Introduction
Parotid cancer (PC) has a low incidence, long
disease-course and numerous histological types. Moreover, the
biological behaviors vary in different histological types (1), which results in complex therapy for
PC. Few studies investigating PC have been conducted thus far
(2,3). In the present study, 135 patients with
PC were recruited from the Department of Head and Neck Surgery of
Zhejiang Cancer Hospital, China, and the prognostic factors and
therapeutic regimens of these patients were retrospectively
analyzed.
Patients and methods
Clinical information
A total of 671 patients with parotid tumors received
surgical treatment in the Department of Head and Neck Surgery of
Zhejiang Cancer Hospital, China, between January 1985 and January
2000. Of the 671 patients, 230 (34.3%) were diagnosed as having
malignant tumors. There were 8 patients with malignant lymphoma, 2
with malignant melanoma, 4 with sarcoma and 63 patients with
metastatic cancer. However, 18 patients with unresectable cancer
were excluded. In the present study, 135 patients with primary PC
were recruited with a median age of 57 years (range, 8–82),
including 77 males and 58 females. Excluding 2 patients, a mass was
found in the parotid gland region on palpation in the remaining
patients. In addition, there were 42 patients with local pain
(31.1%), 14 with facioplegia (10.4%), 6 with difficulty in opening
the mouth (4.4%), and 39 with lymph node enlargement (28.9%).
The patients were pathologically diagnosed as PC and
classified according to the criteria for PC classification
developed by the World Health Organization (4): mucoepidermoid carcinoma (n=36;
well-differentiated cancer in 10, moderately differentiated cancer
in 9 and poorly differentiated cancer in 5), adenocarcinoma (n=19),
acinar cell carcinoma (n=21), malignant pleomorphic adenoma (n=27),
undifferentiated carcinoma (n=10), adenoid cystic carcinoma (n=12),
salivary duct carcinoma (n=7), and squamous cell carcinoma (n=3).
The cancer types were subgrouped into poorly differentiated cancer
(poorly differentiated mucoepidermoid carcinoma, adenocarcinoma,
squamous cell carcinoma, undifferentiated carcinoma, salivary duct
carcinoma, and solid adenoid cystic carcinoma) and
well-differentiated cancer. There were 47 patients with poorly
differentiated and 88 with well-differentiated cancer. The clinical
staging was based on the 1997 UICC classification system (5): stage I (n=25), II (n=47), III (n=45)
and IV (n=18).
Superficial parotidectomy was performed in 30
patients, superficial parotidectomy plus neck lymph node dissection
in 35, total parotidectomy in 15, total parotidectomy plus cervical
lymph node dissection in 43 and palliative surgery in 12 patients
(7 received cervical lymph node dissection). Moreover, 28 patients
received facial nerve resection. Among the 85 patients who
underwent cervical lymph node dissection, therapeutic lymph node
dissection was performed in 39 patients and selective lymph node
dissection in 46 patients. There were 58 patients who received
local radiotherapy following surgery, 31 of whom received cervical
radiotherapy. The radiation dose was as follows: radiotherapy of
the parotid gland region was performed at 1.8–2.0 Gy five times a
week with a total dose of 40–65 Gy, and radiotherapy of the neck at
1.8–2.0 Gy five times a week with a total dose of 40–56 Gy.
Follow-up was performed until January 2005. The study was approved
by the ethics committee of Zhejiang Cancer Hospital. Informed
concent was obtained from all patients involved.
Statistical analysis
Statistical analysis was performed with SPSS version
10.0. Qualitative data were analyzed using the Chi-square test and
the survival rate was calculated using the Kaplan-Meier method and
the log-rank test. Cox stepwise regression was employed for
multivariate analysis. The P-value was defined as 0.1.
