Introduction
Breast cancer is a serious disease that threatens
the health of individuals and is the most common malignant tumor in
females. It is a major disease affecting females in particular, and
its incidence is increasing annually (1–3). The
key therapeutic approach for most breast cancer patients is to find
an effective antitumor drug to assist surgical treatment.
Embelin is a small molecular inhibitor extracted
from Myrsinaceae plants. It is a polyphenolic compound that
inhibits XIAP by binding to the Smac binding site in the BIR3
domain of XIAP protein molecules (4–6).
Previous studies have demonstrated that embelin has
anti-inflammatory and anti-oxidative biological effects (7–10). It
has been demonstrated that embelin has an extensive antitumor role
and is able to restrain the growth of various tumor cells,
including those of breast, colon, prostate and pancreatic cancer
(11–14). However, the detailed mechanism of
the antitumor activity of embelin remains unknown.
This study was designed to investigate the effect of
embelin on cell apoptosis and the cell cycle of MCF-7 breast cancer
cells in vitro, and to explore the embelin-induced cell
apoptosis signaling pathway in MCF-7 cells. We have demonstrated
that by regulating the Bax and Bcl-2 proteins, embelin induces the
release of cytochrome C and activates the caspase family to induce
the apoptosis of breast cancer cells.
Materials and methods
Cell culture
MCF-7 breast cancer cells were purchased from the
American Type Culture Collection (ATCC, Manassas, VA, USA).
Following cell passage, cells were inoculated in RPMI-1640 culture
medium (Gibco-BRL, Grand Island, NY, USA) containing 10% fetal calf
serum (Hyclone Laboratories, Inc., Logan, UT, USA), 100 U/ml
penicillin and 100 U/ml streptomycin. The cells were then cultured
in an incubator containing 5% CO2 and 95% O2,
at 37°C.
Cell viability
MCF-7 cells in the logarithmic growth phase were
collected and cultured in 96-well culture plates inoculated at a
density of 1×105 cells/ml for 24 h. When the cells had
grown to adherence, different doses of embelin were administered to
various groups with 8 duplicate wells for each concentration. A
negative control group without embelin was also created. All cells
were incubated in 5% CO2 for further culture for 24, 48
and 72 h prior to the color reaction. Subsequently, MTT (5 mg/ml;
20 μl) was added to each well, and cells were cultured in
CO2 at 37°C in an incubator for 4 h. The culture
solution was then diposed of. DMSO (150 μl) was added to
each well for room temperature oscillation for 10 min, and the
optical density (OD) values (A570nm) of each well
were measured with a microplate reader (Bio-Rad; Hercules, CA,
USA)
Analysis of apoptosis via flow
cytometry
A 0.25% trypsin digest was used to collect MCF-7
cells of all groups, and the cell density was adjusted to
1×106 cells/ml. Annexin V-FITC (5 μl) and 5 ml of
propidium iodide (PI) were added for 30 min at 4°C to avoid light
dyeing, prior to the flow cytometry analysis.
Analysis of cell cycle by flow
cytometry
MCF-7 cells of all groups were digested and
collected using 0.25% trypsin, and then washed with PBS solution.
Cells were fixed at 4°C with 75% cold ethanol overnight and washed
with PBS solution. The cell density was adjusted to
1×106 cells/ml and the final volume was 100 μl.
DNAStain comprehensive dye liquor (500 ml; Sigma, St. Louis, MO,
USA) was added for storage at room temperature in a dark place for
30 min prior to testing with flow cytometry. The DNAStain contained
RNase, PI and Triton X-100 at end concentrations of 50mg/l, 100
mg/l and 1 ml/l, respectively.
Flow cytometric analysis of mitochondrial
membrane potential
The cell density was adjusted to 1×106
cells/ml and JC-1 dye with an end concentration of 10 mg/ml was
added. This was then mixed and cultured for 30 min at 37°C in the
dark. Analysis with flow cytometry followed. FL1-H indicated a
green fluorescence intensity and FL2-H was a red fluorescence
intensity. Quantitative analysis was conducted using the CellQuest
analysis software.
Western blot analysis
MCF-7 cells of all groups were collected using a
trypsin digest and 2 ml of lysis solution (50 mM Tris-HCl, 137 mM
NaCl, 10% glycerin, 100 mM sodium vanadate, 1 mM PMSF, 10 mg/ml
aprotinin, 10 mg/ml leupeptin, 1% NP-40 and 5 mM cocktail; pH 7.4)
was added for cell lysis to obtain the proteins. Following the BCA
assay, proteins were loaded onto a gel and separated by SDS-PAGE.
