Introduction
The lysyl oxidase (LOX) gene family comprises five
members which act as extracellular modulating enzymes; LOX, LOXL,
LOXL2, LOXL3 and LOXL4 (1). The
first identified and the more studied member of this family is LOX.
The human LOX gene which spans across 15 kb of genomic DNA is
located on chromosome 5 (5q23.3–31.2) and is comprised of seven
exons that encode a 417-amino acid protein. LOX is synthesized as a
48-kDa preproprotein (preproLOX) which includes a 21-amino acid
signal sequence at the amino terminus (2,3).
PreproLOX is N-glycosylated and secreted from the cell as a
catalytically inactive 50-kDa proenzyme protein (proLOX). ProLOX is
subsequently cleaved into a catalytically mature 32-kDa protein
(LOX) and a 180-kDa LOX-PP (1). The
amino terminus of LOX contains the most unique sequence, while the
carboxy terminus is highly conserved among its family members and
is responsible for catalytic activity. The carboxy terminus
contains a copper-binding site, lysyl tyrosyl quinine cofactor
binding residues, a catalytically active site, a cytokine receptor
and growth factor receptor-like domain (2,3).
LOX is a copper-dependent amine oxidase that
maintains the covalent cross-linking of collagens and elastin in
extra-cellular matrices, which is essential for normal function of
connective tissue, embryonic development and adult tissue
remodeling (4). Aberrant LOX
expression or enzymatic activity is correlated with certain
diseases, including cutis laxa, Menkes’ syndrome, spontaneous
coronary artery dissection (5–7),
atherosclerosis, scleroderma, liver cirrhosis and senile plaque
formation in Alzheimer’s and non-Alzheimer’s dementia (8–10).
Recent studies have demonstrated that LOX has
intracellular functions involved in the regulation of cell
differentiation, motility/migration, gene transcription and cell
adhesion. The aberrant LOX expression and activity that have been
observed in various cancerous tissues and neoplastic cell lines are
of interest (11). The role of LOX
in in cancer has been controversial, due to both down- and
upregulation of LOX in tumor tissues and cancer cell lines, which
have been found in initial studies, suggesting a dual role for LOX
as a tumor suppressor, as well as a metastasis-promoting gene
(11–13).
Gastric cancer is one of the most common types of
tumors and remains the second leading cause of cancer mortality in
the world although the diagnosis and treatment of such patients
have improved (14). Surgical
resection is the main treatment modality and is able to cure
patients with early-stage cancer. However, the survival rate of
patients with advanced resectable gastric cancer remains poor,
despite new treatment strategies, such as perioperative
chemotherapy (15) and adjuvant
chemoradiation (16).
Currently, numerous gastric cancer patients are
diagnosed when the tumor is at an unresectable stage. For these
patients, systemic chemotherapy is the main treatment option as it
is able to prolong survival without impacting on quality of life.
Certain single agents and combinations are effective in the
treatment of suchmetastatic disease, but the survival of patients
with advanced gastric cancer treated with palliative chemotherapy
remains low. New therapies are therefore urgently needed.
At present, the TNM stage, tumor differentiation and
histological classification are the primary criteria for predicting
the clinical outcome in gastric cancer. As a highly heterogeneous
tumor, prognosis in gastric cancer, however, often varies among
patients with the same clinicopathological parameters in practice.
Therefore, additional classification parameters need to be defined
in addition to the TNM and the classic pathological characteristics
of the tumor in order to better identify the biological subsets of
this disease. Biological prognostic factors are often derived from
the genetic process, which is thought to represent a crucial step
to gastric cancer. Some of these potential prognostic factors may
also be predictive of response to therapy as they are a molecular
target either to chemotherapeutics or to biological/targeted
therapies (17). With the aid of
microarray technology, the identification of LOX as a potential
modulator of tumorigenesis and/or metastatic tumor progression was
carried out in this study.
Materials and methods
Patients and tissue samples
This study was approved by the Research Ethics
Committee of Ruijin Hospital, Shanghai Jiao Tong University School
of Medicine, China. Written informed consent was obtained from all
the patients enrolled in this study. All specimens were handled and
made anonymous according to the ethical and legal standards.
Tissue specimens for real-time
quantitative reverse transcription polymerase chain reaction
(qRT-PCR)
Fresh specimens for qRT-PCR were obtained from 10
patients who underwent surgery for gastric cancer between March and
May 2010 at the Department of Surgery, Ruijin Hospital, Shanghai
Jiao Tong University School of Medicine. Grossly visible normal and
cancerous portions of the specimens were snap-frozen in liquid
nitrogen and stored at −85°C. None of the patients had received
radiotherapy or chemotherapy prior to surgery.
