Introduction
Triple-negative breast cancer (TNBC) was first
identified by Perou and Sorlie of Stanford University and was
defined as tumors that do not express estrogen receptor (ER),
progesterone receptor (PR) and HER2. Of all breast cancers, 12–20%
are TNBC (1). The majority of these
tumors are high-grade or poorly differentiated tumors and treatment
options for TNBC have been limited by the lack of targeted
therapies, so the prognosis is poorer than for other types of
breast cancer. It has been postulated that their phenotypic and
molecular similarity to BRCA1-associated breast cancers may prove
useful in terms of treatment (2).
The DNA of normal cells may be damaged in a number ways which
activate regulation by the DNA repair-associated protein, BRCA1.
When BRCA1 mutations occur, the DNA repair function is not
regulated and there is an inherited correlation between the BRCA1
gene and the pathogenesis of breast cancer (3). According to previous studies, ∼70% of
breast cancer cases exhibit the correlation between the BRCA1 gene
immune group and TNBC (4). Platinum
is a common second-line antitumor drug in breast cancer
chemotherapy. It has been suggested that platinum may be an
effective drug treatment for breast cancer with genetic mutations
in the BRCA1 gene. An in vitro study concerning the BRCA1
mutation in rat breast epithelial cells showed that platinum and
gemcitabine exhibited superior outcomes compared with the
first-line chemotherapy drugs anthracycline, paclitaxel and
fluorouracil (5). Platinum drugs
for TNBC may also have improved curative effects. A study by Sirohi
et al(6) reported that when
28 patients with TNBC were treated with 4 cycles of cisplatin as
the foundation of neo-adjuvant chemotherapy (75 mg/m2,
day 21), 6 patients (21%) achieved pathological complete response
(pCR) and 18 (64%) achieved clinical complete response (cCR) or
partial response (PR). Another study used cisplatin, epirubicin and
docetaxel in a single solution administered weekly. Of the 74 TNBC
patients, 46 achieved pCR and the total five-year disease-free
survival (DFS) rate was 76% (7).
Although numerous clinical studies have demonstrated the superior
efficacy of platinum treatments for TNBC, the simple clinical
efficacy has been observed mainly in small numbers of TNBC patients
and non-TNBC controls, with each sample size being small and of
variable quality. It is therefore necessary to use a systematic
evidence-based system to evaluate the effect of platinum-based
chemotherapy in treating TNBC and the long-term survival using
previous studies to provide higher quality clinical evidence.
Materials and methods
Identification of trials
The MEDLINE, EMBASE and Cochrane Library databases
were systematically searched until December 2011. Comparative
studies were identified using any of the following keywords:
cisplatin, platinum, carboplatin, paraplatin, oxalipatin,
lobaplatin, breast cancer and breast carcinoma. The search was not
limited to controlled or randomized trials to minimize the chance
of missing a study. A manual search of the relevant references was
performed to identify further relevant trials. There were no date
or language restrictions. Studies involving neo-adjuvant therapy
for advanced/metastatic cancers were excluded. The studies
identified through the search were independently screened by two
authors (M.L. and Q-G.M.) for inclusion. Any disagreements were
arbitrated by a third author (CY.W.).
Outcome measures
The primary outcomes evaluated in the present review
were the complete response (CR), PR, pCR, clinical benefit, DFS,
progression-free survival (PFS) and overall survival (OS) rates.
The secondary outcome was the adverse effects of treatment/toxicity
(including withdrawals and discontinuations).
Quality assessment
The present systematic review was conducted in
accordance with the Quality of Reporting of Meta-analyses (QUOROM)
statement (8). Two authors
independently evaluated all included trials based on randomized
sequence generation, allocation concealment, blinding of outcome
assessors and reporting of an intention-to-treat analysis. Trials
were considered to be of low quality if they reported none of the
items, medium quality if they reported on <3 and of high quality
if they reported on 3 or 4.
Data extraction
Two authors independently extracted the data
concerning the author details, year, methodological quality, number
of patients, patient characteristics, interventions (i.e., drugs,
schedule and number of therapeutic sessions) and outcomes using a
data extraction form. Discrepancies were resolved by consensus.
When multiple publications of the same trial were identified, data
were extracted and reported as a single trial.
Statistical analysis
The Cochrane Collaboration Review Manager 4.2.2
statistical software was used for for meta-analysis. To test for
heterogeneity in the included studies and analyze the statistical
heterogeneity using the χ2 test, the significance level
was set at P≤0.10. When heterogeneity existed between the results,
I2 heterogeneity quantitative analysis was used and the
significance level set at 50%, so I2>50% indicated
heterogeneity in the results. If the test results indicated that
the heterogeneity between the groups was not signficant, then a
fixed-effects model was used for meta-analysis. On the contrary, if
I2> 50%, then there was heterogeneity. After been
processed the heterogeneity still can not be eliminated, and is
intended merger analysis using a random-effects model. A
random-effects model was used for pooled analysis if there was
clinical heterogeneity in a subgroup analysis and treatment
heterogeneity was not eliminated. The standardized mean difference
(SMD) or weighted standard deviation (MD) were the effect
indicators of the present study and the 95% CI was used for the
efficacy analysis statistics.