The proteins were then transferred to a PVDF membrane using a
semi-dry method and sealed with 5% skimmed mild powder at 4°C
overnight. The membrane was washed with Tris-buffered saline
Tween-20 (TBST) and the first antibody was added at 37°C for
hybridization for 1 h, prior to bleaching with TBST. The second
antibody was then added at 37°C for hybridization for 1 h prior to
bleaching with TBST and a color reaction for 5 min with
autoradiography. Quantity One software was used for OD value
analysis and measurements.
Statistical analysis
The SPSS software, version 16.0 was used for
assessing dependence and for variance analysis. Values are
presented as mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference between groups.
Results
Embelin induces inhibition of MCF-7
breast cancer cell growth
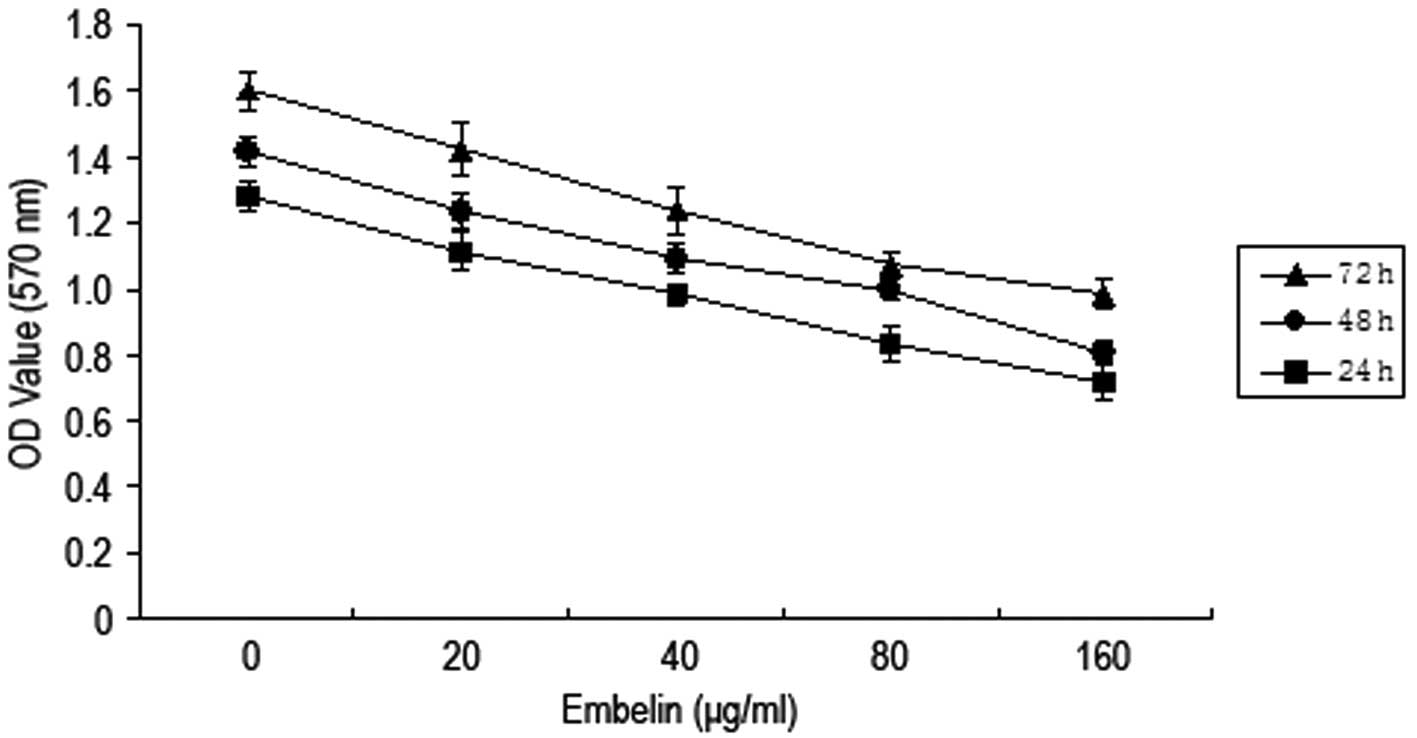
Different concentrations of embelin (0, 20, 40, 80
and 160 μg/ml) were added to MCF-7 breast cancer cells for
24, 48 and 72 h. The MTT method was then employed to determine the
cell activity (Fig. 1). The results
demonstrated that A570 values of breast cancer MCF-7
cells gradually decreased when the concentration of embelin was
increased, and the A570 value decreased most when the
concentration of embelin was 80 μg/ml. This indicates that
embelin has an inhibitory effect on the growth of MCF-7 breast
cancer cells.