Tissue specimens and microarray
construction
A total of 161 patients who had undergone curative
surgery for gastric cancer at Ruijin Hospital, Shanghai Jiao Tong
University School of Medicine, between January 2002 and December
2003 were enrolled in the study. Patient-derived specimens were
collected and archived under protocols approved by the
Institutional Review Boards of Shanghai Jiao Tong University. The
group was composed of 107 males and 54 females with a mean age of
57 (range, 28–80) years at the time of surgery. There were 63 cases
at stage I, 39 at stage II, 46 at stage III and 13 at stage IV. The
diagnoses were confirmed by two pathologists, and the tumor grade
and stage classifications were assigned according to the
International Union Against Cancer guidelines (18). None of the patients had received
radiotherapy or chemotherapy prior to surgery. Patients with
advanced gastric cancer received standard chemotherapeutic
protocols, including 5-fluorouracil postoperatively, according to
the NCCN Practice Guidelines for Gastric Cancer (19). All patients were subjected to close
follow-up observation. The follow-up deadline was October 2010.
For tissue microarray (TMA) construction,
formalin-fixed, paraffin-embedded samples obtained from the above
161 patients that contained primary tumors and adjacent normal
mucosa were retrieved from the archives of the Department of
Pathology in Ruijin Hospital. Hematoxylin and eosin
(H&E)-stained slides were screened for tumor tissue and
non-cancerous tissue adjacent to the tumor (≥2 cm from the tumor).
Representative areas of tissue were established and 2.0-mm diameter
cores were punched from the paraffin blocks. Two cores from each
tumor and paired normal tissue (≥2 cm from the tumor) were arrayed
next to each other to ensure similar reaction conditions. All
specimens were examined by two pathologists to prevent bias. Tumor
and normal mucosa morphology on the arrays were validated as having
high accordance with the whole archived section. TMA slides were
constructed and made in collaboration with Shanghai Biochip
(Shanghai, China).
Real-time qPCR
Total RNA in 10 paired, frozen primary gastric
cancer tissues and adjacent normal mucosa were extracted according
to the manufacturer’s instructions (Life Technologies, Carlsbad,
CA, USA), and then 0.5 μg RNA was reverse transcribed into
cDNA using a PrimerScript™ RT reagent kit (Takara Bio Inc., Dalian,
China). qPCR was performed in a 10-ml total reaction mixture.
Quantitative LOX mRNA levels were assessed using ABI 7500 real-time
PCR System (Applied Biosystems, Carlsbad, CA, USA) with a Master
Mix kit (Takara Bio Inc.) according to the manufacturer’s
instructions. GAPDH was used as an internal control. Each real-time
PCR was repeated 3 times and the 2−ΔΔCt method was used
for normalization with GAPDH as a reference gene. Gene expression
with a ratio of >2 was considered to be upregulated. The
sequences for qRT-PCR primers were as follows: GAPDH forward,
5′-TGTTGCCATCAATGACCCCTT-3′; GAPDH; reverse,
5′-CTCCACGACGTACTCAGCG-3′; LOX forward,
5′-CACAGGACATCATGCGTATGC-3′; LOX reverse,
5′-CCACTTCAGAACACCAGGCAC-3′.
Immunohistochemistry
The TMA sections were deparaffinized in xylene and
rehydrated in graded series of ethanols followed by heat-induced
epitope retrieval in citrate buffer (pH 6.0). LOX expression was
detected using a primary antibody against LOX (anti-LOX antibody,
rabbit polyclonal to LOX, 1/300; Abcam, Cambridge, MA, USA). After
incubation with a biotinylated secondary antibody and DAB (Dako,
Carpinteria, CA, USA), the slides were rinsed and counterstained
with Mayer’s hematoxylin. Staining was scored by two independent
investigators without knowledge of patient outcomes according to
the staining intensity and extent as described previously (20). To evaluate LOX expression,
immuostaining was classified into four groups according to both
intensity and extent. The proportion of cell protein expression was
categorized as follows: 0, 0% immunopositive cells; 1, <10%
positive cells; 2, 11–50% positive cells; 3, 51–75% positive cells;
and 4, >75% positive cells. The staining intensity was
categorized by relative intensity as follows: 0, negative; 1, weak;
3, moderate; 4, strong. The proportion and intensity scores were
then multiplied to obtain a total score. To obtain final
statistical results, scores ≤2 were considered as negative, while
scores of 3–4 were considered as (+), scores of 5–8 as (++) and
scores of 9–16 as (+++).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
statistical software (Chicago, IL, USA). Correlation was assessed
with the Spearman Rho correlation coefficient and Pearson
Chi-square test. Kaplan-Meier survival curves were generated and
survival data were analyzed with the log-rank test and Cox
proportional hazards regression. P<0.05 was considered to
indicate a statistically significant result.