Results
Description of studies
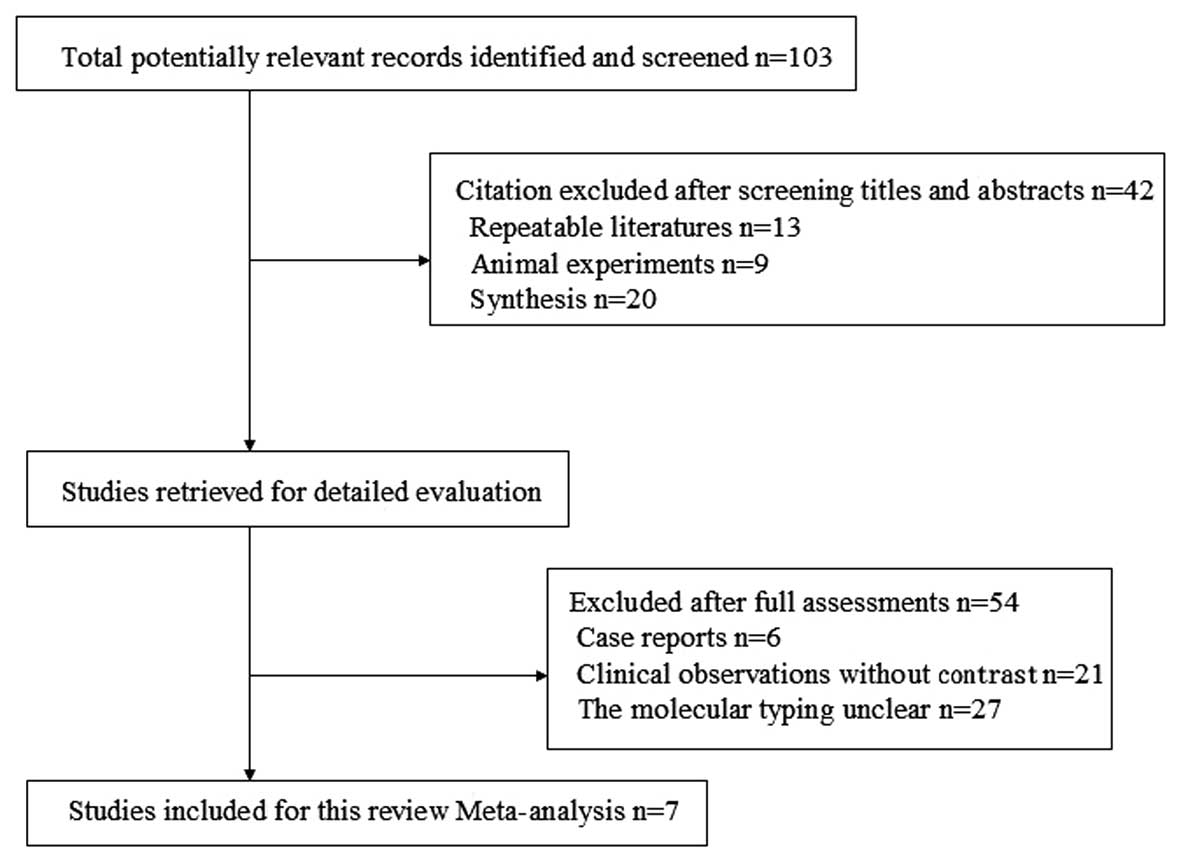
A total of 103 references were identified and 42
studies were excluded by reading the titles and abstracts to
identify the repeated studies, animal experiments and synthesis.
The studies were read further to identify case reports, clinical
observations without contrast and studies where the molecular
typing was unclear, to exculde a total of 54 studies. Ultimately,
seven studies were selected with a total of 717 patients. The
reference flow is shown in Fig.
1.
Quality of included studies
The seven studies were retrospective cohorts,
including 108 patients (9–14). The basic characteristics are shown
in Table I.
 | Table IBaseline characteristics of randomized
controlled trials included the meta-analysis. |
Table I
Baseline characteristics of randomized
controlled trials included the meta-analysis.
| Authors (Ref.) | Year | Country | Clinical stage | Chemotherapy |
|---|
| Sirohi, et al
1 (9) | 2008 | UK | T2–T4, N0–N3, M0 | EPI + DDP + 5-Fu |
| Chang, et
al(10) | 2010 | US | T2–T4, N0–N3, M0 | Carboplatin +
Doc |
| Chen, et
al(11) | 2010 | China | II–IIIC | Paclitaxel +
Carboplatin |
| Koshy, et
al(12) | 2010 | US | Metastatic | Carboplatin +
Gemcitabine |
| Sirohi, et al
2 (9) | 2008 | UK |
Advanced/metastatic | DDP or
Carboplatin |
| Chan, et
al(13) | 2010 | Singapore | Metastatic | Carboplatin +
Gemcitabine |
| Uhm, et
al(14) | 2009 | Korea |
Advanced/metastatic | DDP or
Carboplatin |
| Staudacher, et
al(15) | 2011 | France |
Advanced/metastatic | DDP or
Carboplatin |
The only platinum drugs used in the studies were
cisplatin and carboplatin. The study by Sirohi et
al(9) analyzed neo-adjuvant
chemotherapy in 94 and 155 cases of advanced/metastatic breast
cancer, so the study was divided into Sirohi 1 et al and
Sirohi 2 et al, neo-adjuvant and advanced/metastatic cases,
respectively, to analyze the outcome, as shown in Tables II, III and IV.