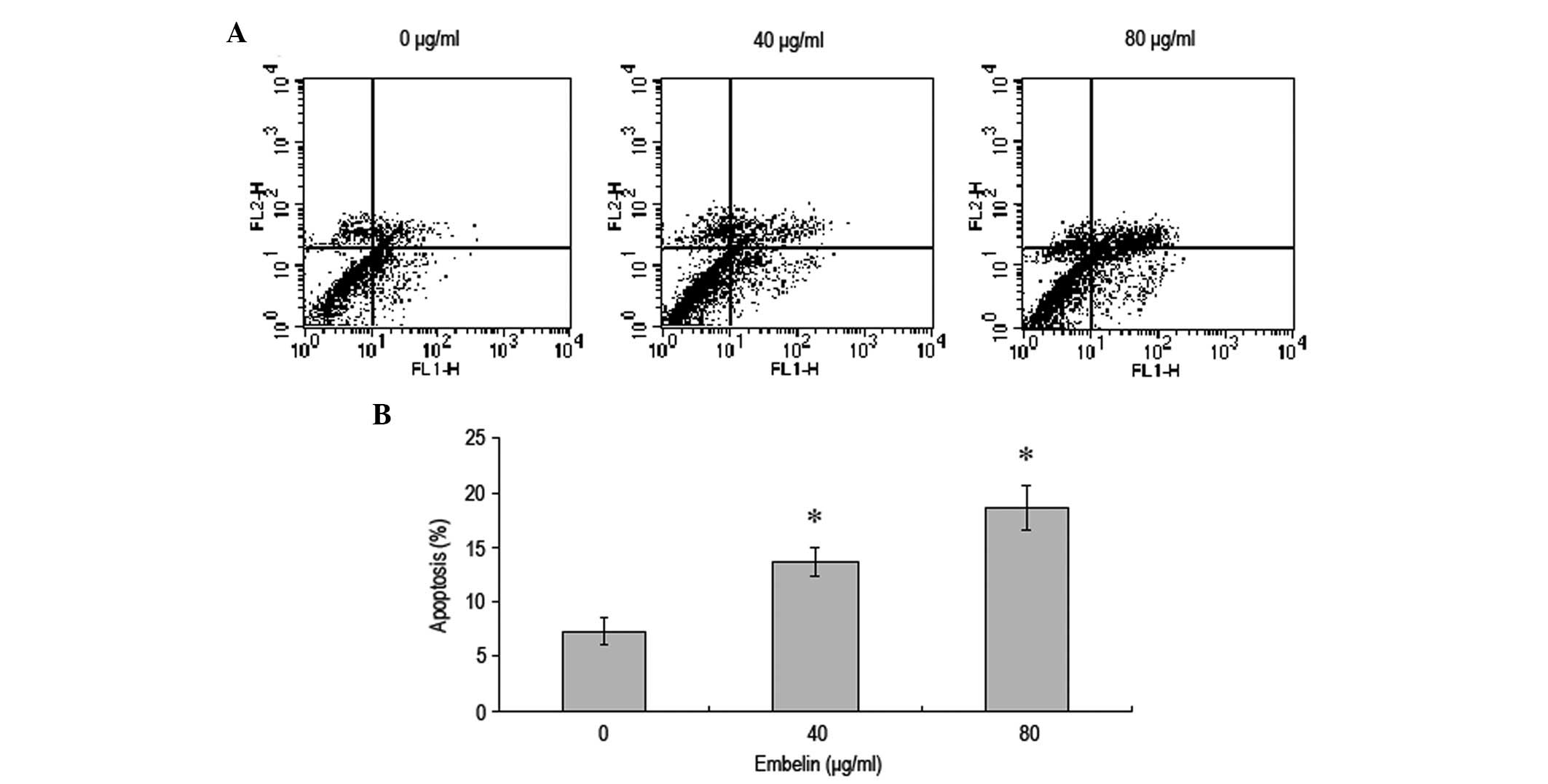
Embelin induces apoptosis of MCF-7 breast
cancer cells
MCF-7 breast cancer cells were treated with
different concentrations of embelin for 48 h and flow cytometry was
used to investigate the effect on the rate of apoptosis (Fig. 2). Our results demonstrated that,
with an increase in the concentration of embelin, the rate of MCF-7
breast cancer cell apoptosis significantly increased in a
dose-dependent manner.
