Introduction
The liver-specific magnetic resonance imaging (MRI)
contrast agent gadolinium-ethoxybenzyl
diethylenetriaminepentaacetic acid (Gd-EOB-DTPA,
Primovist®; Bayer Schering Pharma, Berlin, Germany) is
used to detect focal liver lesions (1–10) and
evaluate the biliary tree (11,12).
Intravenously injected Gd-EOB-DTPA is gradually absorbed by
hepatocytes and finally excreted via the biliary tract. As a result
of hepatocyte uptake, normal liver parenchyma exhibit T1
shortening, unlike focal liver lesions, including those caused by
hepatic metastasis. Certain experimental animal studies have
demonstrated that this contrast material has the potential to
evaluate liver function or detect diffuse liver disease (13–18).
Experimentally induced hepatic dysfunction decreases the degree of
liver enhancement produced by Gd-EOB-DTPA and prolongs the washout
of contrast material (17,18). In rats, liver enhancement with
Gd-EOB-DTPA during MRI is delayed and prolonged following liver
transplantation as compared with controls (14). The correlation between liver
function and Gd-EOB-DTPA kinetics has been evaluated in animal
models, but never in humans. The livers of patients with liver
tumors are occasionally damaged, which might degrade the contrast
between liver and lesion produced by the decreased hepatocyte
uptake of Gd-EOB-DTPA. The present study investigated the
correlation between hepatic function and liver parenchymal
enhancement in Gd-EOB-DTPA-enhanced MRI.
Materials and methods
Patient population
The present study was performed in accordance with
the recommendations of the Declaration of Helsinki. All patients
gave their written, informed consent upon enrollment prior to
undergoing MRI. In total, 49 consecutive patients with chronic
hepatitis were referred to the Interventional Radiology unit at our
institution between March 2, 2008 and June 30, 2009 for curative
treatment of hepatocellular carcinoma (HCC). All patients except
those with MRI contraindications (n=2), including claustrophobia
and presence of a pacemaker, underwent Gd-EOB-DTPA-enhanced MRI
prior to receiving treatment for HCC. Six (12%) of the 49 patients
were considered ineligible for the study due to multifocal HCC
(defined as the presence of >10 tumor nodules, n=5), or
inadequate MRI results (unacceptable image quality due to motion
artifacts, n=1). The remaining 41 patients who were able to undergo
MRI and who met the study criteria (mean age, 71.9 years; range,
38–78 years) comprised the study cohort, which included 32 males
(mean age, 73.5 years; range, 59–86) and 9 females (mean age, 65.9
years; range, 38–78). Three patients had hepatitis B, 36 had
hepatitis C, and two had alcohol-related hepatitis.
MRI
MR images were obtained using a 1.5-T system (Signa
HDx; GE Medical Systems, Milwaukee, WI, USA) with an eight-channel
anteroposterior phased-array surface coil placed around the patient
and covering the entire liver. Imaging protocols included
unenhanced sequences [transaxial T2-weighted fast spin-echo (FSE)
and in- and opposed-phase GRE sequences] and Gd-EOB-DTPA-enhanced
dynamic three-dimensional (3D)-GRE sequences. Images were captured
in the transverse plane during an end-expiratory breath-hold with a
combined eight-coil element, anteroposterior phased-array surface
coil. Using parallel imaging with sensitivity encoding by a factor
of two, the total acquisition time was decreased to approximately
15 sec. A three-quarter field-of-view was used in the
phase-encoding direction. Presaturation pulses were applied above
and below the imaging volume to diminish flow artifacts. The
patients were administered a 30 μmol/kg (0.12 ml/kg body weight)
dose of Gd-EOB-DTPA (Primovist) via the antecubital vein at a rate
of 2 ml/s through a 22-gauge intravenous catheter using a power
injector (Spectris Solaris® EP; Medrad, Indianola, PA,
USA), followed by a 40 ml saline flush at the same injection rate.