Results
Upregulation of LOX expression in primary
gastric cancer tissues compared with adjacent normal mucosa
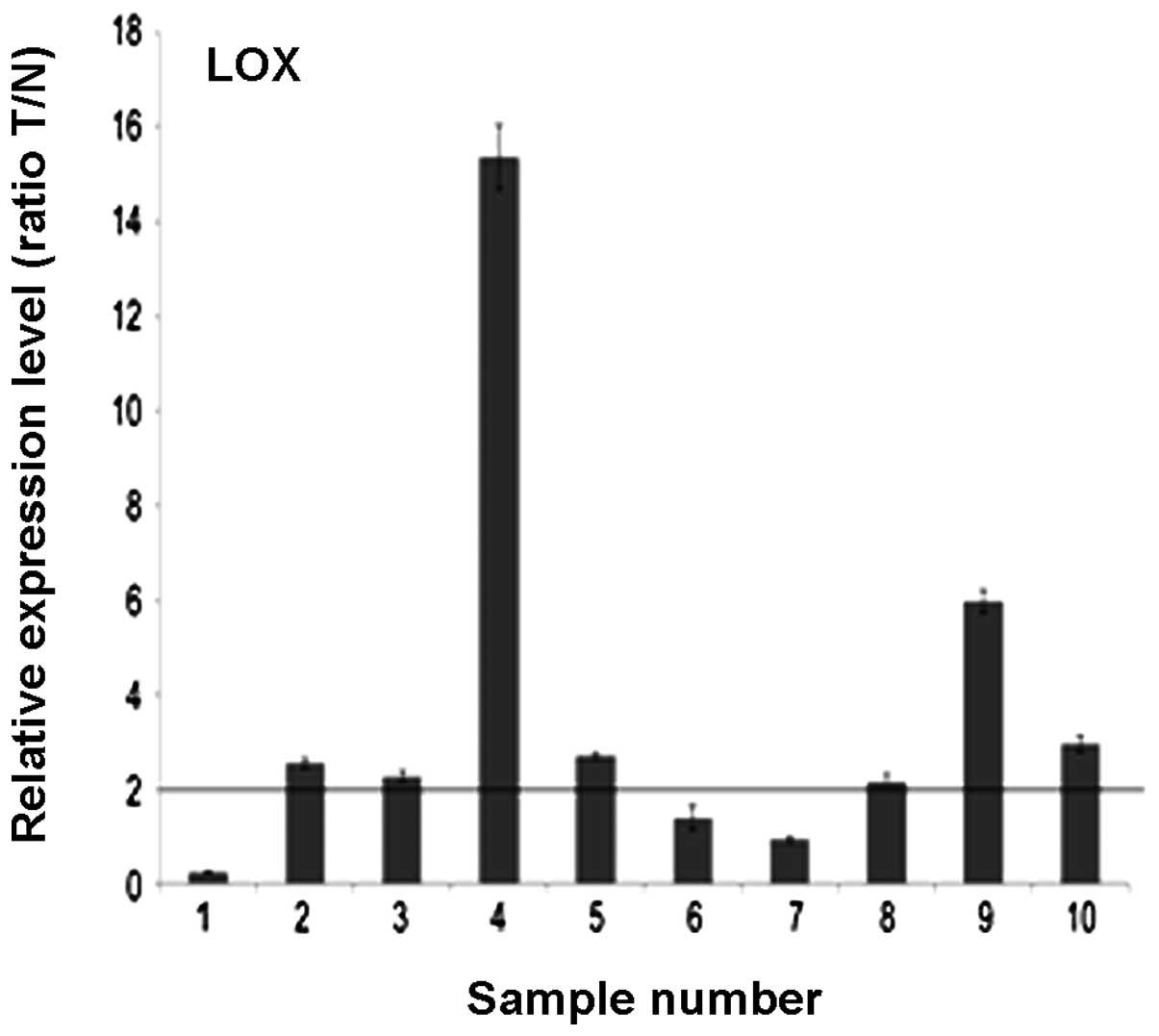
Of the 10 paired cases used for the evaluation of
LOX mRNA and protein expression, 7 (70%) gastric cancer tissues
showed at least a four-fold increase in LOX mRNA level compared
with the adjacent non-cancerous mucosa. The difference in LOX mRNA
expression was significant (70 vs 30%; P<0.05; Fig. 1).
Association of LOX TMA
immunohistochemical staining with patient clinicopathological
parameters
The expression of LOX was predominantly localized in
the cytoplasm and nuclei of tumor cells. Of the 161 TMA specimens,
expression of LOX was observed in 68 (42.2%) of the tumors. Of
these, 54 cases had a score of +, 6 cases had a score of ++ and 8
cases had a score of +++. Meanwhile 20 (12.4%) of the adjacent
noncancerous mucosa displayed positive staining. The difference in
LOX expression between tumor cells and adjacent noncancerous mucosa
was significant (42.2 vs. 12.4%, P<0.05; Fig. 2). Associations between
clinicopathological factors and LOX expression are summarized in
Table I. Increased LOX expression
was significantly correlated with the depth of tumor invasion,
lymph node status and TNM stage (P<0.05; Table I).