Results
Patient follow-up
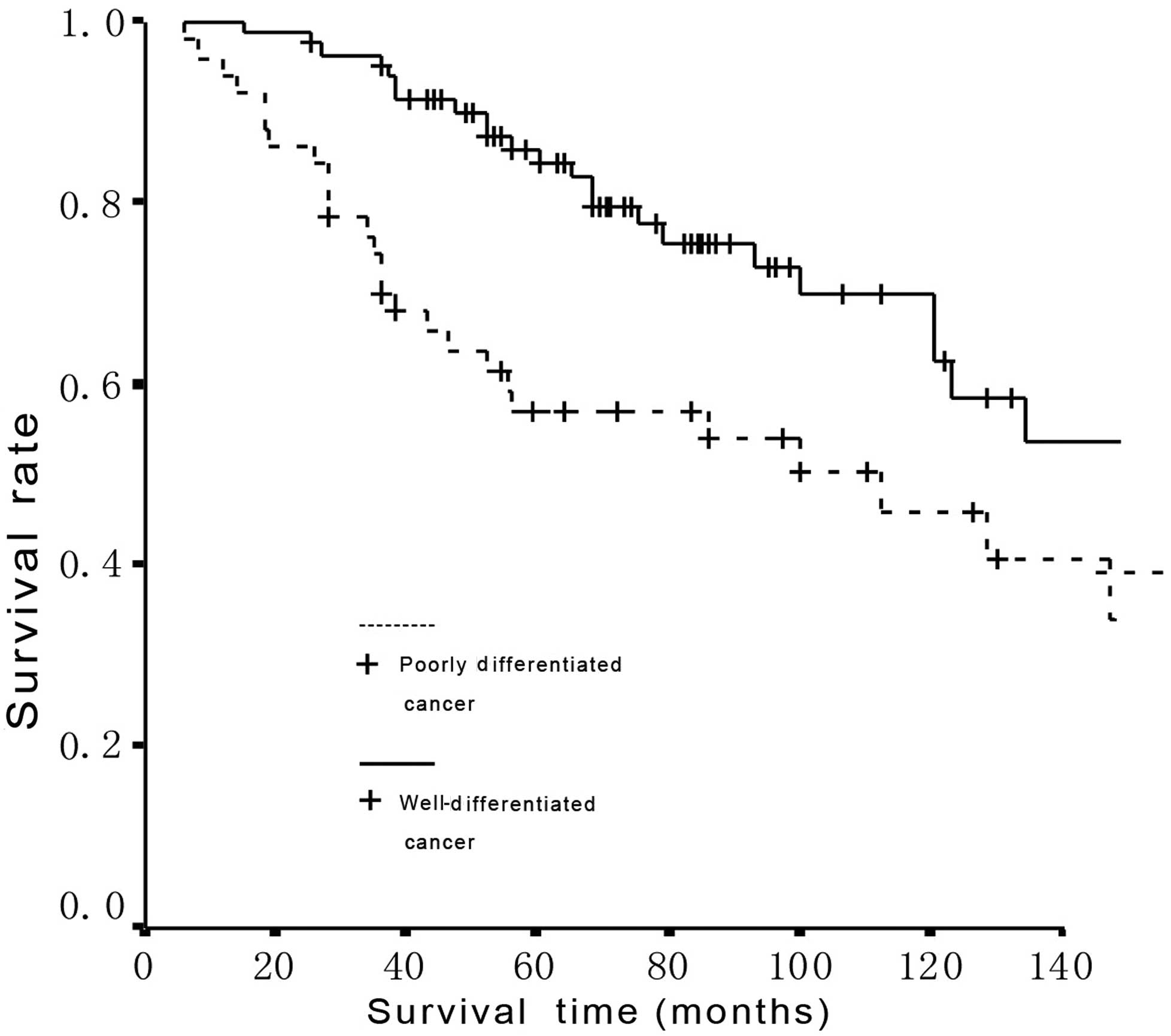
Eighteen patients succumbed to the disease prior to
follow-up and 86.7% of patients completed the follow-up. Survival
time was counted from the day of treatment. The 5- and 10-year
survival rates were 74.0 and 56.4%, respectively, and a significant
difference was found in the survival between patients with
well-differentiated and poorly differentiated PC
(χ2=5.72, P=0.0168) (Fig.