Two studies (10,11) were neo-adjuvant chemotherapy studies
and the remaning four (12–15) were of advanced/metastatic breast
cancer. Of the seven studies of TNBC, five (9–12,14)
had a clear description of ER and PR detection using an
immunohistochemical method, where ER and PR were defined as
negative in TNBC. HER2 testing was performed differently and the
negative standard was different. One study (9) described an immunohistochemical assay
for HER2. HER2 immunohistochemistry or FISH gene amplification were
used to define negative HER2 expression. The neo-adjuvant
chemotherapy, intervention and outcome measures used in the studies
are shown in Table II and another
study of a platinum single-agent neo-adjuvant chemotherapy used in
an observational study of clinical efficacy was added as a
comparison (6). The studies were
divided into groups of three; except the Sirohi 1 study (9) which used doxorubicin, the other 2
studies (10,11) used a drug combination of carboplatin
and paclitaxel. The Sirohi 1 (9)
study reports only the cCR as certain cases did not receive
surgical treatment; the other 2 studies both have pCR data. Sirohi
1 (9) reported that in certain
cases the cisplatin was replaced with carboplatin (AUC=5), due to
adverse reactions causing renal toxicity, neutropenia and
anemia.
 | Table IIStudy characteristics for patients
receiving chemotherapy for neo-adjuvant cancer. |
Table II
Study characteristics for patients
receiving chemotherapy for neo-adjuvant cancer.
| Authors (Ref.) | Sample | Mean age (year) | Clinical stage | Mean tumor size
(cm) | Drugs and dose | Surgery rate | Outcomes | Toxicities of grade
III–IV |
|---|
| Sirohi, et al
1 (9) | TN: 17
Non-TN: 77 | TN: 50
Non-TN: 46 | T3–T4
Nx; M0 | NR | 5 FU: 200
mg/m2
DDP: 60 mg/m2
EPI: 60 mg/m2
day 1 for 21 days*6 | 6 (35%)
P=0.005
54 (70%)
P=0.007 | cCR, PR, pD | NR |
| Chang, et
al(10) | TN: 11
Non-TN: 62 | 49.6 | T2–T4
Nx; M0 | 7.75 | DOC: 75
mg/m2
Carboplatin: AUC=6
day 1 for 21 days*4 | 71 | pCR, cCR,
2-year OS,
5-year OS | Neutropenia,
leukopenia, lymphopenia, febrileneutropenia |
| Chen, et
al(11) | TN: 24
Non-TN: 84 | 51 | II–IIIC | NR | Carboplatin:
AUC=2
Taxol: 180 mg/m2
days 1+8+15 for 28 days*4 | 108 | pCR | Neutropenia,
thrombocytopenia, anemia, skin toxicity |
| Silver, et
al(6) | TN: 28 | 29–69 | II–III | 3.7 (2.0–7.0) | DDP: 75
mg/m2
day 1 for 21 days*4 | 28 | pCR: 6
(21%)
cCR+PR:
18 (64%)
PD: 4 (14%) | Tinnitus,
neutropenia, hyperkalemia, elevation of ALT/AST, nausea, myalgia,
skin toxicity |
 | Table IIIPatient characteristics for those
receiving chemotherapy for advanced/metastatic cancer. |
Table III
Patient characteristics for those
receiving chemotherapy for advanced/metastatic cancer.
| Authors (Ref.) | Mean age
(years) | Sample | Node invasion
(%) | Visceral metastasis
(%) | Bone metastasis
(%) | Brain metastasis
(%) | Drugs and dose |
|---|
| Sirohi, et
al 2 (9) | TN: 47
Non-TN: 53 | TN: 34
Non-TN: 121 | TN: 9
(26)
Non-TN: 9 (8) | TN: 21
(62)
Non-TN: 101 (83) | TN: 4 (12)
Non-TN: 11 (9) | NR | M: 6 or 8
mg/m2
days 1+8+22+36
V: 6 mg/m2
DDP: 50 mg/m2
(or carboplatin: AUC=5)
day 1 for 42 days |
| Chan, et
al(13) | 52 | TN: 5
Non-TN: 36 | NR | NR | 21 (51) | 5 (12) | G: 1000
mg/m2 days 1+8
Carboplatin: AUC=5
day 1 for 21 days |
| Uhm, et
al(14) | TN: 46
Non-TN: 44 | TN: 24
Non-TN: 43 | NR | NR | NR | NR | DDP
or carboplatin |
| Staudacher, et
al(15) | TN: 48.4
Non-TN: 51.5 | TN: 93
Non-TN: 50 | TN: 60
(64.5)
Non-TN: 30 (60.0) | TN: 69
(74.2)
Non-TN: 41 (82) | NR | TN: 18
(19.4)
Non-TN: 7 (14.0) | DDP: 120
(83.9%)
Carboplatin: 23 (16.1%) |
| Koshy, et
al(12) | TN: 47.5
Non-TN: 50.2 | TN: 17
Non-TN: 19 | TN: 12
(71)
Non-TN: 6 (32) | NR | TN: 11
(65)
Non-TN: 9 (47) | TN: 7
(41)
Non-TN: 4 (21) | DDP: 25
mg/m2
G: 1000 mg/m2
days 1+8 for 21 days
(or days 1+8+15 for 28 days) |
| Liedtke, et
al(16) | TN: 58 | TN: 38 | 12 (32) | 33 (87) | 9 (24) | NR | DDP: 30
mg/m2
G: 750 mg/m2
days 1+8 for 21 days |
 | Table IVResults and toxicities for patients
receiving chemotherapy for metastatic/locally recurrent cancer. |
Table IV
Results and toxicities for patients
receiving chemotherapy for metastatic/locally recurrent cancer.