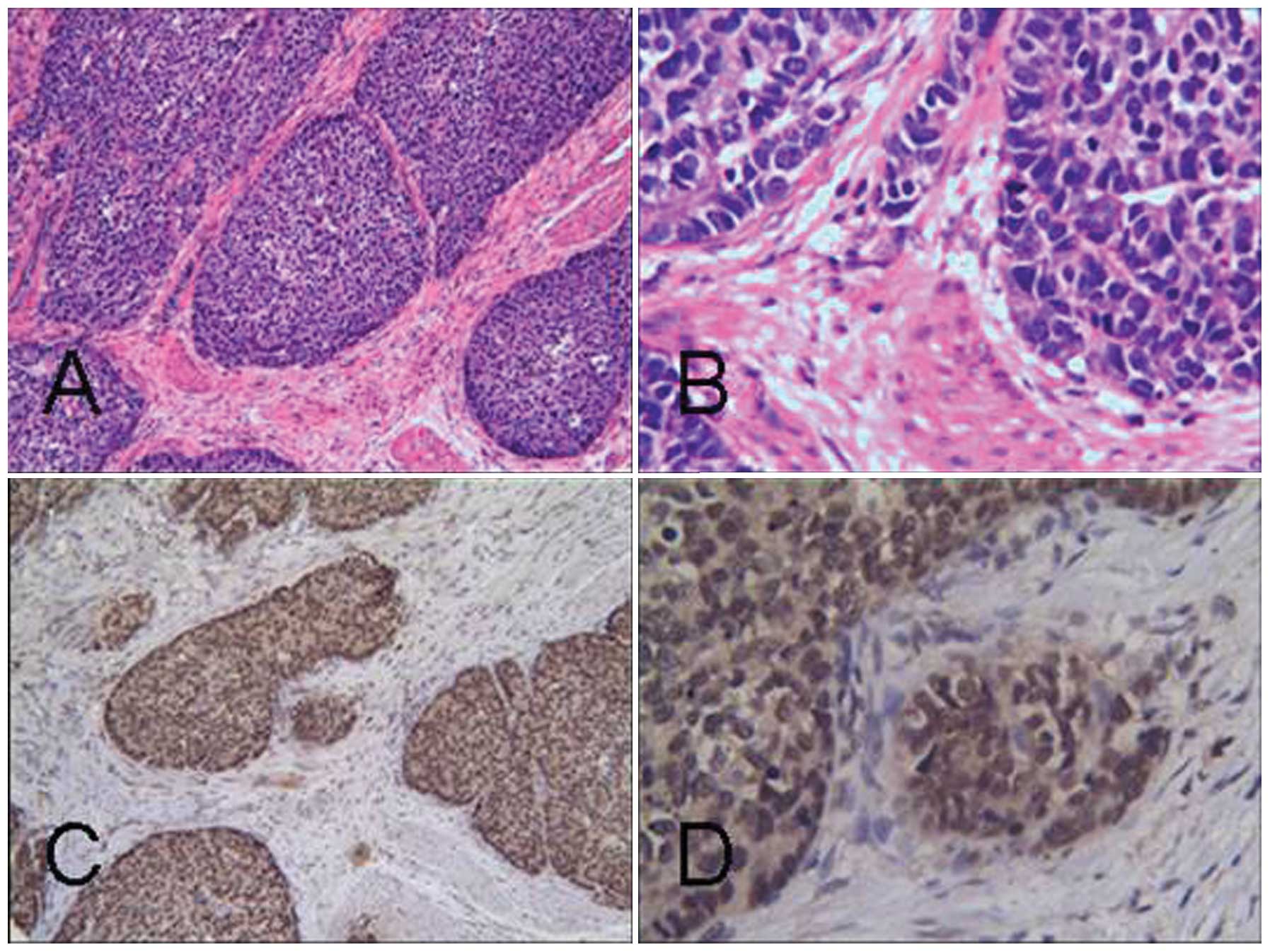 | Figure 2(A) Expression of LOX in primary
gastric carcinoma poorly differentiated adenocarcinoma H&E
staining: (a) magnification, ×100; (b) magnifcation, ×400;
immunohistochemical staining: (c) magnification, ×100; (d)
magnification, ×400. (B) Expression of HOXC6 in primary gastric
carcinoma. Intermediate-differentiated adenocarcinoma H&E
staining: (a) magnification, ×100; (b) magnification, ×400;
immunohistochemical staining: (c) magnification, ×100; (d)
magnification, ×400. LOX, lysyl oxidase; H&E, hematoxylin and
eosin. |
 | Table ICorrelation between expression of LOX
protein with clinicopathological parameters of gastric cancer. |
Table I
Correlation between expression of LOX
protein with clinicopathological parameters of gastric cancer.