Dynamic and delayed images were obtained using a fat-suppressed 3D
T1-weighted GRE sequence with parallel imaging [LAVA™ EFGRE ASSET™
breath-hold; repetition time/echo time (TR/TE), 3.0/1.6 msec; flip
angle, 15°; field of view, 42×42 cm; matrix, 384×256 interpolated
to 512×512; thickness, 5 mm; overlap 2 mm, ASSET acceleration,
2.0]. T1-weighted dynamic GRE breath-hold images were captured at
30 and 180 sec following contrast material administration during
the hepatic arterial dominant and equilibrium phases, respectively,
and during the delayed hepatobiliary phase at 10, 20 and 30 min
following injection.
Quantitative image analysis
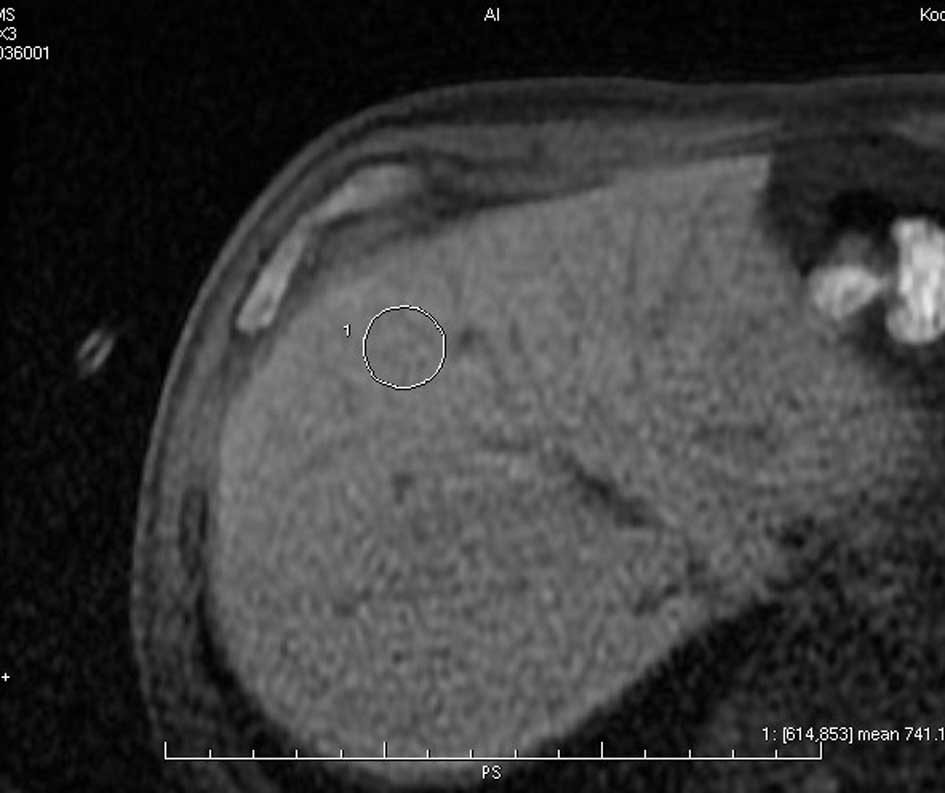
Two radiologists obtained signal intensity values
for the liver using a monitor-defined region of interest (>50
pixels) while avoiding major intrahepatic vessels. The location of
the region of interest was maintained as constant as possible for
individual patients at different time points (Fig. 1). Relative enhancement (RE) of the
liver was calculated using the equation: RE =
SIpostcontrast/SIprecontrast where
SIprecontrast is the signal intensity of the liver on
the precontrast image, and SIpostcontrast is the signal
intensity of the liver on the postcontrast image.
Liver function parameters
Blood serum parameters (total bilirubin, serum
albumin and international normalized ratio of prothrombin time),
the 15 min retention rate of indocyanine green test (ICG-R15 test;
Daiichi-Sankyou, Tokyo, Japan) and the presence or absence of
ascites were recorded to evaluate the degree of liver damage 1–5
days prior to undergoing Gd-EOB-DTPA-enhanced MRI. The degree of
liver damage was assessed according to a system similar to the
Child-Pugh classification except for inclusion of the ICG test;
this evaluation procedure followed the algorithm for HCC treatment
guidelines in Japan (19).
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
were also recorded, as these parameters correlated with the degree
of liver enhancement in animal studies using Gd-EOB-DTPA-enhanced
MRI. Since all 41 patients included were referred from the
Interventional Radiology unit, and MRI was part of the workup prior
to administering treatment for HCC, these laboratory investigations
were undertaken for clinical reasons.