1). Results of the multivariate analysis revealed that age,
stage and pathological grade of PC, and radiotherapy were
independent factors affecting the prognosis of PC patients
(Table I). Age <65 years, PC at
an early stage, well-differentiated PC and post-operative
radiotherapy predicted a good prognosis.
 | Table ICox regression multivariate analysis
of factors affecting the prognosis of PC patients. |
Table I
Cox regression multivariate analysis
of factors affecting the prognosis of PC patients.
| Variables | Regression
coefficient | Standard error | χ2 | P-value | OR | 95% CI |
|---|
| Age | 1.008 | 0.302 | 11.172 | 0.001 | 2.741 | 1.517–4.951 |
| Stage | 0.655 | 0.286 | 5.237 | 0.022 | 1.925 | 1.099–3.373 |
| Grade | 0.549 | 0.285 | 3.712 | 0.054 | 1.732 | 0.991–3.028 |
| Radiotherapy | 0.641 | 0.301 | 4.549 | 0.033 | 1.899 | 1.053–3.424 |
Lymph node metastasis
Among the 39 patients with clinical lymph node
metastasis (CN1 and CN2), 25 had pathologically proven lymph node
metastasis. Of the 46 patients negative for clinical lymph node
metastasis (CN0), who received selective lymph node dissection,
lymph node metastasis was pathologically proven in 10 patients. In
the 35 patients with lymph node metastasis, 19 had poorly
differentiated PC (40.4%) and 16 had well-differentiated PC
(18.2%). In addition, occult metastasis was found in 7 patients in
the poorly differentiated PC group (7/28) and 3 patients in the
well-differentiated PC group (3/18). Among the patients receiving
selective lymph node dissection, there was no marked difference in
the occult metastasis between the well-differentiated and the
poorly differentiated PC groups (χ2=0.447, P=0.
504).
Facial nerve involvement
There were 14 patients with symptoms of facial
paralysis (14/135, 10.4%), of whom 10 had poorly differentiated PC.
Among these 14 patients, 10 received resection of the facial nerve
trunk and 4 received resection of the facial nerve branches. In the
14 patients without symptoms of facial nerve involvement (5 with
poorly differentiated PC and 9 with well-differentiated PC), the
facial nerve was enwrapped by the tumor or there was clear
involvement of the tumor and thus, resection of facial nerve was
performed. Of the 28 patients who received resection of the facial
nerve, facial nerve involvement was pathologically confirmed in all
patients. Furthermore, the proportion of patients with facial nerve
involvement was significantly different between patients with
poorly differentiated PC (15/47) and those with well-differentiated
PC (13/88; χ2=5.477, P=0.019).
Discussion
In the majority of studies (6–9), the
poorly differentiated mucoepidermoid carcinoma, squamous cell
carcinoma, undefined adenocarcinoma, undifferentiated carcinoma and
salivary duct carcinoma were classified into poorly differentiated
cancers. Certain studies classified all types of adenoid cystic
carcinoma into well-differentiated cancer (10), whereas in other studies these types
of carcinoma were classified as poorly differentiated cancer
(6,8). According to our previous studies
(11) on adenoid cystic carcinoma,
the solid adenoid cystic carcinoma is more susceptible to facial
nerve involvement and cervical lymph node metastasis. Thus, we
classified solid adenoid cystic carcinoma into poorly
differentiated cancer, which was consistent with the study of
Pedersen et al (9). In the
present study, the 5- and 10-year survival rates were 74.0 and
56.4%, respectively, and a significant difference was found in the
survival between patients with well-differentiated and poorly
differentiated PC (χ2=5.72, P=0.0168).
Previous studies have demonstrated that pathological
grade is a determinant of survival while in findings of other
studies have suggested that disease stage is of greater
significance as a prognostic factor (12,13).
Pohar et al reported that the factors affecting the
prognosis of PC were age and stage of disease. Multivariate
analysis revealed that age <65 years, early disease and
well-differentiated tumor predicted a good prognosis (13). These findings suggest it is
necessary to investigate the age and severity of disease in further
prospective studies.