| Expression of LOX
protein
| |
|---|
| Clinicopathological
parameters | Negative | Positive | P-value |
|---|
| Gender | | | |
| Male | 58 | 49 | 0.238 |
| Female | 35 | 19 | |
| Age (years) | | | |
| ≤60 | 59 | 32 | 0.053 |
| >60 | 34 | 36 | |
| Differentiation | | | |
| Poor | 62 | 48 | 0.612 |
|
Well+intermediate | 31 | 20 | |
| Tumor site | | | |
| Pylorus | 61 | 45 | 0.795 |
| Gastric corpus | 24 | 19 | |
| Gastric fundus | 8 | 4 | |
| Tumor size (in
diameter) | | | |
| ≤5 cm | 59 | 32 | 0.053 |
| >5 cm | 34 | 36 | |
| Depth of
invasion | | | |
| Mucosa | 16 | 2 | 0.005 |
| Muscular layer | 25 | 16 | |
| Serosa | 52 | 50 | |
| Lymph node
status | | | |
| LN0 | 47 | 21 | 0.015 |
| LN1-3 | 46 | 47 | |
| p-Stage | | | |
| I+II | 65 | 37 | 0.049 |
| III+IV | 28 | 31 | |
Survival analysis and prognostic
significance of LOX expression
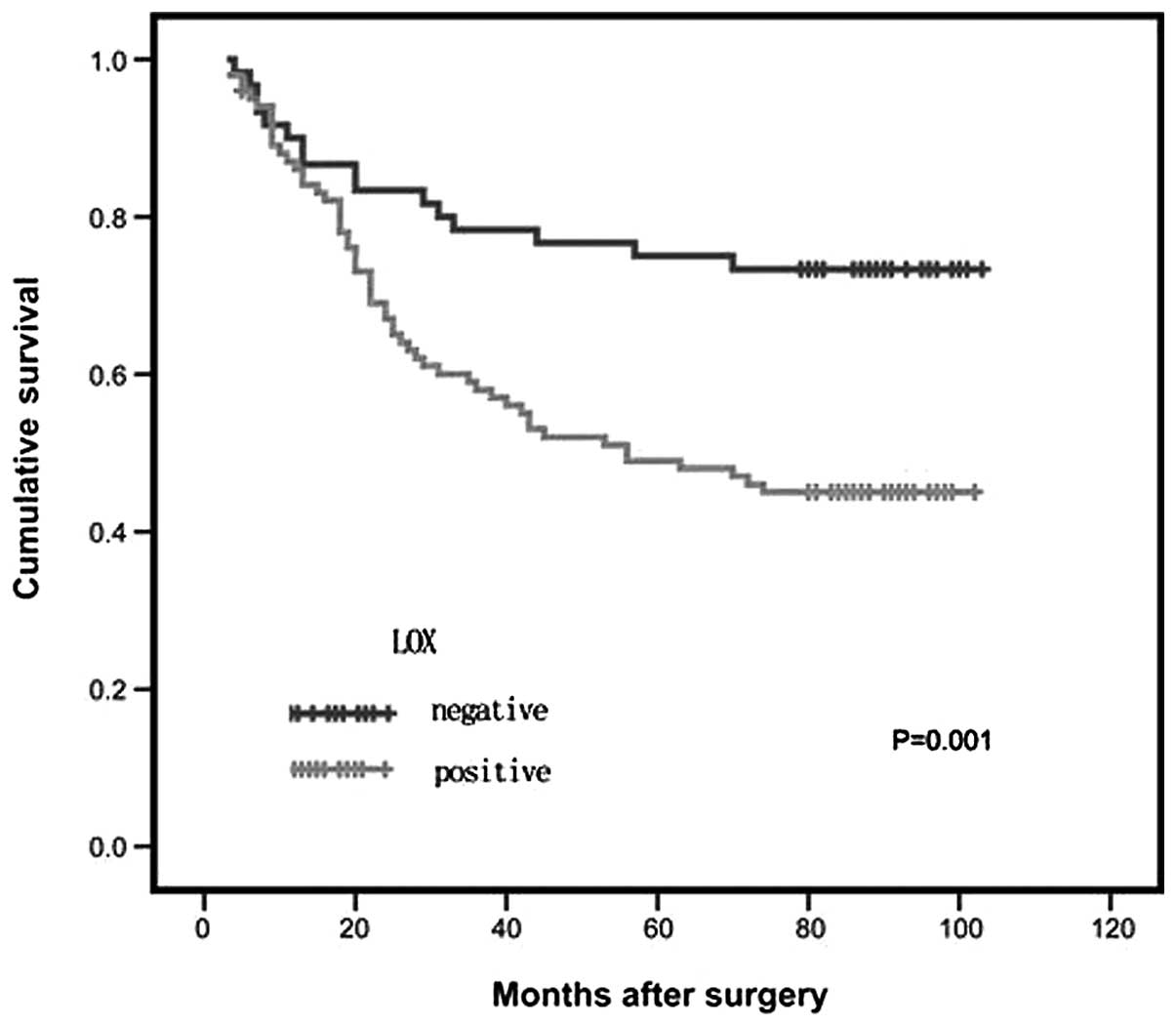
Kaplan-Meier curves showed that patients with
positive LOX expression had a lower overall survival rate than the
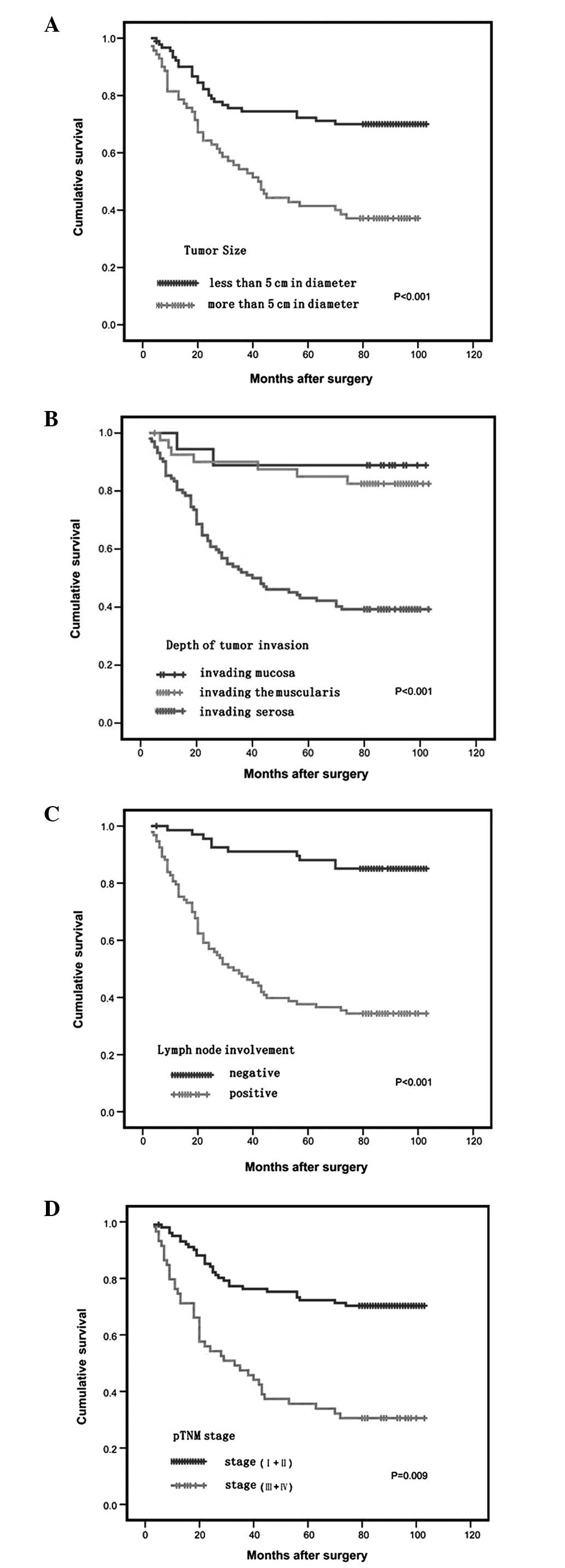
group with negative LOX expression (P<0.05; Fig. 3). As expected, the established
clinical parameters of the tumor size, lymph node status, depth of
tumor invasion and TNM stage significantly affected survival
(P<0.05; Fig. 4).
In the Cox multiple regression analysis, depth of
tumor invasion, histological differentiation, lymph node status and
LOX expression independently significantly affected survival
(Table II).
 | Table IICox multiple regression analysis for
overall survival after surgery. |
Table II
Cox multiple regression analysis for
overall survival after surgery.
