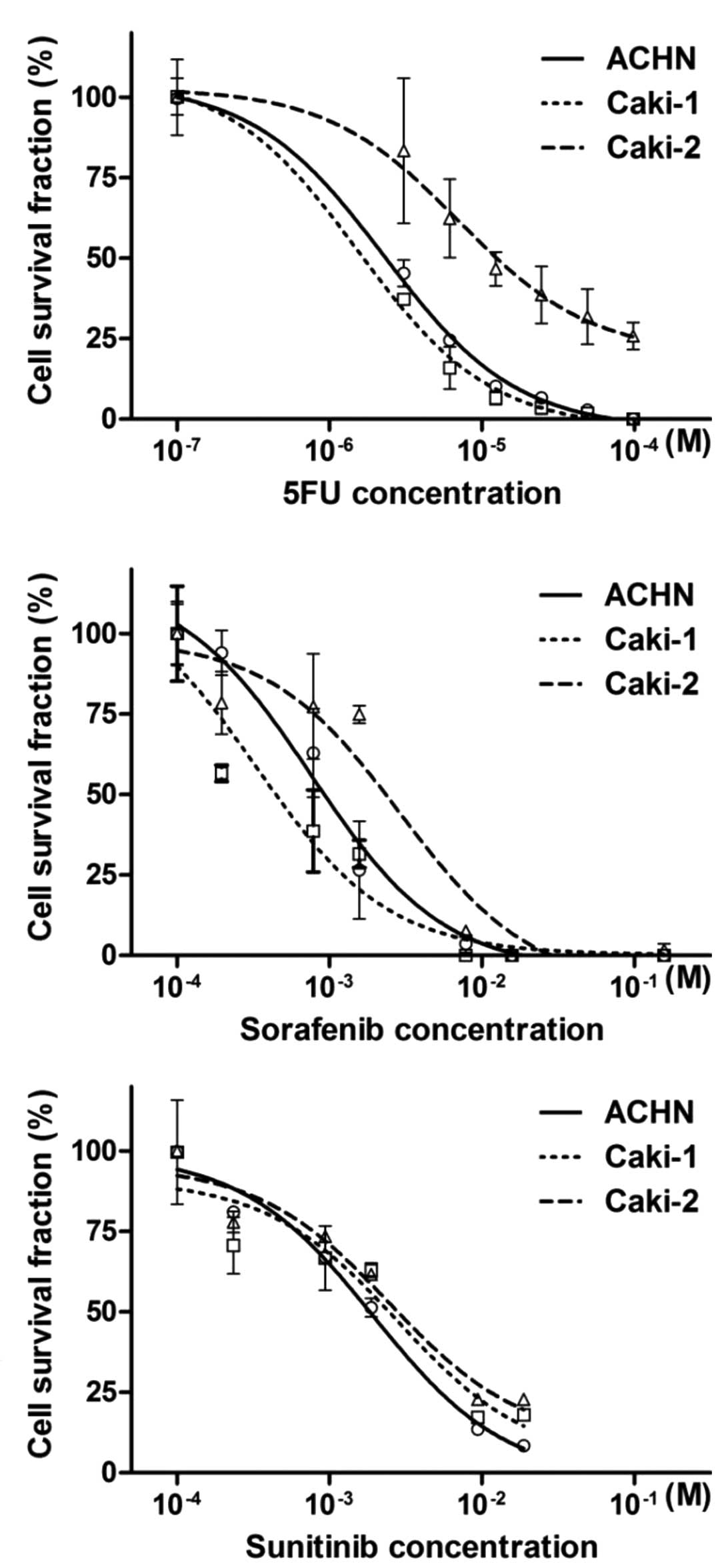
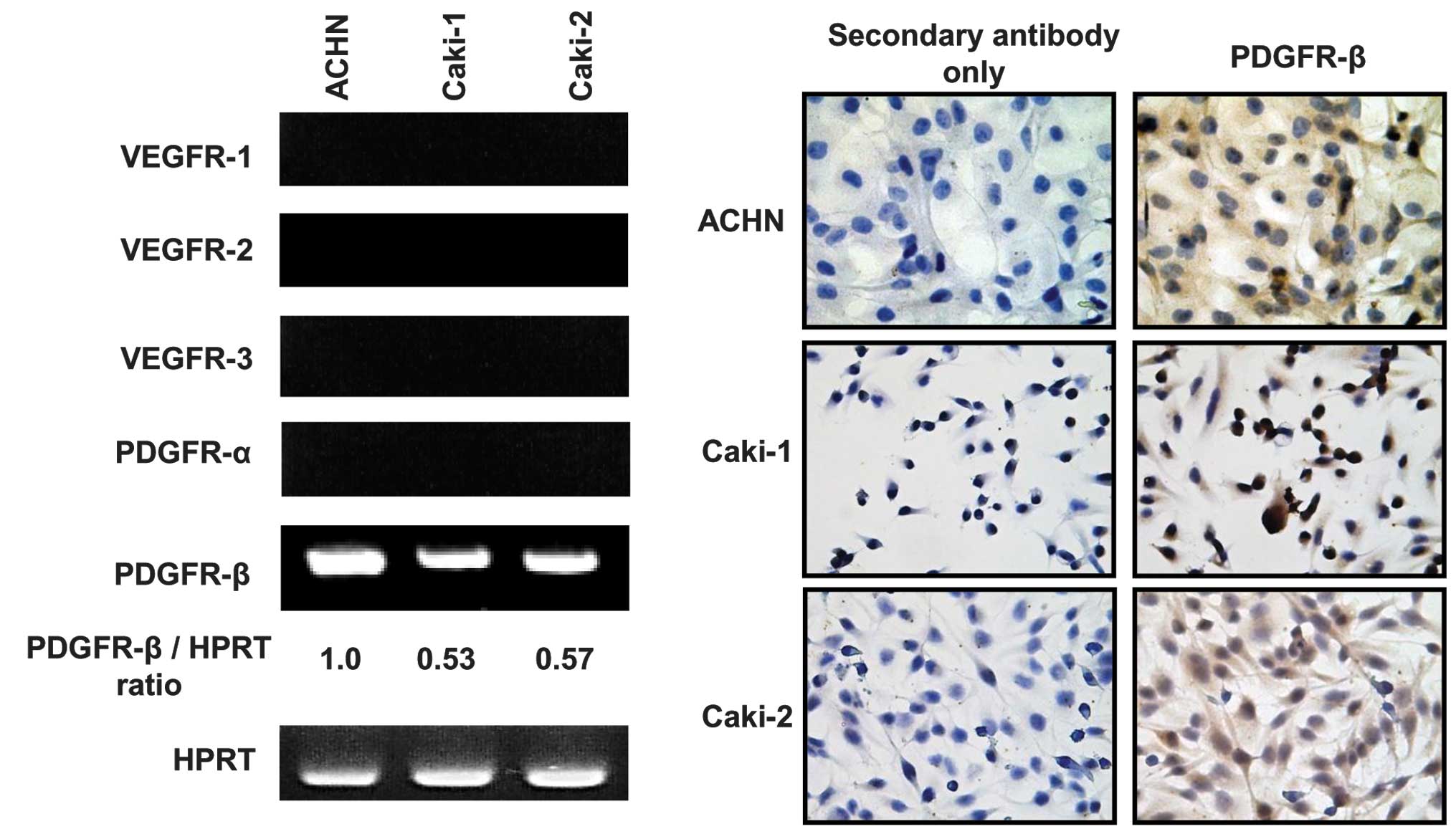
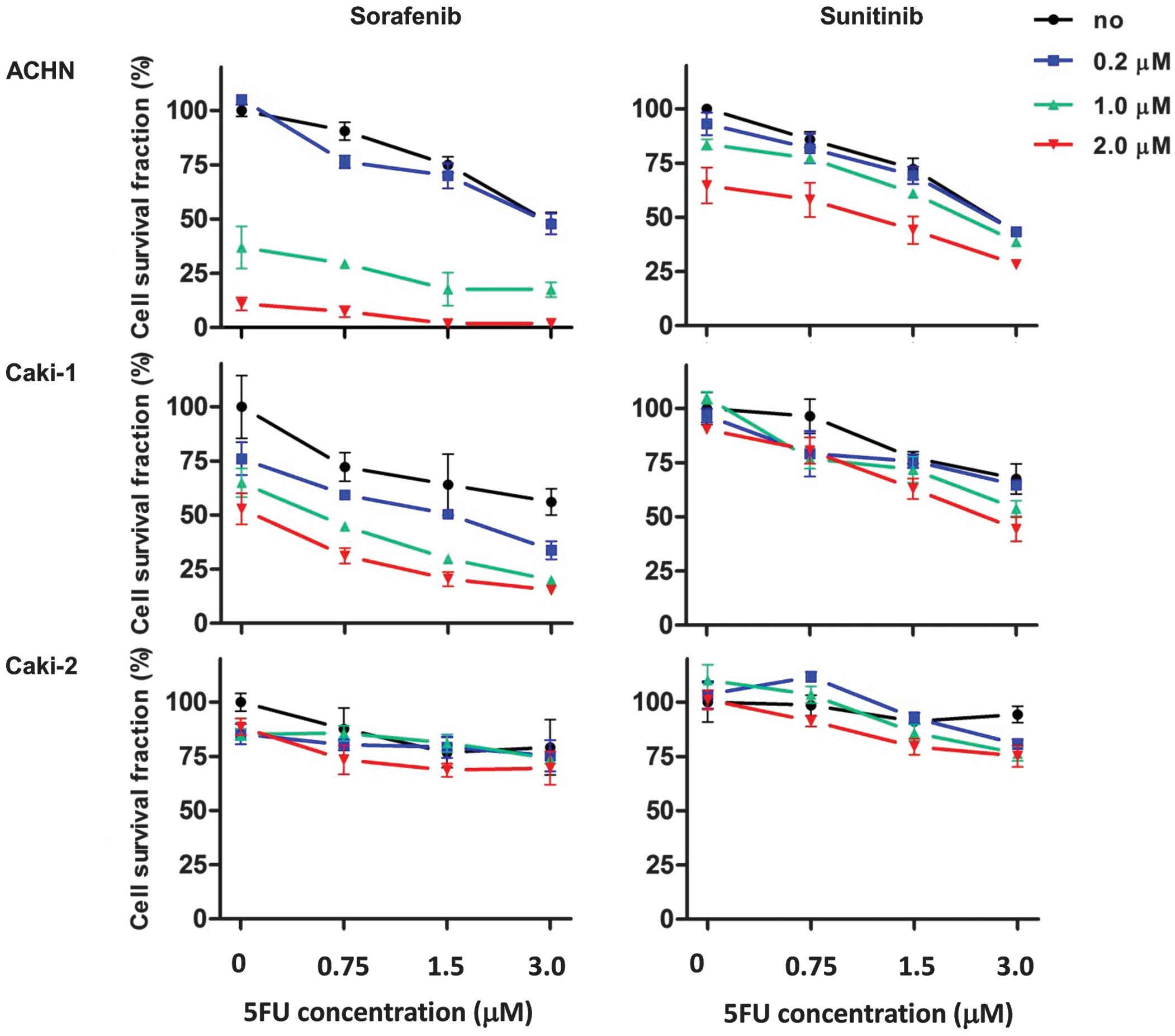
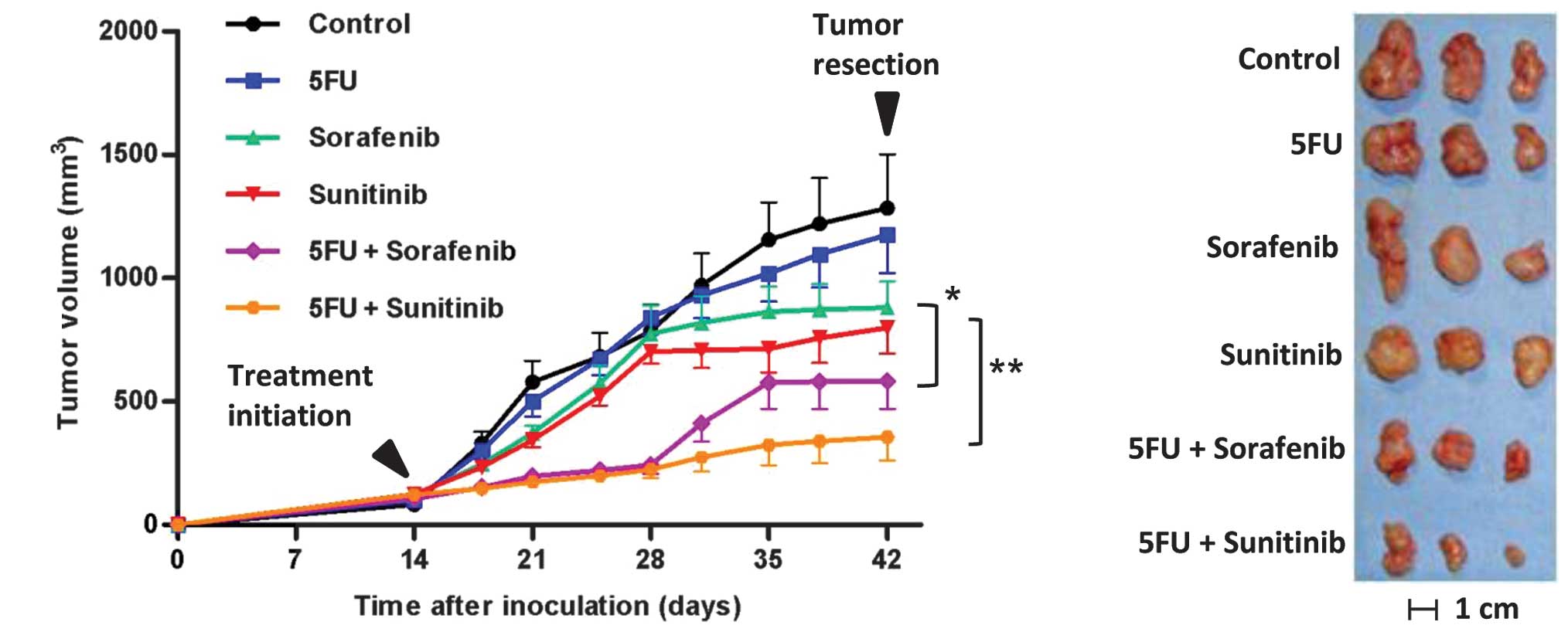
|
1
|
Rini BI: Vascular endothelial growth
factor-targeted therapy in renal cell carcinoma: current status and
future directions. Clin Cancer Res. 13:1098–1106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagstaff J: Renal cell cancer: is
immunotherapy dead? Ann Oncol. 18(Suppl 9): 94–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9(Suppl 1):
2–10. 2004. View Article : Google Scholar
|
|
4
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roskoski R Jr: Sunitinib: a VEGF and PDGF
receptor protein kinase and angiogenesis inhibitor. Biochem Biophys
Res Commun. 356:323–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hutson TE: Targeted therapies for the
treatment of metastatic renal cell carcinoma: clinical evidence.
Oncologist. 16(Suppl 2): 14–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Escudier B, Eisen T, Stadler WM, et al:
Sorafenib for treatment of renal cell carcinoma: final efficacy and
safety results of the phase III treatment approaches in renal
cancer global evaluation trial. J Clin Oncol. 27:3312–3318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Overall survival and updated results for sunitinib compared with
interferon alfa in patients with metastatic renal cell carcinoma. J
Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dal Lago L, D’Hondt V and Awada A:
Selected combination therapy with sorafenib: a review of clinical
data and perspectives in advanced solid tumors. Oncologist.
13:845–858. 2008.PubMed/NCBI
|
|
10
|
Ulahannan SV and Brahmer JR:
Antiangiogenic agents in combination with chemotherapy in patients
with advanced non-small cell lung cancer. Cancer Invest.