| Authors (Ref.) | Outcomes | Median number of
courses (range) | Toxicities in grade
III–IV |
|---|
| Sirohi, et
al(9) | CR, PR, PD, PFS,
OS | 5 (1–8) | NR |
| Chan, et
al(13) | PR, TTP | 4 (1–6) | Leukopenia,
neutropenia, anemia, thrombocytopenia, febrile-neutropenia,
diarrhea, hyponatremia |
| Uhm, et
al(14) | CR, PR, SD, PD | NR | NR |
| Staudacher, et
al(15) | CR, PR, SD, PD, OS,
PFS | 4 (1–9) | Febrileneutropenia,
neutropenia, thrombocytopenia, anemia |
| Koshy, et
al(12) | PFS,
OSa | 3–5 | NR |
| Liedtke, et
al(16) | CR: 2
(5%)
PR: 13 (35%)
SD: 13 (35%)
PD: 10 (27%)
TTP | 5 | Leukopenia,
febrileneutropenia, thrombocytopenia, anemia, alopecia,
nausea/vomiting, asthenia |
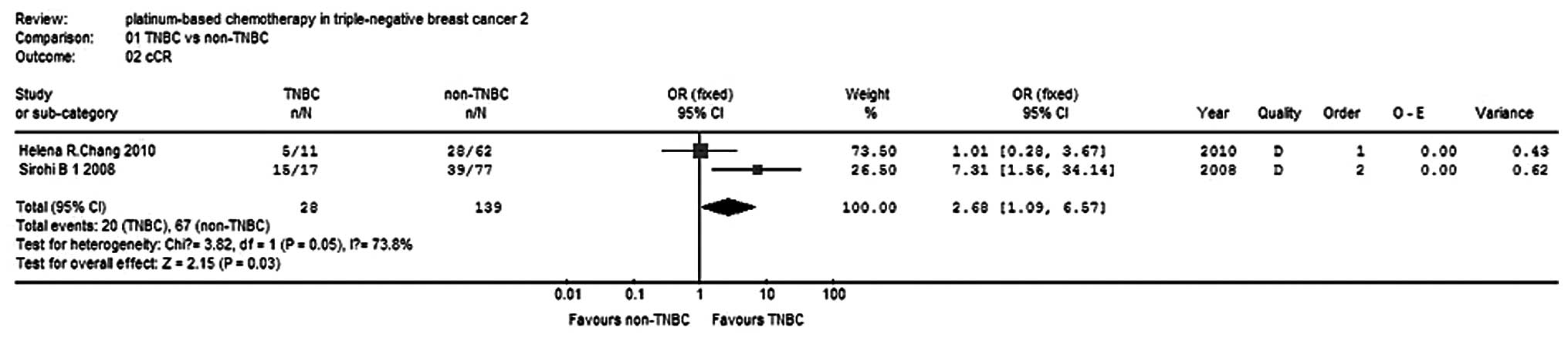
Neo-adjuvant cCR rate
Two studies (9,10)
reported the cCR rate for 167 patients and the test for
heterogeneity was statistically significant (P=0.05,
I2=73.8%), indicating the presence of a large
statistical heterogeneity, so a random-effects model was used for
the pooled analyses. The meta-analysis results showed that the
difference in the cCR rate was statistically significant (OR, 2.68;
95% CI, 1.69–6.57; P= 0.03; Fig. 2)
between the the TNBC group and non-TNBC group with regard to
neo-adjuvant therapy. The cCR rate of the TNBC group was 2.68-fold
higher than that of the non-TNBC group.
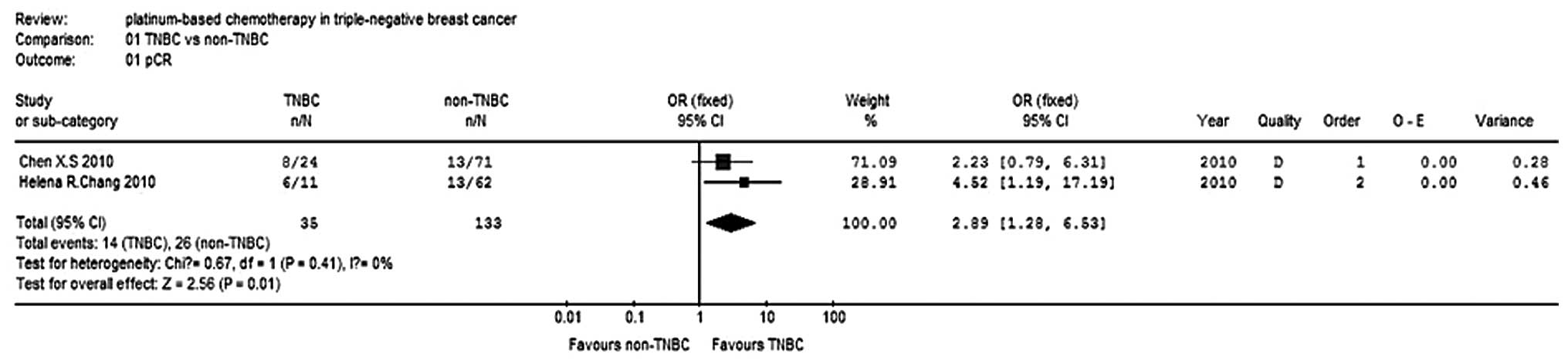
pCR rate
Two studies (10,11)
reported the pCR for 168 patients and the test for heterogeneity
was not statistically significant (P=0.41, I2=0%), so
the fixed-effects model was used. The meta-analysis results
(Fig. 3) showed that the difference
in pCR rates was statistically significant (OR, 2.89; 95% CI,
1.28–6.53; P=0.01) between the TNBC and non-TNBC groups, with
regard to carboplatin-paclitaxel chemotherapy. The effect of the
neo-adjuvant therapy on the TNBC group was superior to that of the
non-TNBC group.