Blood serum parameters, as well as the degree of
liver damage, were compared with the quantitative parameter of
liver enhancement observed on the Gd-EOB-DTPA images.
Statistical analysis
Data were statistically analyzed using the SPSS
software package version 11.0 for Windows (SPSS Inc., Chicago, IL,
USA). Continuous variables are presented as the mean and standard
deviation (SD) as appropriate. The Student’s t-test was used to
compare RE values among respective liver damage scores.
Correlations between the continuous variables and the RE values
were determined using univariate regression analyses. Independent
determinants of RE values were determined by forward multiple
stepwise regression analyses. In the two-tailed test, P<0.05 was
considered to indicate a statistically significant difference.
Results
Time profile of RE values
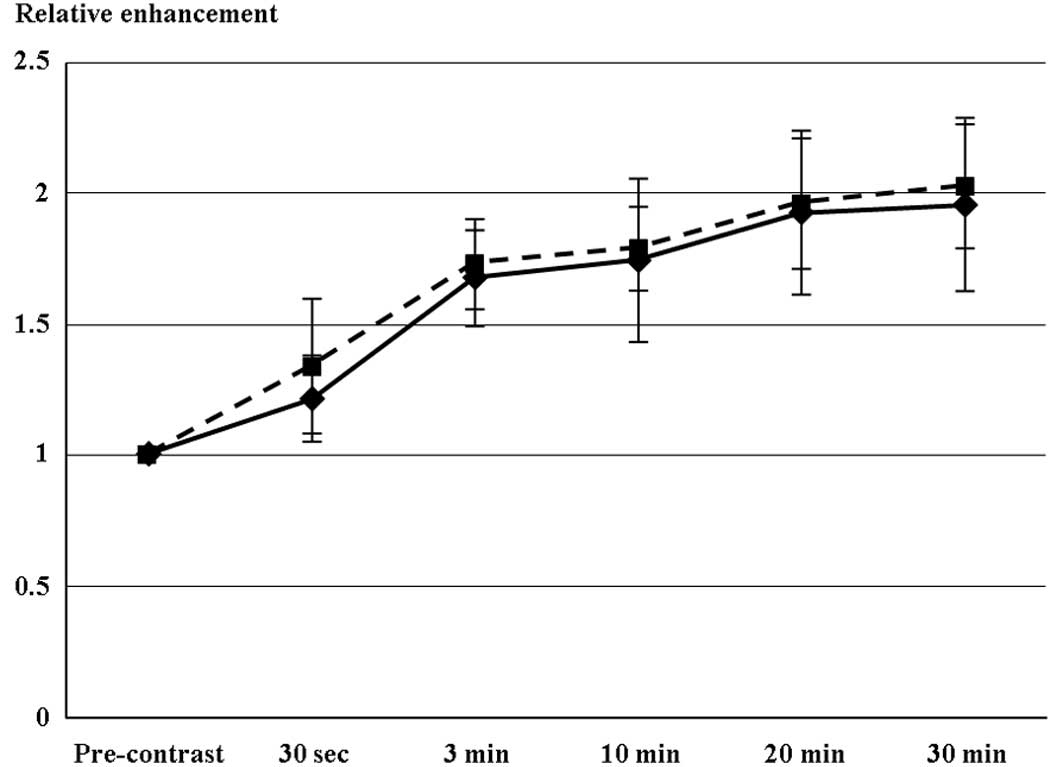
Fig. 2 shows the
time profile of RE values for the respective liver damage scores.
Following injection of Gd-EOB-DTPA, T1-weighted 3D-GRE images
revealed an early contrast effect in the liver parenchyma, with a
steep increase in signal intensity at 30 sec. A further, although
slower, increase for up to approximately 20 min following injection
was followed by a plateau of enhancement in the two groups with
liver damage. The maximal RE value was selected for each patient (8
and 33 patients at 20 and 30 min, respectively). Maximal RE values
did not significantly differ between liver damage levels A and B.
None of the study population had ascites or level C liver
damage.
