Introduction
Cancer patients suffer from various symptoms,
particularly in their final stage of life. Opioids play the main
role in palliative care medicine, among which morphine is most
commonly used (1). Although oral
administration of morphine is preferable, a parenteral route is
often used in terminally ill patients. Due to the inconvenience of
intramuscular injections, either intravenous or subcutaneous
injections are used. Continuous infusion limits the peak
concentration, which reduces the adverse effects of morphine, such
as sedation, and lessens the trough concentration problems of
breakthrough pain or dyspnea. In addition, the dosage is easily
titrated (2).
Lung cancer is the leading cause of cancer-related
mortality, and its incidence is increasing worldwide. Lung cancer,
along with lung cancer treatment and comorbid conditions, may cause
various symptoms that require palliation. There have been
retrospective analyses and reviews regarding continuous morphine
infusion (CMI) for cancer patients (2,3);
however, no such analysis limited to end-stage lung cancer patients
has been reported.
In the present study, we conducted a retrospective
analysis of end-stage lung cancer patients who received CMI at our
hospital based on the indication for CMI.
Patients and methods
A total of 79 patients with end-stage lung cancer
who has been admitted to Kyoto University Hospital, Kyoto, Japan
between 2008 and 2010 received CMI. Firstly, patient
characteristics, including histology, initial treatment patients
received, and infusion route, were analyzed. Then, the patients
were divided by the major indications for CMI, and their clinical
characteristics were compared between the groups. Survival time was
measured from the start of CMI to the time of mortality. All
patient data were obtained from our database. Informed consent
approved by the Institutional Review Board was obtained from all
patients.
Results
Patient characteristics
The patient characteristics are listed in Table I. The median patient age was 67
years (range, 34–86), and there were 55 males (70%) and 24 females
(30%) in total. A total of 63 patients (80%) had been diagnosed
with non-small-cell lung cancer (NSCLC) and 16 (20%) with SCLC.
Sixty-five patients (82%) had undergone aggressive treatment and
the remainder had received best supportive care. Forty-eight
patients (61%) had received pre-infusion opioid administration,
which comprised oxycodone, morphine sulfate and a fentanyl patch
for 26, 11 and 11 patients, respectively. A total of 31 patients
(39%) had received CMI intravenously and 48 (61%) received the
treatment subcutaneously.
 | Table ICharacteristics of the 79 end-stage
lung cancer patients. |
Table I
Characteristics of the 79 end-stage
lung cancer patients.
| Characteristics | Value |
|---|
| Age (years) | |
| Median (range) | 67 (34–86) |
| Gender, n (%) | |
| Male | 55 (70) |
| Female | 24 (30) |
| Histology, n (%) | |
| NSCLC | 63 (80) |
| SCLC | 16 (20) |
| Initial treatment, n
(%) | |
| Chemotherapy | 57 (72) |
|
Chemoradiotherapy | 6 (8) |
| Surgical
resection | 1 (1) |
| Radiation | 1 (1) |
| Best supportive
care | 14 (18) |
| Pre-infusion opioids,
n (%) | |
| Oxycodone | 26 (33) |
| Morphine
sulfate | 11 (14) |
| Fentanyl patch | 11 (14) |
| None | 31 (39) |
| Infusion route, n
(%) | |
| Intravenous | 31 (39) |
| Subcutaneous | 48 (61) |
Indications for CMI
All patients were divided into four groups based on
the indications for CMI: Group A (uncontrolled pain; n=9), group B
(dyspnea; n=44), group C (both dyspnea and pain; n=13) and group D
(an inability to take oral medicine; n=13). The majority of the
pain experienced was associated with cancer spread, and bone
metastasis was most frequently observed (n=22). Dyspnea was
cancer-related in a number of cases (carcinomatous pleurisy, n=22;
airway constriction, n=8; carcinomatous lymphangiosis, n=6);
however, in certain cases it was associated with treatment or
comorbidity, such as interstitial pneumonia (n=12) and radiation
pneumonitis (n=1). The major reason for the inability to take
morphine orally was a decreased level of consciousness due to
carcinomatous meningitis (n=8).