OS and DFS rates
Only one study (9)
reported the OS and DFS rates for neo-adjuvant platinum-based
chemotherapy which included 94 cases with descriptive analysis. The
5-year OS rates in the TNBC and the non-TNBC groups were 0.65 and
0.8, respectively, while the 10-year OS rates in the TNBC and the
non-TNBC groups were 0.53 and 0.65 respectively. The median DFS
times were 68 and 90 months, respectively, with no significant
difference observed between the two groups (P= 0.6), while the
median OS times were 125 and 169 months, respectively. The distant
recurrence and survival of the patients treated using neo-adjuvant
chemotherapy with epirubicin, cisplatin and 5-Fu was not
significantly different between the TNBC and the non-TNBC
groups.
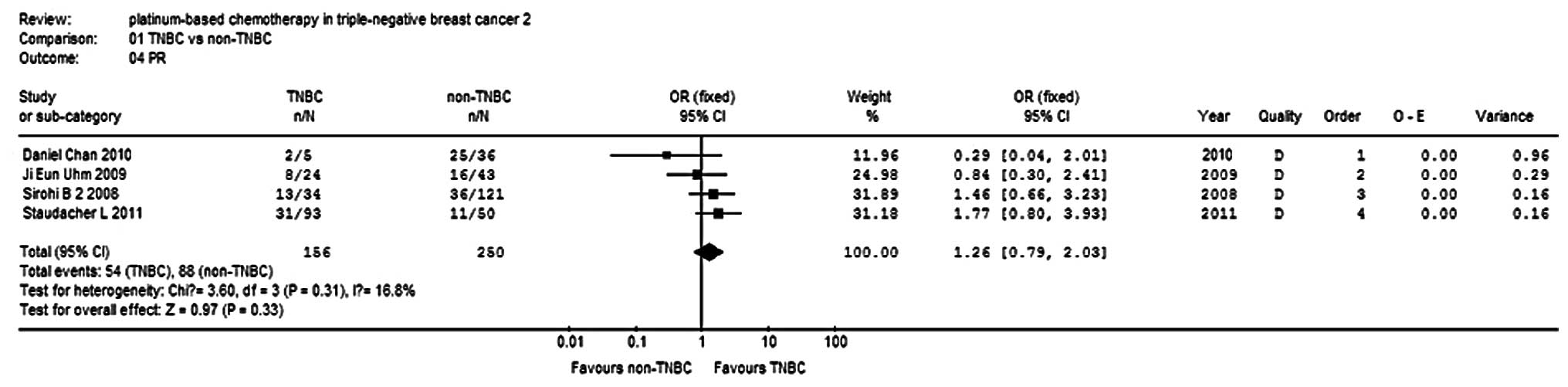
Advanced/metastatic disease
Response
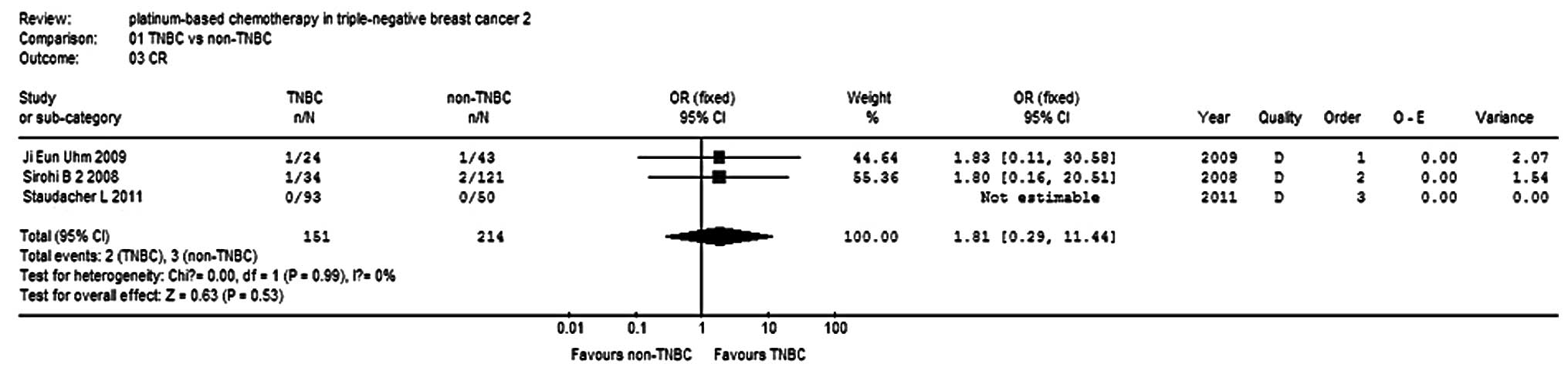
Four studies (9,13–15)
reported PR in 406 patients, and three studies (9,14,15)
reported cCR in 365 patients. There was no statistical
heterogeneity within each sub-group, so a fixed-effects model was
used to analyze the combinations. The results show that the PR and
cCR of the TNBC group were 1.26- and 1.81-fold that of the non-TNBC
group, respectively, but no significant differences were observed
(P=0.33, P=0.53; Figs. 4 and
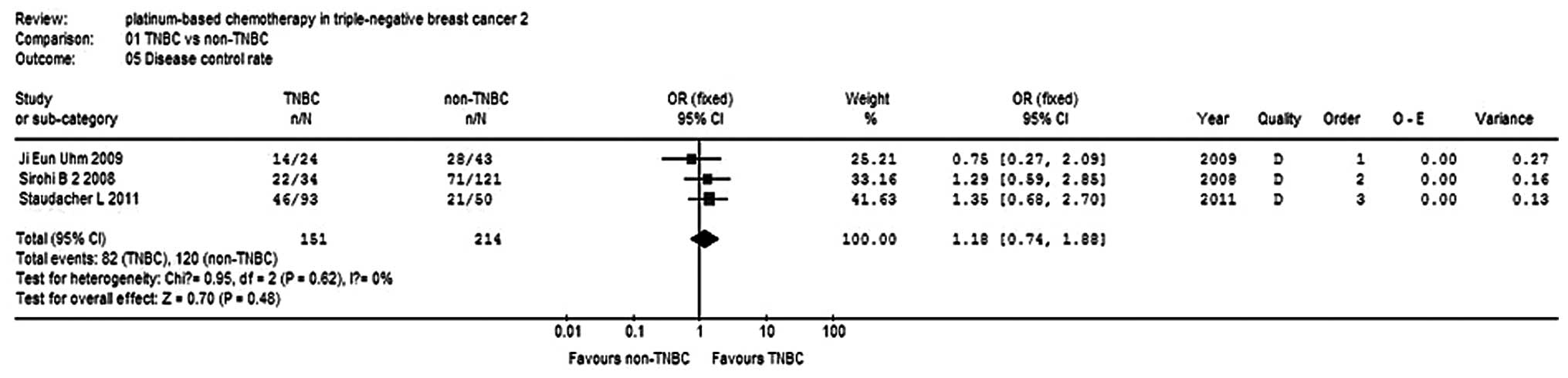
5). The clinical benefit rate (cCR
+ PR + stable disease) of the TNBC group was 1.18-fold higher than
that of the non-TNBC group, but no significant differences were
observed (P=0.48; Fig. 6).
