Introduction
Ultraviolet (UV) radiation from the sun,
particularly its UVB component (290–320 nm), is the major cause of
skin cancer. UV radiation is also known to elicit various other
adverse effects, including erythema, sunburn, inflammation,
hyperplasia, hyper-pigmentation, immunosuppression, premature skin
aging and photocarcinogenesis (1,2). These
cell events are mediated through gene activation or suppression
(3). Despite intensive
investigations into the regulation of gene expression in skin
cells, in the majority of cases the precise molecular events remain
to be elucidated. Novel methods should be investigated in this
area.
MicroRNAs (miRNAs) are short, non-coding RNAs of
approximately 22 nucleotides that are thought to regulate gene
expression through sequence-specific base pairing with the
3′-untranslated region (3′-UTR) of target mRNAs. To elucidate the
molecular mechanisms underlying photodamage, especially skin
carcinogenesis by UVB, miRNA expression profiles in UVB irradiated
cells have been investigated using miRNA microarrays (4–6). Guo
et al investigated the differential expression profiles of
miRNAs in NIH3T3 cells in response to UVB
irradiation (4). Pothof et
al found that miRNA expression changes and stress granule
formation were most pronounced within the first hours following UVB
irradiation, suggesting that miRNA-mediated gene regulation
operates earlier than most transcriptional responses (5). The miRNA response may be related to
the DNA damage response and cell proliferation (6). However, no studies are currently
available on miRNA profiling in response to UVB irradiation in
vivo. In this study, we compared the profiles of miRNA
expression in 3 pairs of UVB irradiated and untreated mice
epidermis, in order to reveal the specific underlying mechanisms
associated with photodamage.
Materials and methods
Animals and UV light source
Animal care and handling complied with protocols
approved by the Nanjing Medical University Institutional Animal
Care and Use Committee and employed measures to minimize pain and
discomfort. Female C57BL/6 mice (12 weeks old) were obtained from
the Chinese Academy of Science, Shanghai SLAC Laboratory Animal Co.
(Shanghai, China) and were maintained in a pathogen-free barrier
facility at Nanjing Medical University. The source of UVB was a
BLE-1T158 (Spectronics Corp., Westbury, NY, USA). A Kodacel filter
(TA401/407; Kodak, Rochester, NY, USA) was used to block
wavelengths of <290 nm (UVC). The UVB dose was quantified using
a Waldmann UV meter (model no. 585100; Waldmann Co.,
VS-Schwenningen, Germany) and 180 mJ/cm2 of UVB was
delivered to the dorsal skin of each mouse.
Animal treatments
The C57BL/6 mice were divided into two groups of
three animals. The mice in the control group did not receive any
treatment. The mice in the second group received UVB (180
mJ/cm2). Following 24 h of UVB irradiation, the dorsal
irradiated skin was collected. The epidermis was harvested for
analysis by heat separation from the dermis.
RNA isolation and miRNA microarray
Total RNA isolation and the miRNA enrichment
procedure were performed using a mirVana miRNA Isolation kit
(Ambion, Austin, TX, USA) according to the manufacturer’s
instructions. RNA concentration was quantified using a NanoDrop
spectrophotometer (Thermo Fisher, Waltham, MA, USA). The integrity
of the RNA was evaluated using an Agilent 2100 Bioanalyzer (Agilent
Technologies, Santa Clara, CA, USA). RNA labeling and hybridization
on the Agilent miRNA microarray chips were performed using a miRNA
labeling reagent and hybridization kit (Agilent Technologies) at
37°C for 30 min. Total RNA samples (100 ng) were treated with calf
intestine alkaline phosphatase (Takara Bio Inc., Dalian, China),
denatured using 100% DMSO (Sigma-Aldrich, St. Louis, MO, USA) at
100°C for 8 min in a thermal cycler, and then transferred to an
ice-water bath to prevent reannealing of the RNA. The RNA samples
were then labeled with pCp-Cy3 using T4 RNA ligase (Ambion) and
incubated at 16°C for 2 h. The labeled samples were hybridized to
Agilent mouse miRNA microarrays, which contained probes for 627
mouse miRNAs and 39 mouse viral miRNAs as catalogued in the Sanger
Centre Database version 10.1 (http://microrna.sanger.ac.uk). Hybridization was
performed in SureHyb chambers (Agilent Technologies) for 24 h at
55°C. The microarrays were then washed using Agilent prepared
buffers. The microarray images were scanned using the Agilent
microarray scanner, and gridded and analyzed using Agilent Feature
Extraction Software, version 9.5.1 (Agilent Technologies).
