Introduction
Curcumin has been proven to be a promising
anti-cancer drug by induction of apoptosis and
apoptosis-independent death, and inhibition of proliferation and
angiogenesis (1–4). Phase I and II studies of this compound
have shown that curcumin is well tolerated and is effective for
cancer patients; however, its benefits may be attenuated due to its
low bioavailability through oral administration for
non-gastrointestinal cancers (5,6).
Therefore, novel strategies are required to overcome these
limitations, which are mostly due to the low water solubility and
low stability of curcumin against gastrointestinal fluids (7). Investigators have recognized that
liposomes have the advantage of improving water insolubility and
enhancing delivery efficacy of drugs (8). At present, various methods have been
reported for the preparation of liposomes (9). Curcumin acts as an anticancer drug
through multiple mechanisms; however, the activity of curcumin may
be changed in a liposomal form. Furthermore, the water solubility
of liposomal curcumin provides a new strategy for intravenous
administration. It is speculated that, systemically, intravenous
administration may exhibit marked inhibitory effects due to
circumvention of the first-pass effect.
Angiogenesis, the process by which capillaries
sprout from pre-existing vasculature, is a hallmark of the majority
of solid tumors (10). Targeting
tumor vascular endothelial cells has proven to be an effective
therapeutic strategy in anti-tumor treatment (11). Therefore, anti-angiogenic agents
have gained increasing importance in cancer research. Mounting
evidence indicates that curcumin inhibits carcinogenesis in various
organs and that the common link between these actions is its
anti-angiogenic effect (4).
Although the anti-cancer and anti-angiogenic effects of curcumin
have been evaluated comprehensively, the anti-angiogenic effect of
the liposomal form of these compounds has not been extensively
studied, particularly when the liposomal curcumin is administered
intravenously.
Previously, we developed water-soluble liposomal
curcumin with the ethanol injection method. In the current study,
we examined the anti-angiogenic and anti-cancer effects of
liposomal curcumin on Lewis lung cancer in vitro and in
vivo. Our study indicated that the liposomal curcumin primarily
inhibits tumor growth due to its anti-angiogenic activity. Our
results also indicate that liposomal curcumin may be used in tumor
treatment for further clinical application.
Materials and methods
Cell lines and animals
Murine Lewis lung carcinoma cell line LL/2 and
endothelial cell line MS1 were purchased from the American Type
Culture Collection (Manassas, VA, USA). The cell lines were
cultured in DMEM supplemented with 10% (vol/vol) fetal bovine serum
and were maintained in a humidified chamber at 37°C in 5%
CO2 atmosphere. C57BL/6 and zebrafish (FLK-1:EGFP) were
purchased from the West China Experimental Animal Center. The study
protocol was reviewed and approved by the institutional animal care
and treatment committee of Sichuan University, Chengdu, China.
Cell proliferation assay
The MTT assay was performed to determine the effect
of curcumin on MS1 and LL/2 viability. Briefly, cells were plated
in a 96-well plate at a density of 3000 cells per well and were
exposed to liposomal curcumin at different concentrations for 48 h.
Cells grown in media without curcumin were used as a control.
Following treatment, the media were carefully removed. Then, 20 μl
MTT (5 mg/ml) was added to each well and incubated with the cells
for 3 h. Dimethyl sulfoxide (150 μl) was added to each well and the
plates were read at 570 nm in an ELISA reader.
Flow cytometry
The percentage of apoptotic cells and the cell cycle
distribution of curcumin-treated cells were analyzed by flow
cytometry. Briefly, cells (1×105/well) were plated in
6-well plates. Following incubation overnight, the cells were
treated with various concentrations of liposomal curcumin (0–40
μg/ml) for 48 h, trypsinized and washed with PBS, and centrifuged.