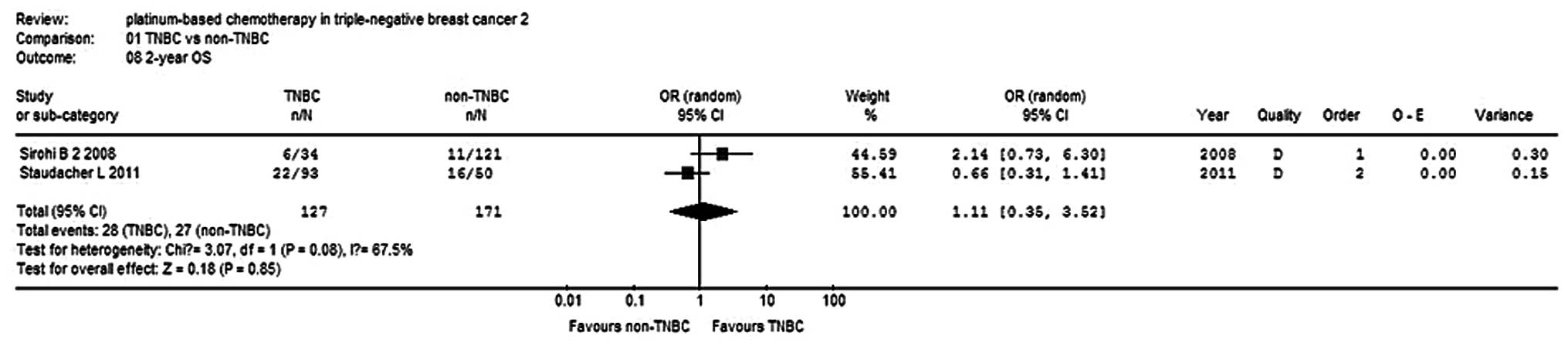
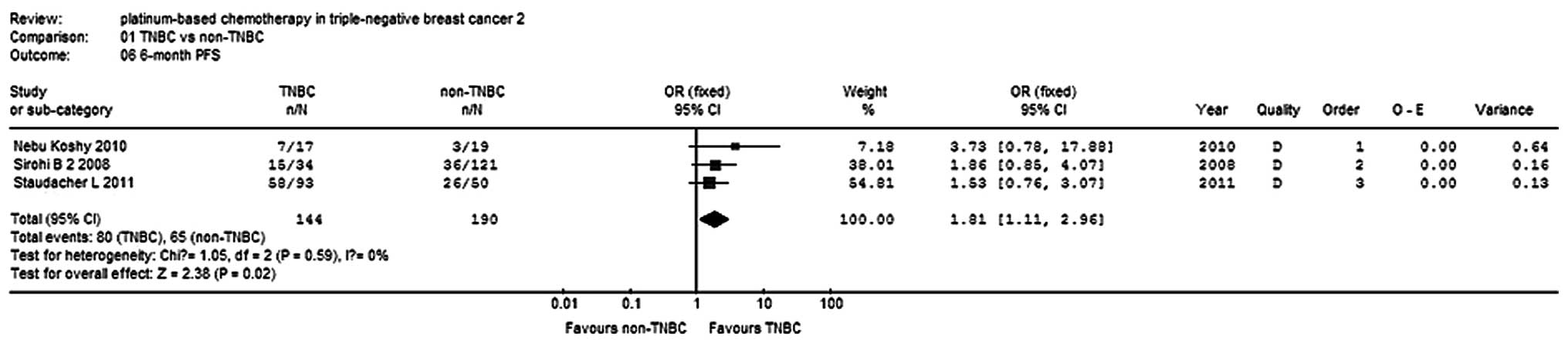
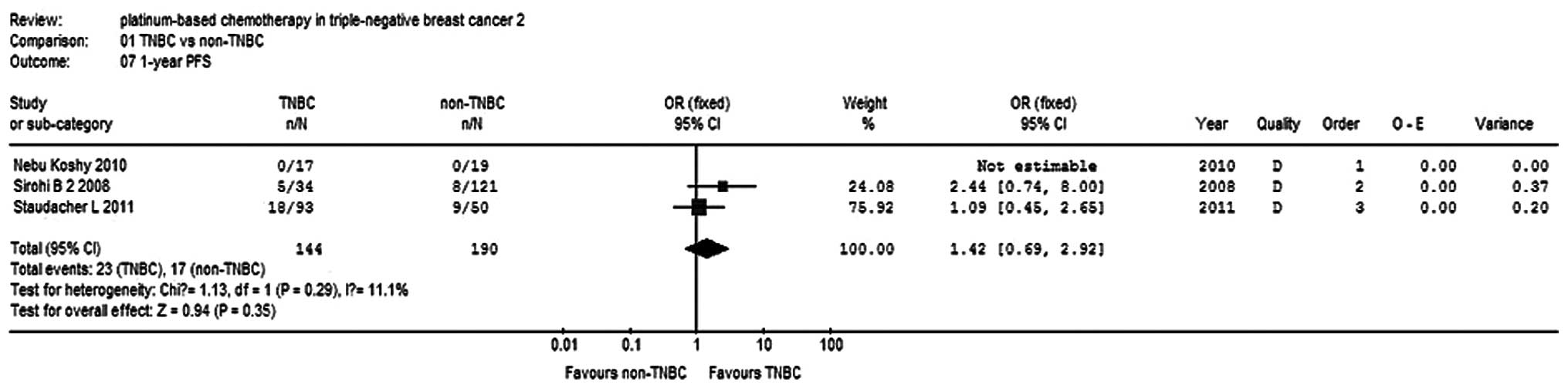
OS and PFS rates
Three studies (9,12,15)
reported 6-month and 1-year PFS rates for a total of 234 patients,
while two studies reported 2-year OS rates for 298 patients. There
was no statistical heterogeneity within the subgroups, so a
fixed-effects model was used to analyze the combinations (Fig. 7). The 6-month PFS rate of the TNBC
group was higher compared with the non-TNBC group (OR, 1.81; 95%
CI, 1.11–2.96; P=0.02; Fig. 8). The
1-year PFS rate was not significantly different between the two
groups (OR, 1.42, 95% CI, 0.69–2.92; P=0.35; Fig. 9).
Discussion
TNBC is a high-risk breast cancer due to the younger
age of patients, poorly differentiated tumors and shortened
survival that lacks the benefit of targeted therapies.
Platinum-based chemotherapy in TNBC is a popular research topic,
although there are significant differences between the various
studies concerning the drug use, dose, cycle, patient ages, tumor
stages and patient physical condition, so the results of the
studies are not the same. The results of the present study show
that, for cases receiving neo-adjuvant or adjuvant chemotherapy
with platinum, the pCR and cCR rates of the TNBC group were
significantly higher than those of the non-TNBC group. However the
long-term recurrence and survival exhibited little difference
between the groups. Sirohi et al(9) reported a 5-year OS rate of 65% in TNBC
patients treated with neo-adjuvant/adjuvant chemotherapy, while
Silver et al(6) reported