Normalization was performed using the per-chip median normalization
method and the median array (7).
Quantitative real-time PCR analysis for
miRNA expression
Expression levels of mmu-miR-233 and mmu-miR-141
were validated using quantitative real-time PCR (qRT-PCR). Primers
for qRT-PCR were synthesized by Invitrogen (Shanghai, China). cDNA
synthesis was performed using a miScript Reverse Transcription kit
(Qiagen, Hilden, Germany) according to the manufacturer’s
instructions. qRT-PCR was performed using a miScript SYBR-Green PCR
kit (Qiagen, Hilden, Germany). The reactions were incubated in a
96-well optical plate at 95°C for 15 min, followed by 40 cycles of
15 sec at 94°C, 30 sec at 55°C and 30 sec at 70°C. Expression
analysis was performed in triplicate for each sample. mmu-Actin was
used as the normalization control. miRNA expression levels were
quantified using an ABI Prism 7300 Sequence Detection System
(Applied Biosystems, Foster City, CA, USA).
Target prediction and function
analysis
TargetScan software was used to predict miRNA
targets. To evaluate the TargetScan target predictions for all
single miRNAs, we searched for significantly over-represented Gene
Ontology (GO) terms among all target genes for all differential
miRNAs separately using GOstat software (http://gostat.wehi.edu.au/cgi-bin/goStat.pl)
(8). In brief, the program
determines all the annotated GO terms and all the GO terms that are
associated (i.e., in the path) with the genes analyzed. It then
counts the number of appearances of each GO term for the genes
inside the group and for the reference genes. First, we pasted the
mouse RefSeq ID of the target genes into the text area, we then
chose ‘mgi’ (Mus musculus) from the available GO
gene-association databases. For the remaining options we selected
the default values. The majority of significant GO terms usually
represent the same subset of genes, since the genes may have
several GO annotations that are similar. Fisher’s exact test was
performed to determine whether the observed difference was
significant. For each GO category, this resulted in a P-value
whereby the observed counts were due to chance. In addition,
pathway analysis of the targets was performed using DAVID
Bioinformatics Resources 2008 (http://david.abcc.ncifcrf.gov/).
Statistical analysis
To identify miRNA that was differentially expressed
among the groups, a Student’s t-test was performed using SPSS
version 12.0 (SPSS, Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
The threshold cycle (Ct) value for the genes was
determined using SDS software, version 1.2 (Applied Biosystems).
The Ct is the cycle number at which fluorescence is generated as a
reaction crosses the threshold. The expression levels of
mmu-miR-188-5p, mmu-miR-22, mmu-miR-233, mmu-miR-125a-5p,
mmu-miR-146a and mmu-miR-141 were normalized by subtracting their
Ct values from that of the internal control mmu-actin, to obtain
ΔCt. The ΔΔCt method for relative quantitation of gene expression
was used to determine the miRNA expression levels. ΔCt was
calculated by subtracting the Ct of actin from the Ct of the miRNA
of interest. ΔΔCt was calculated by subtracting the ΔCt of the
reference sample (control) from the ΔCt of each sample. Fold change
was generated using the equation 2ΔΔCt. Experiments were
repeated in triplicate. Statistical significance was measured using
the Student’s t-test; p<0.05 was considered to indicate a
statistically significant difference.
Results
Significantly differentially expressed
miRNAs
From the miRNA microarray results, six miRNAs
(mmu-miR-188-5p, mmu-miR-223, mmu-miR-22, mmu-miR-125a-5p,
mmu-miR-146a and mmu-miR-141) were found to be differentially
expressed in the UVB treatment group compared with the control
group (P<0.05). The microarray images are shown in Fig. 1.