| Characteristic | HR | 95% CI | P-value |
|---|
| Age (years) | 1.264 | 0.757–2.110 | 0.370 |
| Gender | 1.201 | 0.714–2.021 | 0.490 |
| Tumor site | 1.238 | 0.851–1.801 | 0.265 |
| Tumor size | 1.526 | 0.860–2.705 | 0.148 |
| Lymph node
status | 3.928 | 1.875–8.229 | 0.000 |
| Depth of
invasion | 1.989 | 1.028–3.845 | 0.041 |
| p-Stage | 0.527 | 0.299–0.929 | 0.027 |
|
Differentiation | 1.241 | 0.709–2.171 | 0.450 |
| LOX expression | 1.804 | 1.074–3.027 | 0.026 |
Discussion
Currently, the TNM stage is the most frequently used
for predicting prognosisfor gastric cancer. This classification
system takes into account the depth of invasion of gastric wall
(T), the involvement of lymph nodes (N) and the presence of distant
metastasis (M). In the present study, the results of univariate
analysis showed that tumor size, depth of invasion, involvement of
lymph nodes and TNM stage are associated with the prognosis in
gastric cancer, which was consistent with the results of previous
studies (18).
LOX was initially reported as a copper-dependent
amine oxidase responsible for the catalysis of collagen and elastin
cross-linking within the extracellular matrix. However, previous
studies have shown that LOX may have intracellular functions,
including the regulation of cell differentiation,
motility/migration and gene transcription (11,21).
Since LOX protein structure and function are so complex and involve
vital biological processes, such as cell movement, signal
transduction and gene regulation, it is evident that aberrant
regulation of LOX may lead to tumorigenesis and tumor progression
(11).