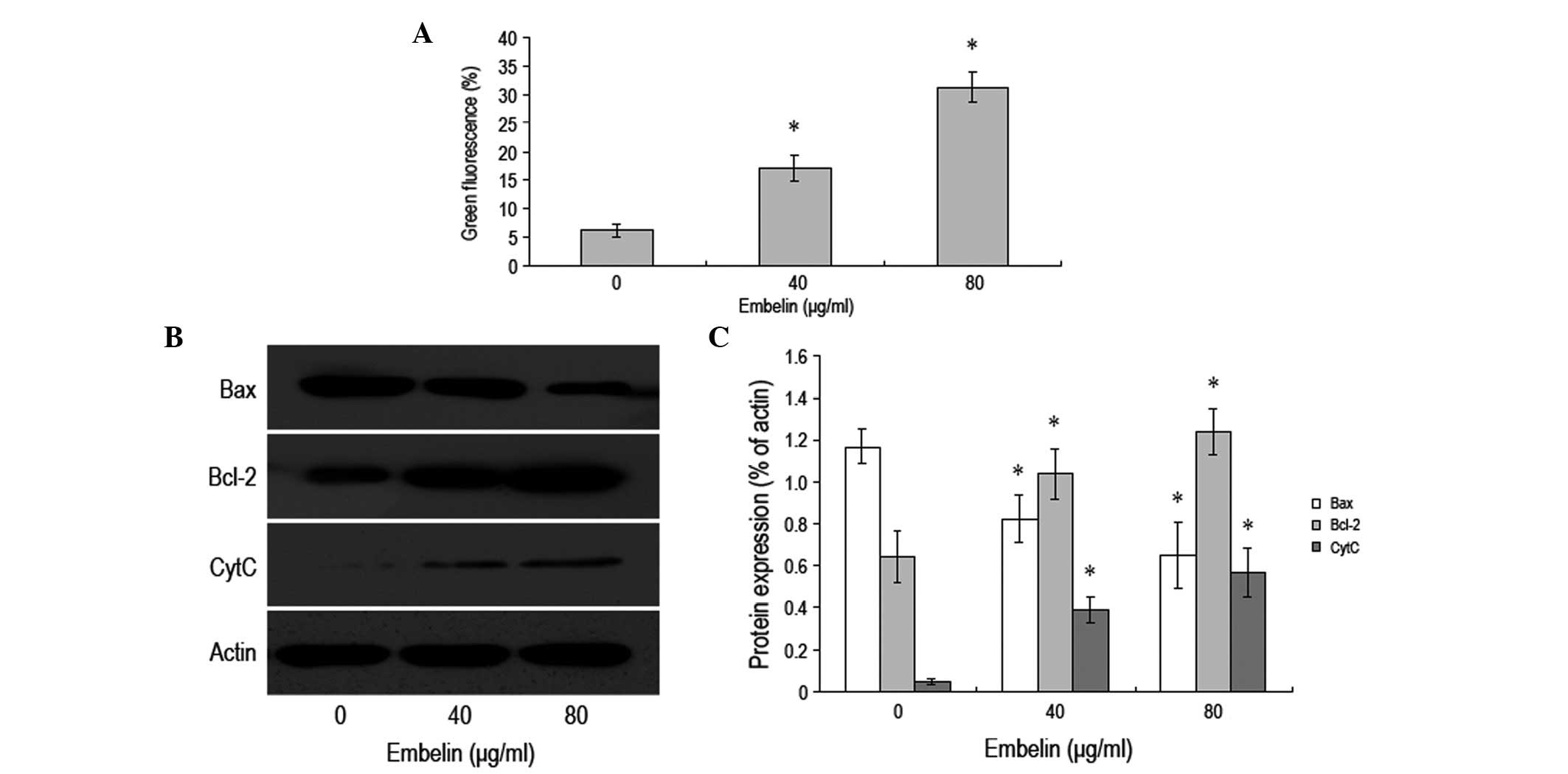
Embelin induces apoptosis of MCF-7 breast
cancer cells via the mitochondrial pathway
JC-1 coloration was used to examine the changes in
the mitochondrial membrane potential. The results demonstrated a
gradual decrease in the mitochondrial membrane potential along with
an increase in the concentration of embelin. Additionally, the
change in the mitochondrial membrane potential was observed prior
to apoptosis (Fig. 3A). Western
blot analysis was employed to examine the levels of Bax, Bcl-2 and
cytochrome C inside the cytoplasm. It was revelaed that when the
concentration of embelin was increased, the level of Bax protein
gradually decreased, while the level of cytochrome C increased
(Fig. 3B and C). Furthermore, the
level of Bcl-2 protein was observed to increase when the
concentration of embelin was increased. Our data demonstrated that
embelin is able to change the mitochondrial membrane potential to
promote a change in the levels of Bax and Bcl-2 as well as the
release of cytochrome C, which results in the apoptosis of MCF-7
breast cancer cells.