Subgroup analysis based on the
indications for CMI
Table II summarizes
the results of the subgroup analysis based on the indications for
CMI. All patients with pain (groups A and C) had received
pre-infusion opioids; however, two-thirds of patients without pain
(group B) had not. With the exception of group D, both the initial
and maximum doses were the lowest in group B, implying that dyspnea
itself required a lower dose of morphine for alleviation compared
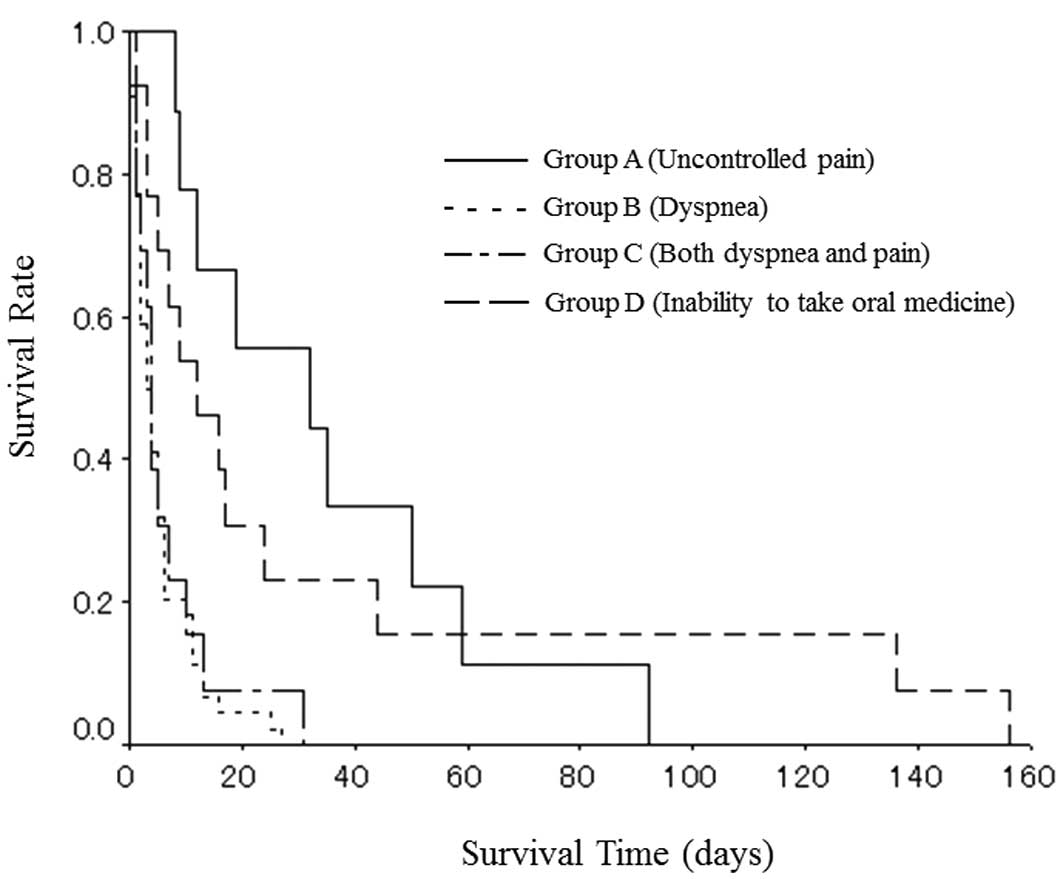
with pain. The median survival time from the start of CMI was 32
days in group A, 3 days in group B, 4 days in group C and 12 days
in group D (Fig. 1).
 | Table IISubgroup analysis based on the
indications for continuous morphine infusion. |
Table II
Subgroup analysis based on the
indications for continuous morphine infusion.
| Characteristic | Group A (n=9) | Group B (n=44) | Group C (n=13) | Group D (n=13) |
|---|
| Age (years), median
(range) | 72 (34–80) | 48 (41–86) | 61 (39–48) | 66 (59–71) |
| Gender,
male/female | 6/3 | 27/17 | 12/1 | 10/3 |
| Pre-infusion opioids,
+/− | 9/0 | 15/29 | 13/0 | 11/2 |
| Infusion route,
IV/SC | 3/6 | 21/23 | 4/9 | 3/10 |
| Starting-dose
(mg/day), median (range) | 50.0 (12.0–80.0) | 25.0 (4.0–100.0) | 25.0 (10.0–75.0) | 10.0 (5.0–60.0) |
| Maximum-dose
(mg/day), median (range) | 60.0
(20.0–250.0) | 25.0
(10.0–200.0) | 50.0
(15.0–240.0) | 15.0 (10.0–60.0) |
| Sedation, +/− | 2/7 | 4/40 | 6/7 | 0/13 |
Discussion
To the best of our knowledge, this is the first
study of lung cancer patients who had received CMI in the final
stage of their lives. In this analysis, uncontrolled pain, dyspnea
and an inability to take oral medicine were identified as
indications for CMI, and the patients were divided into four groups
on this basis: Group A (uncontrolled pain), group B (dyspnea),
group C (both dyspnea and pain) and group D (an inability to take
oral medicine). Among the four groups, group D was an exception;
patients in group D required CMI, not due to severe symptoms, but
due to their inability to take oral medicine, mainly as a result of
carcinomatous meningitis. Therefore, the degree of symptoms (pain
or dyspnea) were observed to be markedly milder in group D compared
with the other groups. With the exception of group D, dyspnea
itself required a lower dose of morphine for alleviation, whereas
the survival time from the start of CMI was shorter in patients
with dyspnea (group B or C) than those without dyspnea (group
A).