Supernatants were removed and the cells were resuspended in 1 ml of
hypotonic fluorochrome solution containing 50 mg/ml propidium
iodide in 0.1% sodium citrate plus 0.1% Triton X-100 and
immediately subjected to flow cytometry (ESP Elite, Beckman Coulter
Fullerton, CA, USA).
Tumor growth inhibition experiment in
vivo
Six-week-old female C57BL/6 mice were acclimatized
for one week and fed with animal chow and water ad libitum.
The mice were injected subcutaneously in the right leg with
5×105 Lewis lung carcinoma cells with a total volume of
50 μl. Seven days later, when the tumors were palpable, the mice
were randomized into two groups (n=6 per group). The experimental
group was treated with liposomal curcumin (10 mg/kg) by intravenous
injection once a day for two weeks. The control mice were
administered normal saline (NS). Tumor dimensions were measured
every three days with calipers. Tumor volume was calculated
according to the formula: volume = width2 × length ×
0.52.
Detection of microvessel density
Frozen sections of the tumor tissue from the mice
were used to determine vessel density with an anti-CD31 antibody,
as described in a previous study in detail (12). The following antibodies and reagents
were used: monoclonal rat anti-mouse CD31 antibody (1:400, Santa
Cruz Biotechnology, Santa Cruz, CA, USA), biotinylated polyclonal
goat anti-rat antibody (1:200, Vector Laboratories, Peterborough,
UK), ABC kit (Boster Biological Engineering Co., Wuhan, China) and
DAB visualization system (ZSJQ Biotechnology, Beijing, China).
Sections were counterstained with hematoxylin and mounted with
glass coverslips. Images were captured using an Olympus
fluorescence microscope at an original magnification of ×200.
Microvessel density (MVD) was assessed within hot spots.
Alginate encapsulation for tumor cell
assay
Alginate bead-containing tumor cell assays were
described in detail in a previous study (13). Briefly, cultured LL/2 cells were
resuspended with 1.5% (m/v) sodium alginate (Sigma-Aldrich, St.
Louis, MO, USA). The tumor cell alginate solution was then dropped
into a swirling bath of 0.25 M CaCl2 in order to form
droplets containing approximately 1×105 tumor cells per
bead. After being anesthetized, the C57BL/6 mice were implanted
subcutaneously with four beads through an incision on the back and
the incisions were sutured with surgical clamps. Treatment with
liposomal curcumin (10 mg/kg) was performed once per day following
bead implantation, with normal saline (NS) as a control. At 14
days, the mice were injected intravenously with 100 μl FITC-dextran
solution (Sigma Chemical) (100 mg/kg) and were sacrificed 20 min
later. Images of the alginate implants were captured using a SPOT
FIEX camera. Alginate beads were transferred to tubes containing 2
ml of saline. The tubes were mixed in a vortex for 20 sec and
centrifuged (3 min; 1000 × g). Finally, the fluorescence of the
supernatant was measured to quantify blood vessel formation.
Zebrafish embryo development assay
FLK-1 promoter EGFP transgenic zebrafish
(FLK-1:EGFP) were used. Fertilized eggs were incubated for 8 h,
after which liposomal curcumin was added to the water at a
concentration of 5 μg/ml. The same water without curcumin was used
as a control. Following incubation for 72 h, the larvae were placed
on glass slides and examined using a Zeiss microscope. Fluorescence
signals were detected and images were captured.