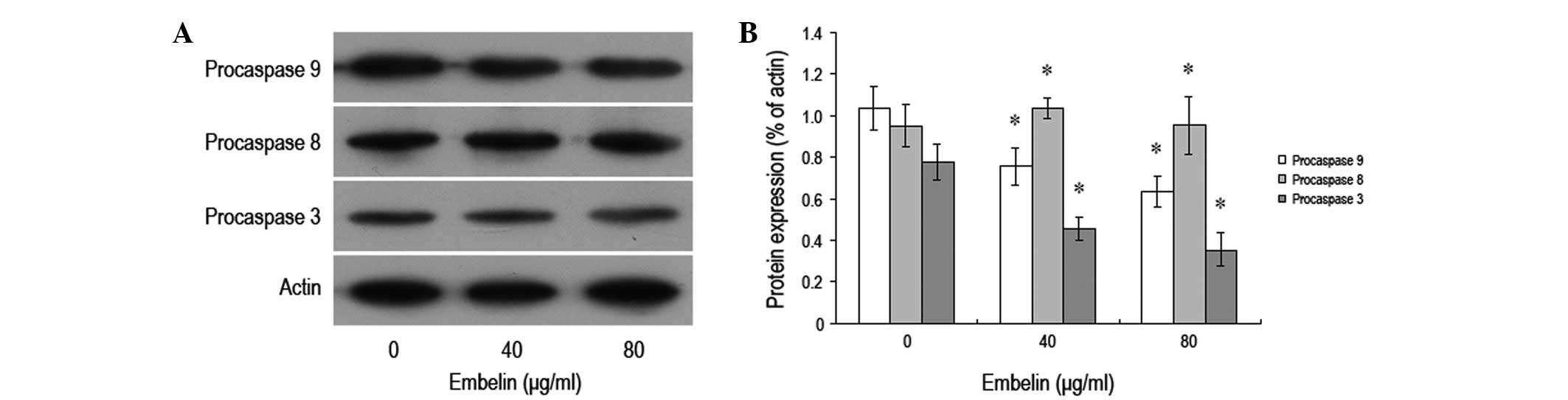
Effect of embelin on the expression
levels of apoptosis-related proteins in MCF-7 breast cancer
cells
In order to study the effect of embelin on the
expression levels of MCF-7 breast cancer cell apoptosis-related
proteins, different concentrations of embelin were administered to
MCF-7 cells for 48 h, and western blot analysis was conducted to
investigate the expression levels of procaspase-3, -8 and -9
proteins (Fig. 4). It was found
that following treatment with different concentrations of embelin,
the expression levels of MCF-7 breast cancer cell procaspase-3 and
-9 proteins signficantly decreased, while no significant
differences were observed in the expression level of procaspase-8
protein. Our data demonstrated that embelin-induced MCF-7 apoptosis
may occurr through the mitochondria-mediated caspase-3 and -9
pathways.