that with the single-agent cisplatin, six (21%) of the 28 patients
achieved pCR and 18 (64%) achieved cCR or PR. However, the 5-year
OS rate in non-TNBC patients has been reported to be 85% higher
than that of TNBC patients (9).
According to two studies (16,17),
the TNBC and non-TNBC patients who achieved pCR had similar OS
rates. Of the neo-adjuvant chemotherapy patients achieving pCR and
the non-pCR cases, the 2-year recurrence-free rates were 93.8 and
78.4%, respectively, while the 3-year recurrence-free rates were
83.3 and 58%, respectively. Therefore, for neo-adjuvant
chemotherapy used to treat TNBC patients, pCR is a significant
indicator of a good prognosis.
In addition, for the platinum treatment in the
metastatic TNBC and non-TNBC groups, the overall response (OR)
rates were similar and the long-term OS and DFS were no
significantly different. It is noteworthy that with the platinum
treatment, the TNBC group often had longer PFS and chemo-therapy
survival times (Table V). Koshy
et al(12) noted that,
compared with non-TNBC patients, the disease progression risk of
TNBC patients was reduced by 47%. However, previous studies appear
to show higher long-term recurrence rates in the TNBC group with
shorter DFS and lower OS. The present study suggests that, overall,
the two groups were not significantly different and it may be
proposed that platinum treatment in the TNBC group patients with
prolonged survival times and improved disease progression times was
superior to that of the non-TNBC group.
 | Table VLong-term effects of
metastatic/locally recurrent cancer. |
Table V
Long-term effects of
metastatic/locally recurrent cancer.