Neoplastic transformation occurs as a result of
genetic and epigenetic alterations in signalling pathways that
mediate cell growth, cell cycle arrest and apoptosis. Genetic
alterations may occur through mutational activation (e.g.,
oncogenes), mutational inactivation and loss of heterozygosity
(e.g., tumor suppressor genes), as well as epigenetically (e.g.,
methylation/ demethylation of CpG dinucleotides) (22). A decrease in LOX mRNA and/or protein
has been observed in basal and squamous cell, bronchogenic, colon,
esophageal, gastric, head and neck squamous cell, pancreatic and
prostatic carcinomas, as well as melanoma (11). The lack of LOX protein in tumor
cells originating from keratinocytes is clearly associated with
human basal cell carcinoma and squamous cell carcinoma skin
cancers. The inhibition of the LOX enzymatic activity in the skin
equivalent model induces basement membrane disruption and
deregulation of filaggrin and K10 expression of the dermis,
preparing a phenotype favorable to tumor development. The loss of
LOX protein adds another step towards invasion (23). As for the role of LOX in gastric
cancer, Kaneda et al(12)
found that loss of heterozygosity and promoter methylation of LOX
were detected in 33% (9 of 27) and 27% (26 of 96) of gastric
cancers, respectively, suggesting that LOX is a tumor suppressor
gene inactivated by methylation in human gastric cancers.
Methylation-associated silencing of LOH was also observed in lung,
colon and ovarian cancer cell lines (11). However, in our study, upregulated
expression of LOX mRNA and protein has been found in gastric
cancer. This finding suggests that there may be another mechanism
by which LOX is involved in gastric cancer progression.
Tumor cell invasion is a complex process that
involves attachment to, degradation of and detachment from an
extracellular matrix, and finally active migration away from the
primary tumor (10). Similar to
tumorigenesis, metastatic progression also emerges as a result of
genetic and epigenetic alterations in pathways that mediate cell
invasion, survival outside of the primary tumor microenvironment
and colonization/growth at a distant organ site (24). Tumor metastasis may be influenced by
both the immediate microenvironment (cell-cell or cell-matrix
interactions) and the extended tumor microenvironment.
An increase in LOX mRNAand/or protein also has been
observed in breast (25), head and
neck squamous cell (25), prostatic
(26) and clear cell renal cell
carcinoma (27), compared with
their normal or non-aggressive neoplastic counterparts. In these
studies, the expression of high levels of LOX mRNA and/or protein
was a poor prognostic factor and was associated with poorly
differentiated, high-grade tumors, increased recurrence rates and
decreased overall survival. In our study, we also found that LOX
protein expression was significantly correlated with depth of tumor
invasion, lymph nodes status, and TNM stage. Upregulated expression
of LOX was an independent prognostic marker of a worse outcome in
gastric cancer patients, in agreement with results from Wilgus
et al in lung adenocarcinoma (28).
As a component of the extracellular matrix, LOX
plays a role in facilitating tumor-stromal interactions that are
important for tumor progression and metastasis. The role of LOX in
tumor progression has been most extensively studied in breast
cancer. In these studies, LOX was considered to be important for
late-stage tumor progression to metastasis, but not for earlier
stages involving tumor formation. LOX was identified to be
upregulated in breast cancer cells with metastatic ability and to
facilitate breast cancer cell migration and adhesion through the
hydrogen peroxide-mediated regulation of the FAK/Src signaling
pathway leading to downstream changes in cell adhesion and
migration (13,25).
Our results showed that LOX staining was
predominantly present in cytoplasm and nuclei of gastric cancer
cell, it has been shown that extracellular LOX was able to enter
into the cytosol and became concentrated in the nuclei of smooth
muscle cells through an unknown mechanism (29).
There have been no published studies on the possible
association between LOX expression and the clinicopathological
features of gastric cancer. Our results revealed significant
correlations between the upregulated expression of LOX and the
depth of tumor invasion, lymph node status and TNM stage. In the
Cox multiple regression analysis, the upregulated expression of LOX
was an independent prognostic marker of a worse outcome in gastric
cancer patients. The strong correlations suggested that LOX
upregulation may promote tumor invasion and metastasis and that LOX
may possibly be used as a biomarker to identify subsets of gastric
cancer with a more aggressive phenotype. These preliminary findings
need to be verified in a larger, prospective, controlled, clinical
study. The mechanism by which LOX is involved in gastric cancer
progression has not been well elucidated in this study.
Acknowledgements
This study was supported by grants
from National Natural Science Foundation of China (Nos. 81172324
and 30900670), Science and Technology Commission of Shanghai
Municipality (Nos. 10jc1411100, 09DZ1950100 and 09DZ2260200),
Shanghai Key Discipline (S30204) and Key Projects in the National
Science & Technology Pillar Program of China (Nos. 2008BA152B03
and 2011BA203191).
References
|
1
|
Panchenko MV, Stetler-Stevenson WG,
Trubetskoy OV, Gacheru SN and Kagan HM: Metalloproteinase activity
secreted by fibrogenic cells in the processing of prolysyl oxidase:
Potential role of procollagen C-proteinase. J Biol Chem.