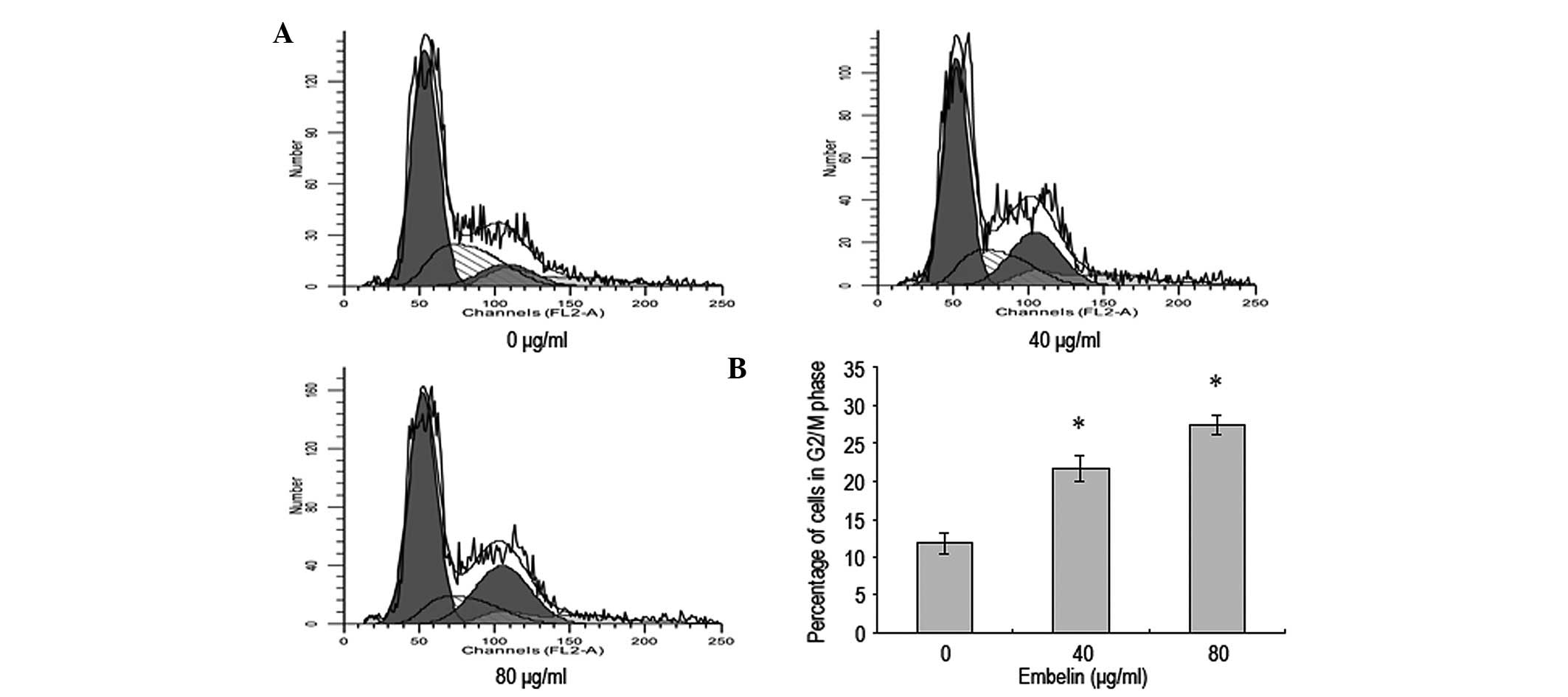
Embelin-induces MCF-7 breast cancer cell
cycle blockade in G2/M phase
Flow cytometry was conducted to investigate whether
embelin affected the cell cycle of MCF-7 breast cells. The results
revealed that 48 h following the addition of different
concentrations of embelin to MCF-7 cells, a cell cycle blockade was
observed in the G2/M phase compared with the control group
(Fig. 5). This suggests that
embelin is able to increase the percentage of MCF-7 cells in the
G2/M phase to decrease the proliferation of breast cancer
cells.
Discussion
Kerr et al(15) first proposed the concept of
apoptosis. Apoptosis is ubiquitous in the majority of tumor cells,
and is important in the genesis and progression of tumors (16). Previous studies have demonstrated
that antitumor drugs typically inhibit tumors by inducing apoptosis
of sensitive tumor cells. Therefore, the intervention in apoptosis
to treat tumors has become a new target in the search for antitumor
drugs and a new development direction in present tumor
pharmacology.