Results
Higher sensitivity to liposomal curcumin
in murine endothelial cell line MS1
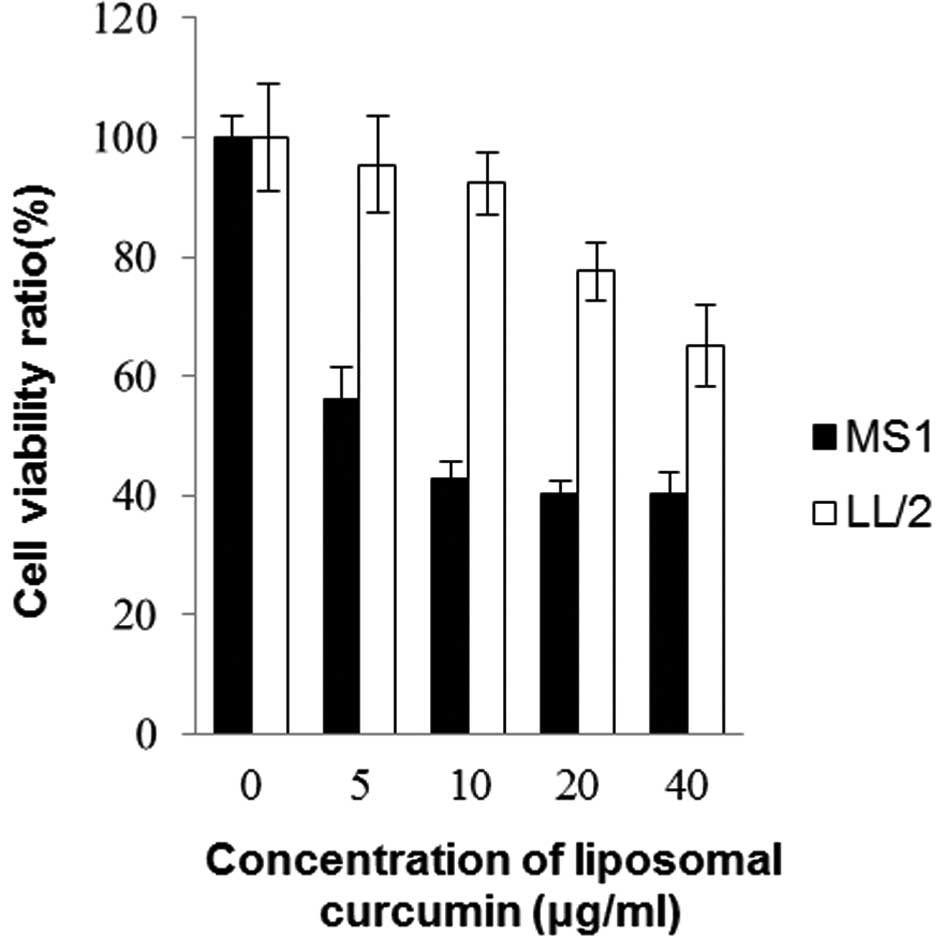
An MTT assay was conducted on murine Lewis lung
carcinoma cell line LL/2 and endothelial cell line MS1. Fig. 1 shows the effects of liposomal
curcumin on cell viability following 48 h of drug exposure. The
cell viability decreased in each cell line with the increasing
concentration of curcumin treatment. The sensitivity to curcumin
differed markedly between MS1 and LL/2 cells. Curcumin had higher
cytotoxic activity towards MS1, but lower cytotoxic activity