271:7113–7119. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Csiszar K: Lysyl oxidases: A novel
multifunctional amine oxidase family. Prog Nucleic Acid Res Mol
Biol. 70:1–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lucero HA and Kagan HM: Lysyl oxidase: an
oxidativeenzyme and effector of cell function. Cell Mol Life Sci.
63:2304–2316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pinnell SR and Martin GR: The
cross-linking of collagen and elastin: enzymatic conversion of
lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde
(allysine) by an extract from bone. Proc Natl Acad Sci USA.
61:708–716. 1968. View Article : Google Scholar
|
|
5
|
Khakoo A, Thomas R, Trompeter R, Duffy P,
Price R and Pope FM: Congenital cutis laxa and lysyl oxidase
deficiency. Clin Genet. 51:109–114. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Royce PM, Camakaris J and Danks DM:
Reduced lysyl oxidase activity in skin fibroblasts from patients
with Menkes’ syndrome. Biochem J. 192:579–586. 1980.PubMed/NCBI
|
|
7
|
Sibon I, Sommer P, Lamaziere JM and Bonnet
J: Lysyl oxidase deficiency: a new cause of human arterial
dissection. Heart. 91:e332005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kagan HM, Raghavan J and Hollander W:
Changes in aortic lysyl oxidase activity in diet-induced
atherosclerosis in the rabbit. Arteriosclerosis. 1:287–291. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kagan HM: Lysyl oxidase: mechanism,
regulation and relationship to liver fibrosis. Pathol Res Prac.
190:910–919. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gilad GM, Kagan HM and Gilad VH: Evidence
for increased lysyl oxidase, the extracellular matrix-forming
enzyme, in Alzheimer’s disease brain. Neurosci Lett. 376:210–214.
2005.PubMed/NCBI
|
|
11
|
Payne SL, Hendrix MJ and Kirschmann DA:
Paradoxical roles for lysyl oxidases in cancer - a prospect. Cell
Biochem. 101:1338–1354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaneda A, Wakazono K, Tsukamoto T, et al:
Lysyl oxidase is a tumor suppressor gene inactivated by methylation
and loss of heterozygosity in human gastric cancers. Cancer Res.
64:6410–6415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Payne SL, Fogelgren B, Hess AR, et al:
Lysyl oxidase regulates breast cancer cell migration and adhesion
through a hydrogen peroxide-mediated mechanism. Cancer Res.
65:11429–11436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cunningham D, Allum WH, Stenning SP, et
al: MAGIC Trial Participants: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. New Eng J
Med. 355:11–20. 2006. View Article : Google Scholar
|
|
16
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Eng J
Med. 345:725–730. 2001. View Article : Google Scholar
|
|
17
|
Scartozzi M, Galizia E, Freddari F,
Berardi R, Cellerino R and Cascinu S: Molecular biology of sporadic
gastric cancer: prognostic indicators and novel therapeutic
approaches. Cancer Treat Rev. 30:451–459. 2004. View Article : Google Scholar
|
|
18
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumors. 6th edition. Wiley-Liss; New
York: 2002
|
|
19
|
Ajani J, D’Amico TA, Hayman JA, Meropol NJ
and Minsky B: National Comprehensive Cancer Network. Gastric
cancer: Clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 1:28–39. 2003.PubMed/NCBI
|
|
20
|
Xu C, Zheng P, Shen S, et al: NMR
structure and regulated expression in APL cell of human SH3BGRL3.
FEBS Lett. 579:2788–2794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kagan HM and Li W: Lysyl oxidase:
properties, specificity, and biological roles inside and outside of
the cell. J Cell Biochem. 88:660–672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bouez C, Reynaud C, Noblesse E, et al: The
lysyl oxidase LOX is absent in basal and squamous cell carcinomas
and its knockdown induces an invading phenotype in a skin
equivalent model. Clin Cancer Res. 12:1463–1469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steeg PS: Tumor metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–903. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erler JT, Bennewith KL, Nicolau M, et al:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lapointe J, Li C, Higgins JP, et al: Gene
expression profiling identifies clinically relevant subtypes of
prostate cancer. Proc Natl Acad Sci USA. 101:811–816. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stassar MJ, Devitt G, Brosius M, et al:
Identification of human renal cell carcinoma associated genes by
suppression subtractive hybridization. Br J Cancer. 85:1372–1382.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilgus ML, Borczuk AC, Stoopler M,
Ginsburg M, Gorenstein L, Sonett JR and Powell CA: Lysyl oxidase: a
lung adenocarcinoma biomarker of invasion and survival. Cancer.
117:2186–2191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Nellaiappan K, Strassmaier T, Graham
L, Thomas KM and Kagan HM: Localization and activity of lysyl
oxidase within nuclei of fibrogenic cells. Proc Natl Acad Sci USA.
94:12817–12822. 1997. View Article : Google Scholar : PubMed/NCBI
|