Correlation between RE values and liver
function parameters
Table I provides
patient demographic data, and Table
II shows the results of univariate and multivariate analyses
for determinants of maximal RE values. The parameters that
significantly related to maximal RE values were serum albumin
(r=0.496, p=0.001), AST (r=−0.366, p=0.023), total bilirubin
(r=−0.487, p=0.002) and ICG-R15 (r=−0.462, p=0.003). Multiple
stepwise regression analysis revealed that serum albumin (p=0.002)
and total bilirubin (p=0.001, inversely) among these parameters
remained significantly (Table II)
and independently related to maximal RE values (R2=0.287).
 | Table IDemographic data of the study subjects
(n=41). |
Table I
Demographic data of the study subjects
(n=41).
| Factors | Mean ± SD |
|---|
| Age (years) | 71.9±9.31 |
| AST (IU/l) | 45.1±24.8 |
| ALT (IU/l) | 37.8±21.3 |
| Total bilirubin | 0.85±0.32 |
| Serum albumin | 3.8±0.43 |
| ICG R15 (%) | 26.5±13.2 |
| Prothrombin time
(%) | 77.9±12.8 |
 | Table IIDeterminants of maximal relative
enhancement values by stepwise regression (forward selection). |
Table II
Determinants of maximal relative
enhancement values by stepwise regression (forward selection).
| Univariatea | Multivariateb |
|---|
|
|
|
|---|
| Factors | β | p | β | F | p |
|---|
| Age (years) | −0.002 | NS | - | - | - |
| AST (IU/l) | −0.366 | 0.023 | - | - | - |
| ALT (IU/l) | −0.002 | NS | - | - | - |
| Total
bilirubin | −0.487 | 0.002 | −0.285 | 4.169 | 0.001 |
| Serum albumin | 0.496 | 0.001 | 0.224 | 4.61 | 0.002 |
| ICG R15 (%) | −0.462 | 0.003 | - | - | - |
| Prothrombin time
(%) | 0.004 | NS | - | - | - |
| R2 | - | - | 0.287 | - | - |
Discussion
Our findings demonstrate that the degree of liver
enhancement by Gd-EOB-DTPA during the hepatic uptake phase in
humans depends on liver function. Certain experimental animal
studies have described the potential of Gd-EOB-DTPA to evaluate
liver function and diffuse hepatic disease (15–18).
Hepatocyte uptake and biliary excretion of Gd-EOB-DTPA, bilirubin
and ICG appear to be mediated by glutathione-s-transferase
(20–22). Kim et al (17) reported that Gd-EOB-DTPA enhancement
in animal livers with chemically induced hepatitis correlates with
plasma bilirubin level and ICG clearance. Our findings in patients
with hepatitis confirmed the results of Kim et al and the
physiological mechanism of Gd-EOB-DTPA. The prognosis of patients
with cirrhosis and HCC depends on residual liver function and tumor
extension (23,24). Various prognostic staging systems
for HCC combine the Child-Pugh liver function classification with
tumor extension (23,24). The preoperative assessment of
functional reserve is significant for estimating the extent of
hepatectomy. Hepatic functional reserve is widely assessed using
the ICG test, but technetium-99m-galactosyl human serum albumin
liver scintigraphy appears to be equally effective for selecting
candidates for hepatectomy (23,24).
Findings of the current study have shown that Gd-EOB-DTPA-enhanced
MRI findings correlated with total bilirubin values and ICG-R15,
and that this modality has potential for use in the same manner as
scintigraphy as a prognostic staging system and as a parameter for
the preoperative assessment of hepatectomy. Shimizu et al
(25) identified regional ischemic
damage in the rat right hepatic lobe during the hepatic uptake
phase of Gd-EOB-DTPA-enhanced MRI. The ability of
Gd-EOB-DTPA-enhanced MRI to evaluate regional liver function might
contribute to the preoperative assessment of hepatectomy. Tsuda
et al (26) reported that
the time to reach maximal enhancement and the half-life of such
enhancement following Gd-EOB-DTPA injection are prolonged in the
livers of rats with non-alcoholic steatohepatitis compared with the
livers of rats with common fatty liver. Non-alcoholic
steatohepatitis is now considered one of the most common types of
chronic liver disease, which induces the development of liver
cirrhosis and tumors (27–29). Although non-alcoholic
steatohepatitis should be diagnosed early so that therapy begins
early, the differences between non-alcoholic steatohepatitis and
common fatty liver are not distinguishable by any radiological
modality, including ultrasonography, computed tomography (CT) and
MRI without contrast material (30,31).