Breast cancer is a potentially life-threatening
malignant tumor; it is important to study the disease to find
effective anti-tumor drugs. Antitumor drugs that originate from
plants have benefits including an extensive variety, low toxicity
and few side-effects and adverse reactions. Therefore, highly
effective antitumor drugs from plants are being explored. Embelin
is a small molecular inhibitor with specific inhibition of XIAP
that affects the proliferation and apoptosis of various tumor
cells. Certain studies have demonstrated that embelin inhibits the
proliferation of various tumor cells; significant effects have been
observed in breast cancer and other solid tumor cells (17). The results of the present study are
concordant with these findings. We demonstrated that when breast
cancer cells had been treated with different concentrations of
embelin for 48 h, the rate of cell apoptosis increased in a
dose-dependent manner, indicating that embelin is able to induce
breast cancer cell apoptosis as opposed to directly causing cell
death. Moreover, embelin has the potent effect of restraining the
cell cycle transition of breast cancer cells to blockade the cell
cycle in G2/M phase, therefore altering the progression of the cell
cycle to induce apoptosis.
There are various signaling pathways of apoptosis
within an organism, of which the mitochondrial pathway is one of
the most important. Bcl-2 family proteins are key regulatory
factors of the mitochondrial pathway (18–20).
We demonstrated that that when breast cancer cells had been treated
with different doses of embelin for 48 h, Bax and Bcl-2 migrated,
the mitochondrial membrane potential increase expression of Bax,
while Bcl-2 expression decreased and cytochrome C was released.
These results indicated that the induction of breast cancer cell
apoptosis by embelin was closely associated with the mitochondrial
pathway. As demonstrated in previous studies, with the stimulation
of pro-apoptosis factors, the Bax protein migrated from the
cytoplasm to the outer mitochondrial membrane, changing the
permeability of the outer mitochondrial membrane to promote the
mitochondrial release of cytochrome C (21). Moreover, Bcl-2 protein is able to
stabilize the mitochondrial permeability transition pore (mPTP) and
maintain the normal functioning of the pore. In the present study,
we demonstrated that with embelin treatment, the Bcl-2 protein
level inside the cytoplasm gradually decreased while the release of
cytochrome C increased in a stepwise manner. This indicates that
embelin induces the activity of Bcl-2, causing the mPTP to open
irreversibly, which further changes the permeability of the
mitochondrial membrane and promotes the release of cytochrome C.
The combination of these factors results in apoptosis.
The caspase family activates apoptosis-related
protease when apoptosis occurs (22,23),
and a series of subsequent biological effects occur in turn.
Therefore, activation of the caspase family is important in the
process of apoptosis. We analyzed the changes in procaspase-3, -8
and -9 proteins following treatment of breast cancer cells with
embelin. A significant decrease in procaspase-3 and -9 expression
was observed when breast cancer apoptosis occured, but no changes
in the level of procaspase 8 expression were identified. The effect
of releasing cytochrome C from the mitochondria to activate
caspase-3 and -9 is an important step of the apoptotic pathway
(24,25). These results suggest that
embelin-induced apoptosis of breast cancer cells is realized via
the endogenous mitochondrial pathway as opposed to via the
exogenous death receptor pathway.
In summary, our study has demonstrated that embelin
releases cytochrome C and activates the caspase family to result in
the induction of breast cancer apoptosis through regulation of the
action of the Bcl-2/Bax family in the mitochondrial pathway.
Embelin may offer important contributions for the development of a
novel drug to prevent and cure breast cancer in the future.
References
|
1
|
McPherson K, Steel CM and Dixon JM: ABC of
breast diseases. Breast cancer-epidemiology, risk factors, and
genetics. BMJ. 321:624–628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson JR and Jatoi I: The global breast
cancer burden. Future Oncol. 8:697–702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson WF, Katki HA and Rosenberg PS:
Incidence of breast cancer in the United States: current and future
trends. J Natl Cancer Inst. 103:1397–1402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reuter S, Prasad S, Phromnoi K, Kannappan
R, Yadav VR and Aggarwal BB: Embelin suppresses osteoclastogenesis
induced by receptor activator of NF-κB ligand and tumor cells in
vitro through inhibition of the NF-κB cell signaling pathway. Mol
Cancer Res. 8:1425–1436. 2010.PubMed/NCBI
|
|
5
|
Hu R, Zhu K, Li Y, Yao K, Zhang R, Wang H,
Yang W and Liu Z: Embelin induces apoptosis through down-regulation
of XIAP in human leukemia cells. Med Oncol. 28:1584–1588. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikolovska-Coleska Z, Xu L, Hu Z, Tomita
Y, Li P, Roller PP, Wang R, Fang X, Guo R, Zhang M, Lippman ME,
Yang D and Wang S: Discovery of embelin as a cell-permeable,
small-molecular weight inhibitor of XIAP through structure-based
computational screening of a traditional herbal medicine
three-dimensional structure database. J Med Chem. 47:2430–2440.