Validation of microarray data by
miRNA-specific qRT-PCR analysis
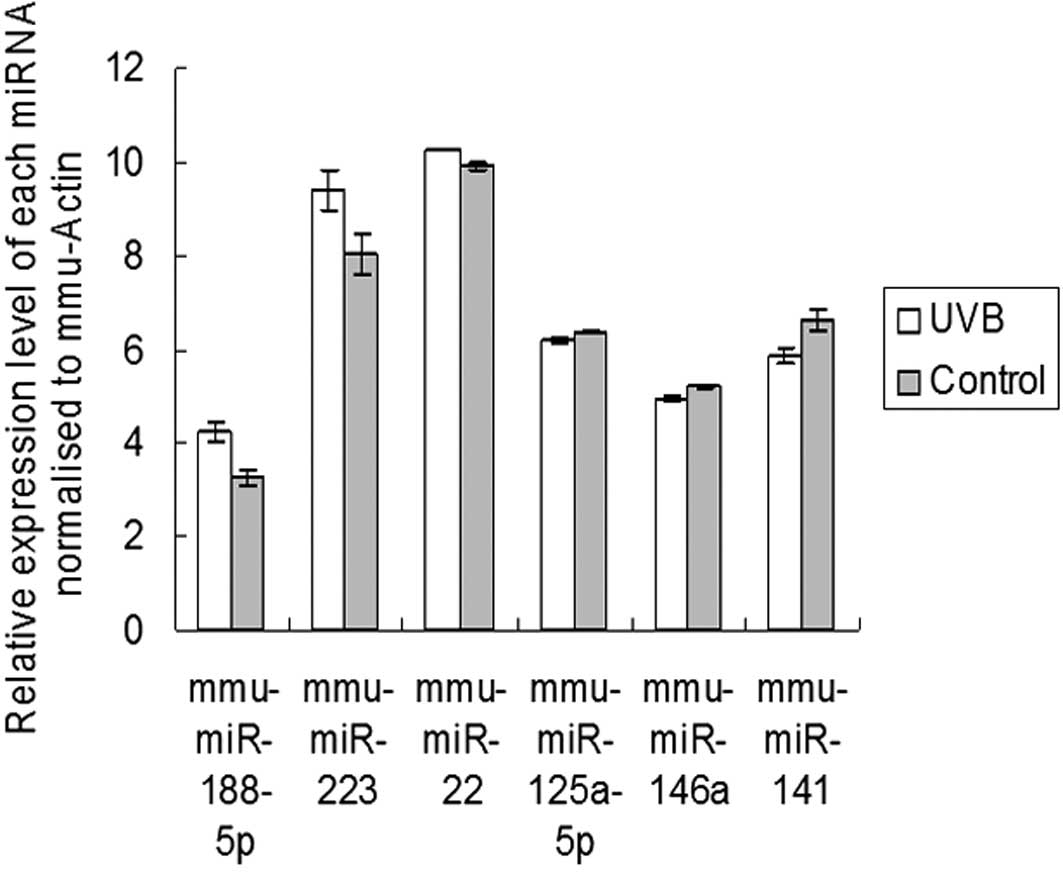
To confirm the microarray findings, we measured the
expression levels of six differentially expressed miRNAs
(mmu-miR-188-5p, mmu-miR-223, mmu-miR-22, mmu-miR-125a-5p,
mmu-miR-146a and mmu-miR-141), using qRT-PCR. The expression of
mmu-miR-188-5p, mmu-miR-223 and mmu-miR-22 was increased in the
UVB-treated epidermis compared with the control. mmu-miR-125a-5p,
mmu-miR-146a and mmu-miR-141 were downregulated following UVB
irradiation. These results suggest that the expression levels of
the four miRNAs observed in the arrays were consistent with those
observed using qRT-PCR (Fig.
2).