Gd-EOB-DTPA-enhanced MRI has the potential to differentially
diagnose human non-alcoholic steatohepatitis and common fatty
liver. Our study results demonstrate that Gd-EOB-DTPA-enhanced MRI
was capable of estimating liver function in humans similar to that
observed in animal studies.
The gadolinium-diethylenetriaminepentaacetic
(Gd-DTPA) derivative Gd-EOB-DTPA is comparable to Gd-DTPA in terms
of being highly hydrophilic and water-soluble. In addition, a
lipophilic ethoxybenzyl group enables selective intracellular
uptake by hepatocytes (2,18). Therefore, images of the early
dynamic perfusion and late hepatocyte uptake phases can be captured
following a single injection of Gd-EOB-DTPA (1,2). The
first phase within approximately 3 min following a bolus injection
is equivalent to that of Gd-DTPA (1,2). Focal
lesions are more effectively identified using Gd-EOB-DTPA than
contrast-enhanced dynamic-CT, with high diagnostic reliability and
superiority (4–10). Although valid direct comparisons of
Gd-DTPA and Gd-EOB-DTPA are rare, at least one study has proven
that there are similar perfusion-phase tumor enhancement
characteristics following the injection of the two contrast
materials (2). Focal lesions with
hepatocellular function, including focal nodular hyperplasia,
adenoma and well-differentiated HCC, all absorb Gd-EOB-DTPA during
the hepatocyte uptake phase. However, conditions without such
hepatocellular function, including moderately or poorly
differentiated HCC and liver metastases, do not absorb contrast
material during the uptake phase (4–6,9). These
imaging features are useful in characterizing focal lesions
(4–6,9).
Consequently, the early dynamic perfusion and late hepatocyte
uptake phases are useful for detecting and characterizing focal
hepatic lesions. In addition, Gd-EOB-DTPA has the same favorable
safety profile as Gd-DTPA (1,3,6,7,22).
However, the delay in the hepatocyte uptake phase wastes more than
20 min (4,6,8–10).
Certain investigators achieve the hepatocyte uptake phase at 10 and
20 min following injection (4,8,9). The
uptake rates of Gd-EOB-DTPA are similar at 10 and 20 min in 88 and
90% of focal nodular hyperplasia lesions, respectively (9). Huppertz et al (8) interpreted the enhancement
characteristics of focal liver lesions at 10 and 20 min without a
distinction in image quality. Differences in tumor-liver
contrast-to-noise ratios in 23 liver metastases were not
significant between 10 and 45 min following injection (4). The results of the present study
suggest that a normally functioning liver uptakes considerable
amounts of contrast material during the early period (10 min)
following injection, which generates favorable contrast between
focal lesions and the surrounding liver. Further studies are
required to determine the image acquisition time appropriate to
individual liver functions to decrease the duration required during
the procedure. Once the time that is currently wasted obtaining the
hepatocyte uptake phase image is shortened, Gd-EOB-DTPA-enhanced
MRI may become as significant as ultrasonography, CT and MRI in the
diagnosis of focal hepatic lesions (32–35).
This study has two limitations. Firstly, as the
patient population comprised candidates for HCC therapy, no study
subjects had extremely poor liver function (level C liver damage).
Secondly, we did not observe Gd-EOB-DTPA washout from the liver.
Although Gd-EOB-DTPA is absorbed by hepatocytes and excreted from
the rat liver within approximately 60 min (16,17,26),
these processes occur over 6 h in humans (1). The washout of Gd-EOB-DTPA is also
prolonged in animals with a damaged liver (16,17,26).
Human patients would not be able to tolerate such protracted
procedures.
In conclusion, the degree of liver enhancement with
Gd-EOB-DTPA correlates with the level of liver function. Future
clinical investigations are required to further evaluate the
usefulness of Gd-EOB-DTPA-enhanced MRI as a test for diffuse liver
disease and as a prognostic staging system for HCC. Decreasing the
examination duration with rapid hepatocyte uptake phase images
should also be investigated for patients with normal liver
function.