2004. View Article : Google Scholar
|
|
7
|
Joshi R, Kamat JP and Mukherjee T: Free
radical scavenging reactions and antioxidant activity of embelin:
biochemical and pulse radiolytic studies. Chem Biol Interact.
167:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sreepriya M and Bali G: Effects of
administration of Embelin and Curcumin on lipid peroxidation,
hepatic glutathione antioxidant defense and hematopoietic system
during N-nitrosodiethylamine/Phenobarbital-induced
hepatocarcinogenesis in Wistar rats. Mol Cell Biochem. 284:49–55.
2006. View Article : Google Scholar
|
|
9
|
Mahendran S, Badami S, Ravi S, Thippeswamy
BS and Veerapur VP: Synthesis and evaluation of analgesic and anti-
inflammatory activities of most active free radical scavenging
derivatives of embelin-A structure-activity relationship. Chem
Pharm Bull. 59:913–919. 2011. View Article : Google Scholar
|
|
10
|
Thippeswamy BS, Mahendran S, Biradar MI,
Raj P, Srivastava K, Badami S and Veerapur VP: Protective effect of
embelin against acetic acid induced ulcerative colitis in rats. Eur
J Pharmacol. 654:100–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Danquah M, Li F, Duke CB III, Miller DD
and Mahato RI: Micellar delivery of bicalutamide and embelin for
treating prostate cancer. Pharm Res. 26:2081–2092. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai Y, Qiao L, Chan KW, Yang M, Ye J, Ma
J, Zou B, Gu Q, Wang J, Pang R, Lan HY and Wong BC: Peroxisome
proliferator-activated receptor-gamma contributes to the inhibitory
effects of Embelin on colon carcinogenesis. Cancer Res.
69:4776–4783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aird KM, Ding X, Baras A, Wei J, Morse MA,
Clay T, Lyerly HK and Devi GR: Trastuzumab signaling in
ErbB2-overexpressing inflammatory breast cancer correlates with
X-linked inhibitor of apoptosis protein expression. Mol Cancer
Ther. 7:38–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mori T, Doi R, Kida A, Nagai K, Kami K,
Ito D, Toyoda E, Kawaguchi Y and Uemoto S: Effect of the XIAP
inhibitor Embelin on TRAIL-induced apoptosis of pancreatic cancer
cells. J Surg Res. 142:281–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiarugi P and Giannoni E: Anoikis: a
necessary death program for anchorage-dependent cells. Biochem
Pharmacol. 76:1352–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allensworth JL, Aird KM, Aldrich AJ,
Batinic-Haberle I and Devi GR: XIAP inhibition and generation of
reactive oxygen species enhances TRAIL sensitivity in inflammatory
breast cancer cells. Mol Cancer Ther. 11:1518–1527. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang B, Zhang Y, Liang R, Yuan P, Du J,
Wang H and Wang L: Activation of the δ-opioid receptor inhibits
serum deprivation-induced apoptosis of human liver cells via the
activation of PKC and the mitochondrial pathway. Int J Mol Med.
28:1077–1085. 2011.
|
|
19
|
Mattson MP and Kroemer G: Mitochondria in
cell death: novel targets for neuroprotection and cardioprotection.
Trends Mol Med. 9:196–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saito M, Korsmeyer SJ and Schlesinger PH:
BAX-dependent transport of cytochrome c reconstituted in pure
liposomes. Nat Cell Biol. 2:553–555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nicholson DW, Ali A, Thornberry NA,
Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle
M and Lazebnik YA: Identification and inhibition of the ICE/CED-3
protease necessary for mammalian apoptosis. Nature. 376:37–43.
1995. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hyman BT and Yuan J: Apoptotic and
non-apoptotic roles of caspases in neuronal physiology and
pathophysiology. Nat Rev Neurosci. 13:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi LG, Zhang GP and Jin HM: Inhibition of
microvascular endothelial cell apoptosis by angiopoietin-1 and the
involvement of cytochrome C. Chin Med J (Engl). 119:725–730.
2006.PubMed/NCBI
|