In the present study, 25.9% of patients had lymph
node metastasis. In patients in the CN0 stage receiving cervical
lymph node dissection, the incidence of occult lymph node
metastasis was 21.7%. Armstrong et al proposed that the
treatment of metastatic cervical lymph nodes (including lymph node
dissection during surgery and post-operative radiotherapy of
cervical lymph nodes) should be selective for patients with PC
(14). We consider that the
cervical lymph nodes should be treated for patients with PC, as the
incidence of occult lymph node metastasis is high in these
patients. For patients negative for clinical lymph node metastasis,
therapeutic cervical lymph node dissection is recommended. For
patients in the CN0 stage, selective lymph node dissection can be
performed according to the intra-operative pathological
examination. For patients with poorly differentiated PC, removal of
level II and III lymph nodes is recommended. A previous study
demonstrated that the detection rate of metastatic lymph node was
90% following the removal of level II and III lymph nodes (14). For patients with well-differentiated
PC, removal of the lymph nodes surrounding the parotid gland as the
well-differentiated PC has a low incidence of local lymph node
metastasis and PC mainly invades the surrounding lymph nodes
directly. Our results also demonstrate that lymph node metastasis
was closely correlated with the histological type. In patients with
poorly differentiated PC, the incidence of cervical lymph node
metastasis was 40.4%, but was only 18.2% in those with
well-differentiated PC, demonstrating a significant difference
(χ2=7.893, P=0.005). In addition, the pathological grade
following cervical lymph node dissection is also able to provide
evidence for the radiotherapy of cervical lymph nodes.
In the surgical treatment of malignant parotid gland
tumors, the balance between radical dissection of cancer and the
preservation of the facial nerve has been a challenge for surgeons.
Surgery for the parotid gland may in certain cases be regarded as
surgery for the facial nerve. Dissection of the parotid gland tumor
may occur close to the facial nerve. When cancer invades the
tissues surrounding the facial nerve, it has the potential to
involve the facial nerve. If separation of the facial nerve is
complete in surgery, the facial nerve can be preserved.
Post-operative radiotherapy can remove the minimal residual tumor.
Thus, surgeons should pay attention to the preservation of the
facial nerve in surgery, unless the nerve itself is involved in
cancer. In the present study, dissection of the facial nerve was
performed in 20.7% of patients, which was consistent with previous
studies (6,15). Facial nerve dysfunction is a
contributing factor of distant metastasis and also a predictive
factor of recurrence (15). In the
present study, among the 28 patients with pathologically proven
facial nerve involvement, 13 patients developed local recurrence
(46.4%). Of the 107 patients without facial nerve involvement, 20
patients had local recurrence (18.7%). A significant difference was
revealed in the incidence of recurrence between patients with and
without facial nerve involvement (χ2=9.244,
P=0.002).
In the past two decades, radiotherapy has become a
significant component in the comprehensive treatment of PC.
Although certain studies revealed that the survival rate following
surgery plus post-operative radiotherapy was comparable to that
following surgery alone, PC in the early stage, small PC and
movable PC were not included in these studies for radiotherapy. Our
results reveal that post-operative radiotherapy was an independent
prognostic factor. Pohar et al retrospectively analyzed 163
patients with PC in two medical centers in the USA between 1960 and
2000 (13). In 56 patients
receiving surgery alone, the incidence of local recurrence was 37%.
Among the 91 patients receiving surgery and post-operative
radiotherapy, the incidence of local recurrence was 11%.
Multivariate analysis demonstrated that post-operative radiotherapy
did not improve the overall survival rate, but reduced the local
recurrence and increased the recurrence-free survival rate.
Recently, a study revealed that post-operative radiotherapy was
also beneficial for patients with PC in stages T1 and T2. Zbaren
et al (16) retrospectively
analyzed 58 patients with PC in stages T1 and T2. Of the 34
patients receiving post-operative radiotherapy, only 1 developed
recurrence (3%), while 8 had recurrence in 24 patients without
post-operative radiotherapy (33%). In patients with and without
recurrence, the 5-year survival rates were 93 and 83%,
respectively, and the 5-year tumor-free survival rates were 92 and
70%, respectively. Thus, prospective studies are required to
confirm these findings. For patients with recurrence following
surgery, salvage surgery commonly causes dissection of the facial
nerve, resulting in unacceptable disfigurement. Thus, we speculate
that post-operative radiotherapy is necessary for patients with PC
in stages T3 and T4, PC close to the surgical and positive margin,
PC in combination with facial nerve involvement, deep lobe PC,
poorly differentiated PC and PC with local lymph node
metastasis.