References
|
1
|
Hamm B, Staks T, Muhler A, et al: Phase I
clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast
agent: safety, pharmacokinetics, and MR imaging. Radiology.
195:785–792. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogl TJ, Kummel S, Hammerstingl R, et al:
Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and
Gd-DTPA. Radiology. 200:59–67. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reimer P, Rummeny EJ, Shamsi K, et al:
Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects,
and pulse sequence. Radiology. 199:177–183. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reimer P, Rummeny EJ, Daldrup HE, et al:
Enhancement characteristics of liver metastases, hepatocellular
carcinomas, and hemangiomas with Gd-EOB-DTPA: preliminary results
with dynamic MR imaging. Eur Radiol. 7:275–280. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stern W, Schick F, Kopp AF, et al: Dynamic
MR imaging of liver metastases with Gd-EOB-DTPA. Acta Radiol.
41:255–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huppertz A, Balzer T, Blakeborough A, et
al: Improved detection of focal liver lesions at MR imaging:
multicenter comparison of gadoxetic acid-enhanced MR images with
intraoperative findings. Radiology. 230:266–275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bluemke DA, Sahani D, Amendola M, et al:
Efficacy and safety of MR imaging with liver-specific contrast
agent: U.S. multicenter phase III study. Radiology. 237:89–98.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huppertz A, Haraida S, Kraus A, et al:
Enhancement of focal liver lesions at gadoxetic acid-enhanced MR
imaging: correlation with histopathologic findings and spiral CT -
initial observations. Radiology. 234:468–478. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zech CJ, Grazioli L, Breuer J, Reiser MF
and Schoenberg SO: Diagnostic performance and description of
morphological features of focal nodular hyperplasia in
Gd-EOB-DTPA-enhanced liver magnetic resonance imaging: results of a
multicenter trial. Invest Radiol. 43:504–511. 2008. View Article : Google Scholar
|
|
10
|
Hammerstingl R, Huppertz A, Breuer J, et
al: Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI
and spiral CT for a therapeutic strategy: comparison with
intraoperative and histopathologic findings in focal liver lesions.
Eur Radiol. 18:457–467. 2008. View Article : Google Scholar
|
|
11
|
Bollow M, Taupitz M, Hamm B, Staks T, Wolf
KJ and Weinmann HJ: Gadolinium-ethoxybenzyl-DTPA as a hepatobiliary
contrast agent for use in MR cholangiography: results of an in vivo
phase-I clinical evaluation. Eur Radiol. 7:126–132. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asbach P, Warmuth C, Stemmer A, et al:
High spatial resolution T1-weighted MR imaging of liver and biliary
tract during uptake phase of a hepatocyte-specific contrast medium.
Invest Radiol. 43:809–815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clement O, Muhler A, Vexlar V, Berthezene
Y and Brasch RC: Gadolinium-ethoxybenzyl-DTPA, a new liver-specific
magnetic resonance contrast agent. Kinetic and enhancement patterns
in normal and cholestatic rats. Invest Radiol. 27:612–619. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muhler A, Freise CE, Kuwatsuru R, et al:
Acute liver rejection: evaluation with cell-directed MR contrast
agents in a rat transplantation model. Radiology. 186:139–146.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muhler A, Heinzelmann I and Weinmann HJ:
Elimination of gadolinium-ethoxybenzyl-DTPA in a rat model of
severely impaired liver and kidney excretory function. An
experimental study in rats. Invest Radiol. 29:213–216. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmitz SA, Muhler A, Wagner S and Wolf
KJ: Functional hepatobiliary imaging with gadolinium-EOB-DTPA. A
comparison of magnetic resonance imaging and 153gadolinium-EOB-DTPA
scintigraphy in rats. Invest Radiol. 31:154–160. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim T, Murakami T, Hasuike Y, et al:
Experimental hepatic dysfunction: evaluation by MRI with
Gd-EOB-DTPA. J Magn Reson Imaging. 7:683–688. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryeom HK, Kim SH, Kim JY, et al:
Quantitative evaluation of liver function with MRI using
Gd-EOB-DTPA. Korean J Radiol. 5:231–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Makuuchi M, Kokudo N, Arii S, et al:
Development of evidence-based clinical guidelines for the diagnosis
and treatment of hepatocellular carcinoma in Japan. Hepatol Res.