29:325–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sweeney CJ, Chiorean EG, Verschraegen CF,
Lee FC, Jones S, Royce M, Tye L, Liau KF, Bello A, Chao R and
Burris HA: A phase I study of sunitinib plus capecitabine in
patients with advanced solid tumors. J Clin Oncol. 28:4513–4520.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castillo-Avila W, Piulats JM, Garcia Del
Muro X, Vidal A, Condom E, Casanovas O, Mora J, Germà JR, Capellà
G, Villanueva A and Viñals F: Sunitinib inhibits tumor growth and
synergizes with cisplatin in orthotopic models of
cisplatin-sensitive and cisplatin-resistant human testicular germ
cell tumors. Clin Cancer Res. 15:3384–3395. 2009. View Article : Google Scholar
|
|
13
|
Zhang D, Hedlund EM, Lim S, Chen F, Zhang
Y, Sun B and Cao Y: Antiangiogenic agents significantly improve
survival in tumor-bearing mice by increasing tolerance to
chemotherapy-induced toxicity. Proc Natl Acad Sci USA.
108:4117–4122. 2011. View Article : Google Scholar
|
|
14
|
Yoon CY, Lee JS, Kim BS, Jeong SJ, Hong
SK, Byun SS and Lee SE: Sunitinib malate synergistically
potentiates anti-tumor effect of gemcitabine in human bladder
cancer cells. Korean J Urol. 52:55–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hartmann JT and Bokemeyer C: Chemotherapy
for renal cell carcinoma. Anticancer Res. 19:1541–1543.
1999.PubMed/NCBI
|
|
16
|
Ravaud A, Trufflandier N, Ferrière JM,
Debled M, Palussière J, Cany L, Gaston R, Mathoulin-Pélissier S and
Bui BN: Subcutaneous interleukin-2, interferon alpha-2b and
5-fluorouracil in metastatic renal cell carcinoma as second-line
treatment after failure of previous immunotherapy: a phase II
trial. Br J Cancer. 89:2213–2218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyake M, Fujimoto K, Tanaka M, Hirao Y,
Uemura H, Otani T and Yoshii M: Immunochemotherapy with
interferon-α, interleukin-2, 5-fluorouracil, and cimetidine for
patients with advanced renal cell carcinoma. J Nara Med Assoc.
60:37–47. 2009.
|
|
18
|
Naito S, Eto M, Shinohara N, Tomita Y,
Fujisawa M, Namiki M, Nishikido M, Usami M, Tsukamoto T and Akaza
H: Multicenter phase II trial of S-1 in patients with
cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol.
28:5022–5029. 2010. View Article : Google Scholar
|
|
19
|
Miyake M, Fujimoto K, Anai S, Ohnishi S,
Nakai Y, Inoue T, Matsumura Y, Tomioka A, Ikeda T, Okajima E,
Tanaka N and Hirao Y: Inhibition of heme oxygenase-1 enhances the
cytotoxic effect of gemcitabine in urothelial cancer cells.
Anticancer Res. 30:2145–2152. 2010.PubMed/NCBI
|
|
20
|
Miyake M, Fujimoto K, Anai S, Ohnishi S,
Kuwada M, Nakai Y, Inoue T, Matsumura Y, Tomioka A, Ikeda T, Tanaka
N and Hirao Y: Heme oxygenase-1 promotes angiogenesis in urothelial
carcinoma of the urinary bladder. Oncol Rep. 25:653–660. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koch I, Depenbrock H, Danhauser-Riedl S,
Rastetter JW and Hanauske AR: Interleukin 1 modulates growth of
human renal carcinoma cells in vitro. Br J Cancer. 71:794–800.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeuchi A, Shiota M, Tatsugami K,
Yokomizo A, Eto M, Inokuchi J, Kuroiwa K, Kiyoshima K and Naito S:
Sorafenib augments cytotoxic effect of S-1 in vitro and in vivo
through TS suppression. Cancer Chemother Pharmacol. 68:1557–1564.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naito S, Yokomizo A and Koga H: Mechanisms
of drug resistance in chemotherapy for urogenital carcinoma. Int J
Urol. 6:427–439. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibayama Y, Nakano K, Maeda H, Taguchi M,
Ikeda R, Sugawara M, Iseki K, Takeda Y and Yamada K: Multidrug
resistance protein 2 implicates anticancer drug-resistance to
sorafenib. Biol Pharm Bull. 34:433–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang EJ and Johnson WW: The farnesyl
protein transferase inhibitor lonafarnib (SCH66336) is an inhibitor
of multidrug resistance proteins 1 and 2. Chemotherapy. 49:303–308.
2003. View Article : Google Scholar : PubMed/NCBI
|