towards LL/2 (p<0.05). It was clear that mouse endothelial cells
were more sensitive to curcumin in comparison to Lewis lung cancer
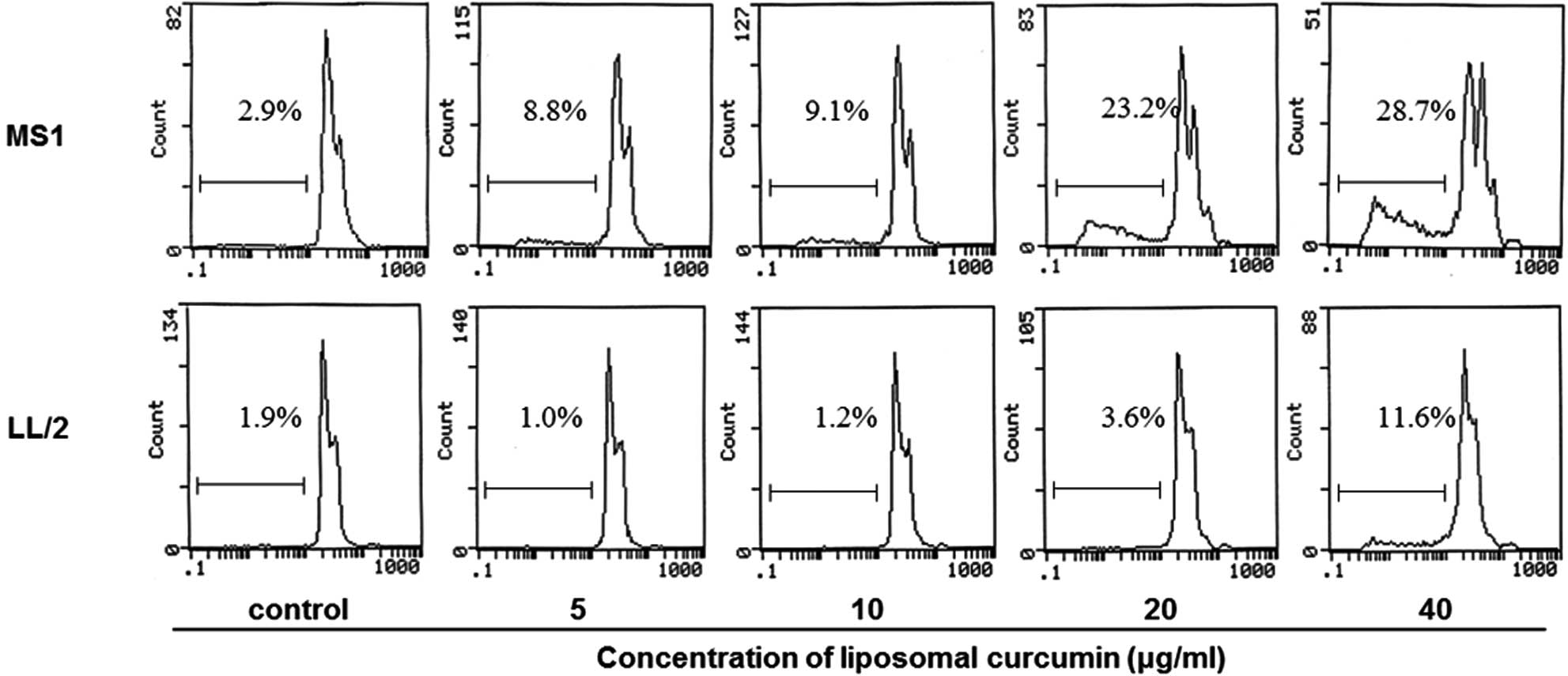
cells. In addition, flow cytometry was performed to investigate
whether liposomal curcumin induced MS1 and LL/2 cell apoptosis. The
quantitative assessment of sub-G1 cells by flow cytometry was used
to estimate the number of apoptotic cells. Liposomal curcumin was
found to increase the number of sub-G1 cells compared with the
control groups (Fig. 2). Notably,
no marked pro-apoptotic effect of liposomal curcumin was observed
against LL/2 cells.