Target prediction and function analysis
of differentially expressed miRNA
The prediction of miRNA-regulated gene targets is a
crucial step in understanding the functions of miRNA. We used
TargetScan to obtain predicted gene targets for all differentially
expressed miRNAs. As expected, these miRNA genes could potentially
regulate several hundred targets. We then examined the significant
GO categories and Kyoto Encyclopedia of Genes and Genomes pathways
(KEGG) (Tables I-III).
 | Table IDifferentially expressed miRNAs and
putative targets. |
Table I
Differentially expressed miRNAs and
putative targets.
| miRNA | Published
targets | Putative targets |
|---|
| Three miRNAs
expressed significantly more in UVB group than in control
group | mmu-miR-188-5p | | RSPO3, PHF3, RNF144A,
ZFP91, RAP2C |
| mmu-miR-223 | Mef2c | PHF20L1, GPR155,
RHOB, FBXW7, NFAT5 |
| mmu-miR-22 | | FUT9, CLIC4, SNX30,
PTGS1, TET2 |
| Three miRNAs
expressed significantly less in UVB group than in control
group | mmu-miR-125a-5p | | MSRB3, EIF1AD, IRF4,
TMEM170B, ENPEP |
| mmu-miR-146a | | IRAK1, TRAF6, APPL1,
FAM62B, MED1 |
| mmu-miR-141 | ZEB1/TCF8 | AKAP2, PALM2-AKAP2,
ZEB2, FAT3, TRHDE |
 | Table IIIPathway analysis of target genes of
treatment responsive miRNAs using DAVID Bioinformatics Resources
2008. |
Table III
Pathway analysis of target genes of
treatment responsive miRNAs using DAVID Bioinformatics Resources
2008.
| KEGG pathway | P-value |
|---|
| Three miRNAs
expressed significantly more in UVB group than in control
group | ErbB signaling
pathway | 9.45 |
| MAPK signaling
pathway | 8.01 |
| Dorsoventral axis
formation | 6.67 |
| Prostate cancer | 6.53 |
| Chronic myeloid
leukemia | 5.94 |
| Three miRNAs
expressed significantly less in UVB group than in control
group | Chronic myeloid
leukemia | 13.54 |
| Notch signaling
pathway | 9.32 |
| Dorsoventral axis
formation | 7.83 |
| MAPK signaling
pathway | 6.29 |
| Pancreatic
cancer | 5.67 |
Discussion
Our present study revealed miRNAs that are sensitive
to UVB and baicalin treatment. We analyzed skin tissues from mice
in two groups (those irradiated with UVB and controls) 24-h
post-irradiation, using an miRNA microarray platform that was able
to assess the expression of 627 mouse miRNAs and 39 mouse viral
miRNAs. To select differentially expressed miRNAs from the
microarray data, we set a cut-off limit at p<0.05. The
differentially expressed miRNAs were mmu-miR-188-5p, mmu-miR-223,
mmu-miR-22, mmu-miR-125a-5p, mmu-miR-146a and mmu-miR-141.
Among the UVB downregulated miRNAs, miR-141 has been
described as a member of the miR-200 family. Korpal et al
(9) found that the miR-200 family
miRNAs inhibit epithelial-mesenchymal transition and cancer cell
migration by direct targeting of the E-cadherin transcriptional
repressors ZEB1 and ZEB2. These findings suggested that the
downregulated expression of miR-141 induced by UVB irradiation is
involved in the repression of E-cadherin, thereby enhancing
migration and invasion during cancer progression. The base excision
repair protein MED1 is a predicted target of mmu-miR-146a. MED1
interacts with the mismatch repair protein MLH1 and has a key role
in the maintenance of genomic stability, with dual functions in DNA
damage response and repair (10,11).
MED1 acts as a thymine and uracil DNA N-glycosylase on T:G and U:G
mismatches that occur at cytosine-phosphate-guanine (CpG)
methylation sites due to the spontaneous deamination of
5-methylcytosine and cytosine, respectively. This event indicates
that MED1 is involved in the removal of methylated DNA (12). Abnormal DNA methylation (including
hypermethylation and hypomethylation) is a hallmark of the majority
of cancers, including colon, lung, prostate and breast cancers, and
contributes to carcinogenesis by silencing tumor suppressor genes,
upregulating oncogenes and/or reducing genomic stability (13,14).
Mittal et al observed global DNA hypomethylation and reduced
maintenance methylation in UV-exposed mouse skin (15). In view of these findings, whether or
not the downregulated expression of miR-146a contributes to
UV-induced DNA hypomethylation via MED1 requires further
investigation.