In conclusion, our results have shown that the
overall survival rate and incidence of cervical lymph node
metastasis varied between the patients with poorly differentiated
PC and well-differentiated PC. Facial nerve involvement is a
significant factor affecting local recurrence. Multivariate
analysis revealed that age, stage of PC, pathological grade and
post-operative radiotherapy are independent prognostic factors. We
recommend selective regional lymph node dissection in patients with
primary PC, with the extent of lymph node dissection depending on
the pathological grade. Post-operative radiotherapy is useful in
improving the quality of life and survival. However, this is a
retrospective study and a randomized control group was not
included. Further clinical randomized trials are required to
clarify which patients can benefit from post-operative
radiotherapy.
References
|
1
|
Renehan AG, Gleave EN, Slevin NJ, et al:
Clinico-pathological and treatment-related factors influencing
survival in parotid cancer. Br J Cancer. 80:1296–1300. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alterio D, Jereczek-Fossa BA, Griseri M,
et al: Three-dimensional conformal postoperative radiotherapy in
patients with parotid tumors: 10 years’ experience at the European
Institute of Oncology. Tumori. 97:328–334. 2011.PubMed/NCBI
|
|
3
|
Croce A, D’Agostino L, Moretti A and
Augurio A: Parotid surgery in patients over seventy-five years old.
Acta Otorhinolaryngol Ital. 28:231–238. 2008.PubMed/NCBI
|
|
4
|
Seifert G and Sobin LH: Histological
typing of salivary gland tumours. 2nd edition. Springer-Verlag;
Berlin, Germany: 1991, View Article : Google Scholar
|
|
5
|
Sobin LH and Wittekind CH: UICC TNM
classification of malignant tumors. 5th edition. Wiley-Liss; New
York: 1997
|
|
6
|
Gallo O, Franchi A, Bottai GV, et al: Risk
factors for distant metastases from carcinoma of the parotid gland.
Cancer. 80:844–851. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Magnano M, Gervasio CF, Cravero L, et al:
Treatment of malignant neoplasms of the parotid gland. Otolaryngol
Head Neck Surg. 121:627–632. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van der Poorten VL, Balm AJ, Hilgers FJ,
et al: The development of a prognostic score for patients with
parotid carcinoma. Cancer. 85:2057–2067. 1999.PubMed/NCBI
|
|
9
|
Pedersen D, Overgaard J, Søgaard H, et al:
Malignant parotid tumors in 110 consecutive patients: treatment
results and prognosis. Laryngoscope. 102:1064–1069. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Squillaci S, Marchione R and Piccolomini
M: Cystic sebaceous lymphadenoma of the parotid gland: case report
and review of the literature. Pathologica. 103:32–39.
2011.PubMed/NCBI
|
|
11
|
Jin SN, Shang JB and Wang KJ: Expression
and significance of nerve growth factor receptor in human salivary
adenoid cystic carcinoma. Zhejiang Med J. 32:471–472. 2010.
|
|
12
|
Lima RA, Tavares MR, Dias FL, et al:
Clinical prognostic factors in malignant parotid gland tumors.
Otolaryngol Head Neck Surg. 133:702–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pohar S, Gay H, Rosenbaum P, et al:
Malignant parotid tumors: presentation, clinical/pathologic
prognostic factors, and treatment outcomes. Int J Radiat Oncol Biol
Phys. 61:112–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Armstrong JG, Harrison LB, Thaler HT, et
al: The indications for elective treatment of the neck in cancer of
the major salivary glands. Cancer. 69:615–619. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Régis De Brito Santos I, Kowalski LP,
Cavalcante De Araujo V, et al: Multivariate analysis of risk
factors for neck metastases in surgically treated parotid
carcinomas. Arch Otolaryngol Head Neck Surg. 127:56–60.
2001.PubMed/NCBI
|
|
16
|
Zbaren P, Nuyens M, Caversaccio M, et al:
Postoperative radiation therapy for T1 and T2 primary parotid
carcinoma: is it useful? Otolaryngol Head Neck Surg. 135:140–143.
2006. View Article : Google Scholar : PubMed/NCBI
|