Changes of cell cycle phase distribution
mediated by liposomal curcumin
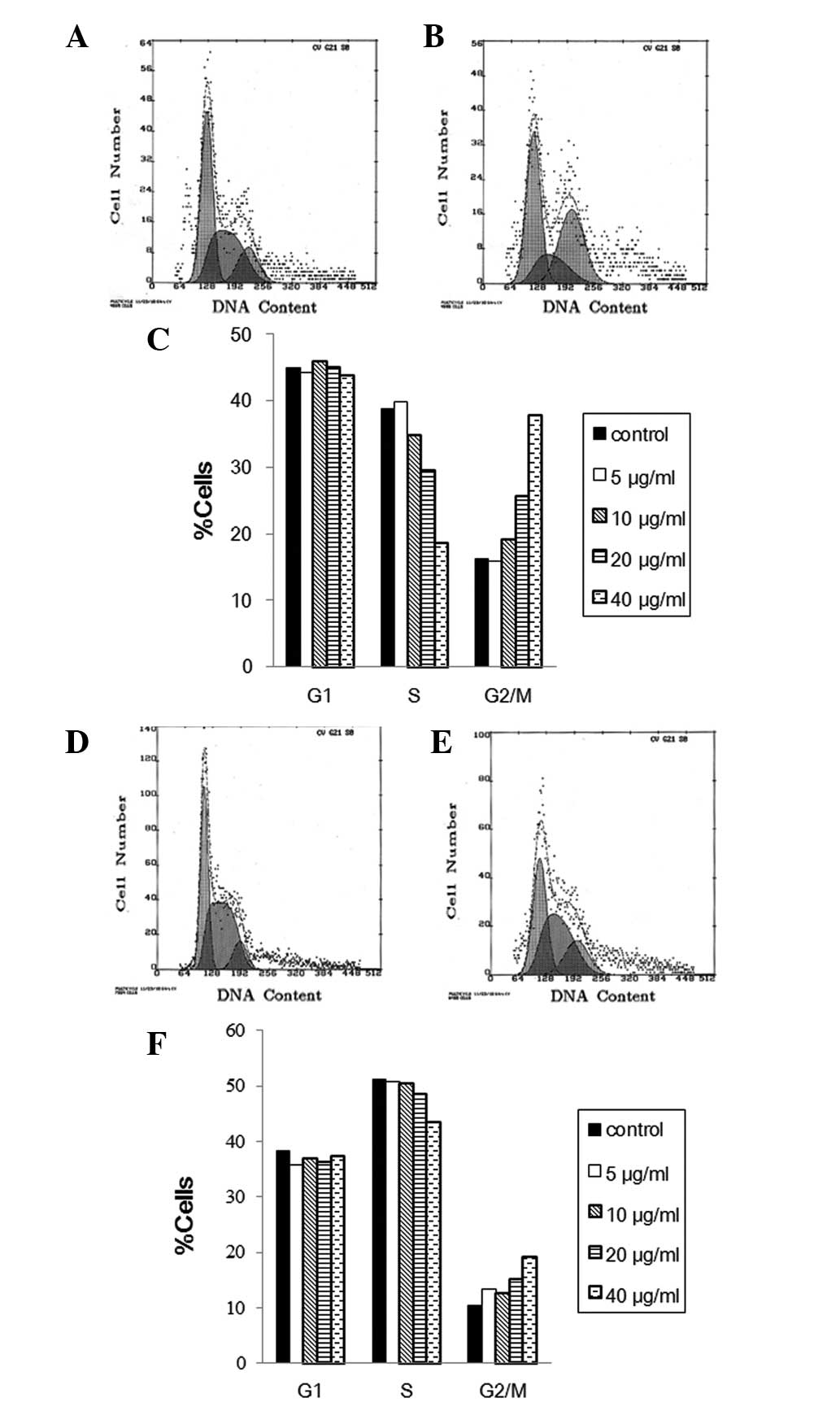
To evaluate the cell cycle phase distribution of MS1
and LL/2 cells with curcumin treatment, the DNA content was
measured using flow cytometry. FACS analysis of MS1 cells revealed
that exposure to liposomal curcumin from 5 to 40 μg/ml for 48 h
caused an increase of the G2/M-phase population from 15.8 to 37.8%,
compared to control cells with 16.2% G2/M phase cells (Fig. 3A). This increase was accompanied by
a significant decrease in the percentage of S-phase cells, whereas
the fraction of G1-phase cells was mainly unchanged (Fig. 3C). This result demonstrates that
curcumin induces growth inhibitory effects on MS1 at least in part
via G2/M phase arrest. Results of the FACS analysis of propidium
iodide-stained LL/2 cells showed that liposomal curcumin also
reduced S phase and increased G2/M percentages in LL/2 compared to
the control cells (Fig. 3D).