| Study (Ref.) | PFS (months) | OS (months) | OS after start of
PBCT | 6 month PFS
(%) | 1 year PFS (%) | 2 year OS (%) |
|---|
| Sirohi 2 et
al(9) | TN: 6
Non-TN: 4
P=0.05 | NR | TN: 11
Non-TN: 7 | TN: 15
(44)
Non-TN: 36 (30) | TN: 5
(14)
Non-TN: 8 (7) | TN: 6
(17)
Non-TN: 11 (9) |
| Uhm et
al(14) | NR | TN: 21
Non-TN: 56 | NR | NR | NR | NR |
| Staudacher et
al(15) | NR | TN: 22
Non-TN: 40
P=0.008 | NR | TN: 58
(62)
Non-TN: 26 (51) | TN: 18
(19)
Non-TN: 9 (18) | TN: 22
(24)
Non-TN: 16 (33) |
| Koshy et
al(12) | TN: 5.3
Non-TN: 1.7
P=0.058 | TN: 47.8
Non-TN: 66.8
P=0.25 | TN: 10.8
Non-TN: 4.3
P=0.10 | TN: 7
(41)
Non-TN: 3 (15) | TN: 0
Non-TN: 0 | NR |
| Liedtke et
al(16) | TN: 6 | NR | 13.5 | 21 (52.5) | 6 (15) | 7 (17.5) |
The present analysis included seven retrospective
cohort studies of varying quality and had the following
limitations. Since the observation time was long, there may be bias
as many cases were lost in follow-up. Study exposure factors that
affect the drug treatment, dose and cycle, including cases where
non-TNBC patients receive endocrine therapy and targeted therapy,
which the TNBC cases lack, cause a large bias. There were certain
differences between the studies with regard to the source of
subjects, disease classification, age, illness, physical fitness
and primary or secondary baseline information, which lead to larger
clinical heterogeneity. A lack of detailed data on the DFS and PFS
period data prevented the analysis of count data, such as the OS
and PFS times.
In summary, the efficacy of platinum in TNBC
treatment was demonstrated in the short- and long-term, subject to
further research and feasibility studies.
The present systematic review included studies where
the overall quality was not high and it requires more rigorous
design of high-quality randomized controlled studies to reduce and
remove bias that may exist in the study results. Future research
should focus more on comparisons of the therapeutic effects of
platinum compared with other chemotherapy drugs, in order to allow
more valuable quality of life studies of TNBC patients.
References
|
1
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, et al: Molecular portraits of human breast
tumours. Nature. 406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner N, Tutt A and Ashworth A: Hallmarks
of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 4:814–819.
2004.
|
|
3
|
Schneider BP, Winer EP, Foulkes WD, Garber
J, Perou CM, Richardson A, et al: Triple-negative breast cancer:
risk factors to potential targets. Clin Cancer Res. 14:8010–8018.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, et al: Breast cancer molecular
subtypes respond differently to preoperative chemotherapy. Clin
Cancer Res. 11:5678–5685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alli E, Sharma VB, Hartman AR, Lin PS,
McPherson L and Ford JM: Enhanced sensitivity to cisplatin and
gemcitabine in Brca1-deficient murine mammary epithelial cells. BMC
Pharmacol. 11:72011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silver DP, Richardson AL, Eklund AC, Wang
ZC, Szallasi Z, Li Q, et al: Efficacy of neoadjuvant Cisplatin in
triple-negative breast cancer. J Clin Oncol. 28:1145–1153. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frasci G, D’Aiuto G, Comella P, D’Aiuto M,
Di Bonito M, Ruffolo P, et al: Preoperative weekly cisplatin,
epirubicin, and paclitaxel (PET) improves prognosis in locally
advanced breast cancer patients: an update of the Southern Italy
Cooperative Oncology Group (SICOG) randomised trial 9908. Ann
Oncol. 21:707–716. 2010. View Article : Google Scholar
|
|
8
|
Moher D, Cook DJ, Eastwood S, Olkin I,
Rennie D and Stroup DF: Improving the quality of reports of
meta-analyses of randomised controlled trials: the QUOROM
statement. Quality of Reporting of Meta-analyses. Lancet.
354:1896–1900. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sirohi B, Arnedos M, Popat S, Ashley S,
Nerurkar A, Walsh G, et al: Platinum-based chemotherapy in
triple-negative breast cancer. Ann Oncol. 19:1847–1852. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang HR, Glaspy J, Allison MA, Kass FC,
Elashoff R, Chung DU and Gornbein J: Differential response of
triple-negative breast cancer to a docetaxel and carboplatin-based
neoadjuvant treatment. Cancer. 116:4227–4237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen XS, Nie XQ, Chen CM, Wu JY, Wu J, Lu
JS, et al: Weekly paclitaxel plus carboplatin is an effective
nonanthracycline-containing regimen as neoadjuvant chemotherapy for
breast cancer. Ann Oncol. 21:961–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koshy N, Quispe D, Shi R, Mansour R and
Burton GV: Cisplatin-gemcitabine therapy in metastatic breast
cancer: Improved outcome in triple negative breast cancer patients
compared to non-triple negative patients. Breast. 19:246–248. 2010.
View Article : Google Scholar
|
|
13
|
Chan D, Yeo WL, Tiemsim CM, Wong CI, Chuah
B, Soo R, et al: Phase II study of gemcitabine and carboplatin in
metastatic breast cancers with prior exposure to anthracyclines and
taxanes. Invest New Drugs. 28:859–865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uhm JE, Park YH, Yi SY, Cho EY, Choi YL,
Lee SJ, et al: Treatment outcomes and clinicopathologic
characteristics of triple-negative breast cancer patients who
received platinum-containing chemotherapy. Int J Cancer.
124:1457–1462. 2009. View Article : Google Scholar
|
|
15
|
Staudacher L, Cottu PH, Diéras V,
Vincent-Salomon A, Guilhaume MN, Escalup L, et al: Platinum-based
chemotherapy in metastatic triple-negative breast cancer: the
Institut Curie experience. Ann Oncol. 22:848–856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, et al: Response to neoadjuvant therapy and
long-term survival in patients with triple-negative breast cancer.
J Clin Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rouzier R, Pusztai L, Garbay JR, Delaloge
S, Hunt KK, Hortobagyi GN, et al: Development and validation of
nomograms for predicting residual tumor size and the probability of
successful conservative surgery with neoadjuvant chemotherapy for
breast cancer. Cancer. 107:1459–1466. 2006. View Article : Google Scholar : PubMed/NCBI
|