The prevalence of dyspnea varies with the primary
tumor site. Dyspnea is one of the most commonly reported symptoms
in lung cancer, with 15% of patients presenting with dyspnea at
diagnosis and 65% suffering from it at some point during their
illness. Close to death, 90% of patients with NSCLC suffer from
dyspnea (4). In the present
analysis, ∼70% of patients received CMI due to dyspnea (groups B
and C). The efficacy of systemic morphine administration, both
orally and parenterally, has been established in cancer patients
with dyspnea (5,6).
In this study, 61% of patents received CMI
intravenously and 39% received the treatment subcutaneously. The
method of administration employed was selected by the doctor. In a
prospective, within-patient, crossover study, continuous
intravenous and subcutaneous morphine were demonstrated to be
equally effective with a similar adverse effect profile (7). The parenteral route is desirable,
either intravenously or subcutaneously, considering the respective
advantages and disadvantages (8).
In conclusion, in our limited experience, terminally
ill lung cancer patients required CMI due to uncontrolled pain,
dyspnea and an inability to take oral medicine, and dyspnea was the
major indication for CMI. Dyspnea required a lower dose of morphine
for alleviation than uncontrolled pain; however, the survival time
of patients who required CMI due to dyspnea was extremely short
compared with patients without dyspnea. There has been no
established strategy with regard to when to commence CMI and how to
escalate the morphine dose. We predict that the strategy is likely
to differ according to the indications for CMI, considering the
apparent differences in the required morphine dose and the survival
time between the patient subgroups in the present study. Further
studies are required to facilitate the effective and appropriate
use of CMI in end-stage lung cancer patients.
References
|
1
|
Qaseem A, Snow V and Shekelle P, Casey DE
Jr, Cross JT Jr, Owens DK; Clinical Efficacy Assessment
Subcommittee of the American College of Physicians; Dallas P, Dolan
NC, Forciea MA, Halasyamani L, Hopkins RH Jr and Shekelle P:
Evidence-based interventions to improve the palliative care of
pain, dyspnea, and depression at the end of life: a clinical
practice guideline from the American College of Physicians. Ann
Intern Med. 148:141–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson SL and Shreve ST: Continuous
subcutaneous infusion of opiates at end-of-life. Ann Pharmacother.
38:1015–1023. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glare P, Walsh D, Groh E and Nelson KA:
The efficacy and side effects of continuous infusion intravenous
morphine (CIVM) for pain and symptoms due to advanced cancer. Am J
Hosp Palliat Care. 19:343–350. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kvale PA, Selecky PA and Prakash UB:
Palliative care in lung cancer: ACCP evidence-based clinical
practice guidelines (2nd edition). Chest. 132(3 Suppl): 368S–403S.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ben-Aharon I, Gafter-Gvili A, Paul M,
Leibovici L and Stemmer SM: Interventions for alleviating
cancer-related dyspnea: a systematic review. J Clin Oncol.
26:2396–2404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Viola R, Kiteley C, Lloyd NS, Mackay JA,
Wilson J and Wong RK; Supportive Care Guidelines Group of the
Cancer Care Ontario Program in Evidence-Based Care: The management
of dyspnea in cancer patients: a systematic review. Support Care
Cancer. 16:329–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nelson KA, Glare PA, Walsh D and Groh ES:
A prospective, within-patient, crossover study of continuous
intravenous and subcutaneous morphine for chronic cancer pain. J
Pain Symptom Manage. 13:262–267. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mercadante S: Intravenous morphine for
management of cancer pain. Lancet Oncol. 11:484–489. 2010.
View Article : Google Scholar : PubMed/NCBI
|