33:37–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clement O, Muhler A, Vexler VS, et al:
Evaluation of radiation-induced liver injury with MR imaging:
comparison of hepatocellular and reticuloendothelial contrast
agents. Radiology. 185:163–168. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaplowitz N: Physiological significance of
glutathione S-transferases. Am J Physiol. 239:G439–G444.
1980.PubMed/NCBI
|
|
22
|
Schuhmann-Giampieri G, Schmitt-Willich H,
Press WR, Negishi C, Weinmann HJ and Speck U: Preclinical
evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the
hepatobiliary system. Radiology. 183:59–64. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwon AH, Ha-Kawa SK, Uetsuji S, Inoue T,
Matsui Y and Kamiyama Y: Preoperative determination of the surgical
procedure for hepatectomy using technetium-99m-galactosyl human
serum albumin (99mTc-GSA) liver scintigraphy. Hepatology.
25:426–429. 1997. View Article : Google Scholar
|
|
24
|
Lau H, Man K, Fan ST, Yu WC, Lo CM and
Wong J: Evaluation of preoperative hepatic function in patients
with hepatocellular carcinoma undergoing hepatectomy. Br J Surg.
84:1255–1259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimizu J, Dono K, Gotoh M, et al:
Evaluation of regional liver function by
gadolinium-EOB-DTPA-enhanced MR imaging. Dig Dis Sci. 44:1330–1337.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsuda N, Okada M and Murakami T: Potential
of gadolinium- ethoxybenzyl-diethylenetriamine pentaacetic acid
(Gd-EOB- DTPA) for differential diagnosis of nonalcoholic
steatohepatitis and fatty liver in rats using magnetic resonance
imaging. Invest Radiol. 42:242–247. 2007. View Article : Google Scholar
|
|
27
|
Ono M and Saibara T: Clinical features of
nonalcoholic steatohepatitis in Japan: Evidence from the
literature. J Gastroenterol. 41:725–732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada M, Hashimoto E, Taniai M, et al:
Hepatocellular carcinoma in patients with non-alcoholic
steatohepatitis. J Hepatol. 37:154–160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Asanuma T, Ono M, Kubota K, et al: Super
paramagnetic iron oxide MRI shows defective Kupffer cell uptake
function in non-alcoholic fatty liver disease. Gut. 59:258–266.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saadeh S, Younossi ZM, Remer EM, et al:
The utility of radiological imaging in nonalcoholic fatty liver
disease. Gastroenterology. 123:745–750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murata Y, Ogawa Y, Saibara T, et al:
Tamoxifen-induced non-alcoholic steatohepatitis in patients with
breast cancer: Determination of a suitable biopsy site for
diagnosis. Oncol Rep. 10:97–100. 2003.PubMed/NCBI
|
|
32
|
Kubota K, Hisa N, Fujiwara Y, Fukumoto M,
Yoshida D and Yoshida S: Evaluation of the intratumoral vasculature
of hepatocellular carcinoma by power Doppler sonography: advantages
and disadvantages versus conventional color Doppler sonography.
Abdom Imaging. 25:172–178. 2000. View Article : Google Scholar
|
|
33
|
Kubota K, Hisa N, Nishikawa T, Ohnishi T,
Ogawa Y and Yoshida S: The utility of tissue harmonic imaging in
the liver: A comparison with conventional gray-scale sonography.
Oncol Rep. 7:767–771. 2000.PubMed/NCBI
|
|
34
|
Kubota K, Hisa N, Nishikawa T, et al:
Evaluation of hepatocellular carcinoma after treatment with
transcatheter arterial chemoembolization: comparison of
Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom
Imaging. 26:184–190. 2001. View Article : Google Scholar
|
|
35
|
Kubota K, Yamanishi T, Itoh S, et al: Role
of diffusion-weighted imaging in evaluating therapeutic efficacy
after transcatheter arterial chemoembolization for hepatocellular
carcinoma. Oncol Rep. 24:727–732. 2010. View Article : Google Scholar
|