the pancreas and related tumors. J Clin
Oncol. 20:4673–4678. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt CM, Matos JM, Bentrem DJ,
Talamonti MS, Lillemoe KD and Bilimoria KY: Acinar cell carcinoma
of the pancreas in the United States: prognostic factors and
comparison to ductal adenocarcinoma. J Gastrointest Surg.
12:2078–2086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antoine M, Khitrik-Palchuk M and Saif MW:
Long-term survival in a patient with acinar cell carcinoma of
pancreas. A case report and review of literature. JOP. 8:783–789.
2007.PubMed/NCBI
|
|
9
|
Dewald GW, Smyrk TC, Thorland EC, et al:
Fluorescence in situ hybridization to visualize genetic
abnormalities in interphase cells of acinar cell carcinoma, ductal
adenocarcinoma and islet cell carcinoma of the pancreas. Mayo Clin
Proc. 84:801–810. 2009. View
Article : Google Scholar
|
|
10
|
Van Custem E, Peeters M, Siena S, et al:
Open-label phase III trial of panitumumab plus best supportive care
compared with best supportive care alone in patients with
chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol.
25:1658–1664. 2007.PubMed/NCBI
|