In the present study, it is clear that
mmu-miR-188-5p, mmu-miR-223 and mmu-miR-22 were upregulated
following UVB irradiation. Johnnidis et al found that
miR-223 mutant mice have an expanded granulocytic compartment
resulting from a cell-autonomous increase in the number of
granulocyte progenitors (16).
These authors also revealed that Mef2c, a transcription factor that
promotes myeloid progenitor proliferation, is a target of miR-223
(16–18). Their data support a model in which
miR-223 acts as a fine-tuner of granulocyte production and the
inflammatory response. We demonstrated that miR-223 is expressed in
the skin of untreated mice, and markedly increases following UVB
irradiation. These findings suggest that mmu-miR-223 is sensitive
to UVB and elicits a direct or indirect effect of UVB-induced
inflammation. RhoB, a predicted target of miR-223, has been
reported to protect human keratinocytes from UVB-induced apoptosis
through epidermal growth factor receptor signaling (19), suggesting that miR-223 plays a role
in regulating keratinocyte survival following UVB exposure. Little
is known about the function of miR-188-5p and miR-22.
To further analyze the correlation between patterns
of target gene expression and their functional implications, we
classified the target genes of all differentially expressed miRNAs
into several function categories using GOstat software (http://gostat.wehi.edu.au/cgi-bin/goStat.pl) (8). GO terms concerning the regulation of
cellular and biological processes are listed in Table II. The targets of these nine miRNAs
were selected for pathway analysis using DAVID Bioinformatics
Resources 2008 (http://david.abcc.ncifcrf.gov/) (Table III).
 | Table IIGene Ontology (GO) terms for the
target genes of treatment responsive miRNAs. |
Table II
Gene Ontology (GO) terms for the
target genes of treatment responsive miRNAs.
| GO term | Function | P-value |
|---|
| Three miRNAs
expressed significantly more in UVB group than in control
group | 0043283 | Biopolymer metabolic
process | 3.64E-18 |
| 0050794 | Regulation of
cellular process | 3.64E-18 |
| 0050789 | Regulation of
biological process | 3.56E-17 |
| 0065007 | Biological
regulation | 3.56E-17 |
| 0031323 | Regulation of
cellular metabolic process | 4.37E-15 |
| Three miRNAs
expressed significantly less in UVB group than in control
group | 0043283 | Biopolymer metabolic
process | 9.13E-16 |
| 0050789 | Regulation of
biological process | 3.06E-13 |
| 0005515 | Protein binding | 3.06E-13 |
| 0050794 | Regulation of
cellular process | 3.06E-13 |
| 0010468 | Regulation of gene
expression | 3.06E-13 |
It should be noted that certain miRNAs reported to
be involved in the response to UVB irradiation were not observed in
this study. For example, miR-21 is known to be involved in the
progression of cancer and has been described as an oncogenic miRNA
(20), but when it appears together
with miR-24, it inhibits growth. Guo et al found that miR-21
and miR-24 appeared together following 12-h exposure to 50
J/m2 UVB (4) and their
observations of the cell cycle and apoptosis appeared to reveal a
sub-G1 DNA content fraction and apoptotic cells 12-h
post-irradiation, suggesting that miR-21 and miR-24 together
inhibited growth. However, the present study results did not show
any changes in miR-21 or miR-24 in the UVB group. This finding may
be due to differences in animal selection, UVB dose, chip
fabrication or a weakness in the microarray technology.
In conclusion, the focus of this study was to
investigate the differential expression profiles of miRNAs in the
skin of mice following UVB irradiation. Although our investigation
is at a preliminary stage, we believe this study provides a basis
for further investigation of the function of expression profiles in
miRNAS in signal transduction pathways induced by UVB treatment.
miRNAs may therefore be new research hotspots for the prevention or
treatment of skin cancer caused by UVB.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81000700).
References
|
1
|
Marrot L and Meunier JR: Skin DNA
photodamage and its biological consequences. J Am Acad Dermatol.
58:S139–S148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Timares L, Katiyar SK and Elmets CA: DNA
damage, apoptosis and langerhans cells-Activators of UV-induced
immune tolerance. Photochem Photobiol. 84:422–436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heck DE, Gerecke DR, Vetrano AM and Laskin
JD: Solar ultraviolet radiation as a trigger of cell signal
transduction. Toxicol Appl Pharmacol. 195:288–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo L, Huang ZX, Chen XW, Deng QK, Yan W,
Zhou MJ, Ou CS and Ding ZH: Differential expression profiles of
microRNAs in NIH3T3 cells in response to UVB
irradiation. Photochem Photobiol. 85:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pothof J, Verkaik NS, Hoeijmakers JH and
van Gent DC: MicroRNA responses and stress granule formation
modulate the DNA damage response. Cell Cycle. 8:3462–3468. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pothof J, Verkaik NS, van IW, Wiemer EA,
Ta VT, van der Horst GT, Jaspers NG, van Gent DC, Hoeijmakers JH
and Persengiev SP: MicroRNA-mediated gene silencing modulates the
UV-induced DNA-damage response. EMBO J. 28:2090–2099. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu CG, Calin GA, Meloon B, Gamliel N,
Sevignani C, Ferracin M, Dumitru CD, Shimizu M, Zupo S, Dono M,
Alder H, Bullrich F, Negrini M and Croce CM: An oligonucleotide
microchip for genome-wide microRNA profiling in human and mouse
tissues. Proc Natl Acad Sci USA. 101:9740–9744. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castoldi M, Schmidt S, Benes V, Noerholm
M, Kulozik AE, Hentze MW and Muckenthaler MU: A sensitive array for
microRNA expression profiling (miChip) based on locked nucleic
acids (LNA). RNA. 12:913–920. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bellacosa A: Role of MED1 (MBD4) gene in
DNA repair and human cancer. J Cell Physiol. 187:137–144. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Howard JH, Frolov A, Tzeng CW, Stewart A,
Midzak A, Majmundar A, Godwin AK, Heslin MJ, Bellacosa A and
Arnoletti JP: Epigenetic downregulation of the DNA repair gene
MED1/MBD4 in colorectal and ovarian cancer. Cancer Biol Ther.
8:94–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turner DP, Cortellino S, Schupp JE,
Caretti E, Loh T, Kinsella TJ and Bellacosa A: The DNA
N-glycosylase MED1 exhibits preference for halogenated pyrimidines
and is involved in the cytotoxicity of 5-iododeoxyuridine. Cancer
Res. 66:7686–7693. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baylin SB, Esteller M, Rountree MR,
Bachman KE, Schuebel K and Herman JG: Aberrant patterns of DNA
methylation, chromatin formation and gene expression in cancer. Hum
Mol Genet. 10:687–692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goodman JI and Watson RE: Altered DNA
methylation: a secondary mechanism involved in carcinogenesis. Annu
Rev Pharmacol Toxicol. 42:501–525. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mittal A, Elmets CA and Katiyar SK:
Dietary feeding of proanthocyanidins from grape seeds prevents
photocarcinogenesis in SKH-1 hairless mice: relationship to
decreased fat and lipid peroxidation. Carcinogenesis. 24:1379–1388.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnnidis JB, Harris MH, Wheeler RT,
Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD and
Camargo FD: Regulation of progenitor cell proliferation and
granulocyte function by microRNA-223. Nature. 451:1125–1129. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Li H, Park EJ and Chen JD: SMRTE
inhibits MEF2C transcriptional activation by targeting HDAC4 and 5
to nuclear domains. J Biol Chem. 276:24177–24185. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paroni G, Mizzau M, Henderson C, Del Sal
G, Schneider C and Brancolini C: Caspase-dependent regulation of
histone deacetylase 4 nuclear-cytoplasmic shuttling promotes
apoptosis. Mol Biol Cell. 15:2804–2818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Canguilhem B, Pradines A, Baudouin C, Boby
C, Lajoie-Mazenc I, Charveron M and Favre G: RhoB protects human
keratinocytes from UVB-induced apoptosis through epidermal growth
factor receptor signaling. J Biol Chem. 280:43257–43263. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|