However, the change of cell cycle distribution with the increasing
concentration of liposomal curcumin treatment was relatively
insignificant in the LL/2 cell line (Fig. 3F).
Tumor growth inhibition in vivo
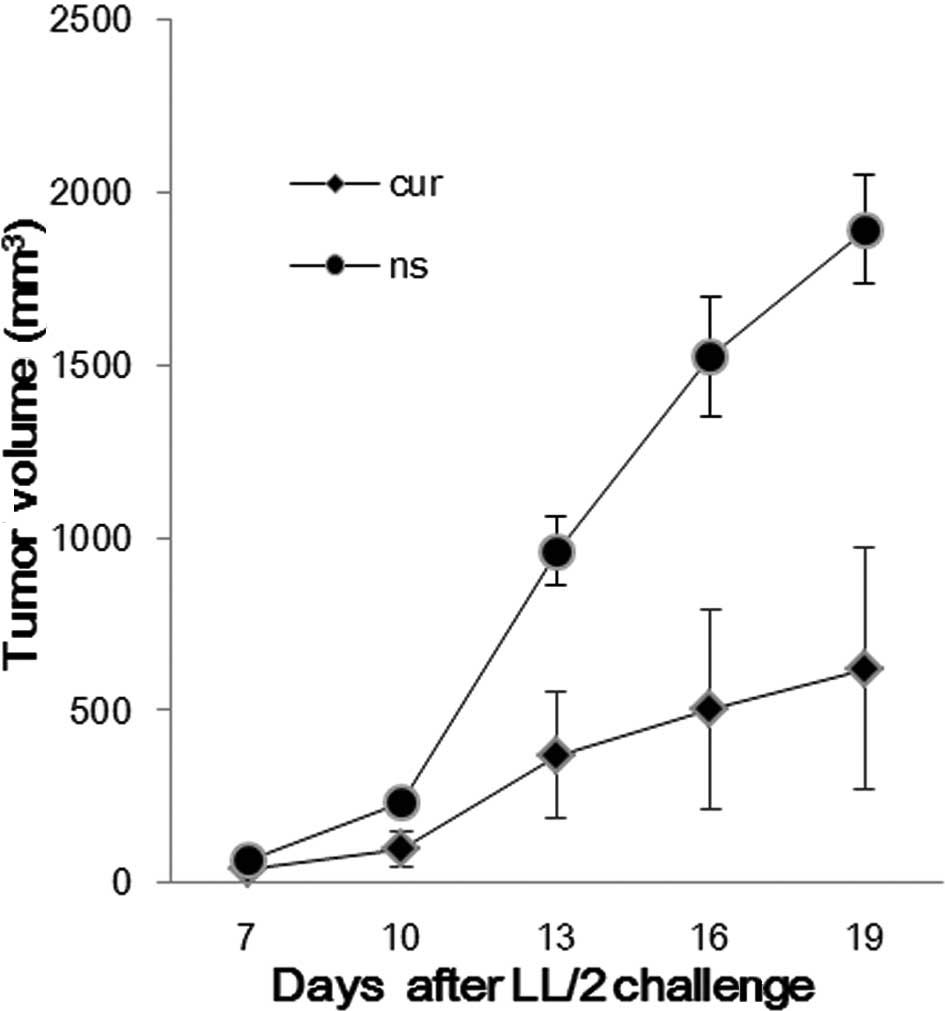
The established LL/2 tumor model was used to observe
the effect of liposomal curcumin on the tumor burden of mice. The
treatment regimens were carried out as described in Materials and
methods. Compared with the control group, the liposomal
curcumin-treated group was found to significantly inhibit tumor
growth (Fig. 4). The tumor volume
of the control and treated groups was (1892.26±158.03
mm3) vs. (618.64±350.26 mm3) on day 19.
Inhibition of tumor-induced
angiogenesis
Tumor sections from each group were stained with
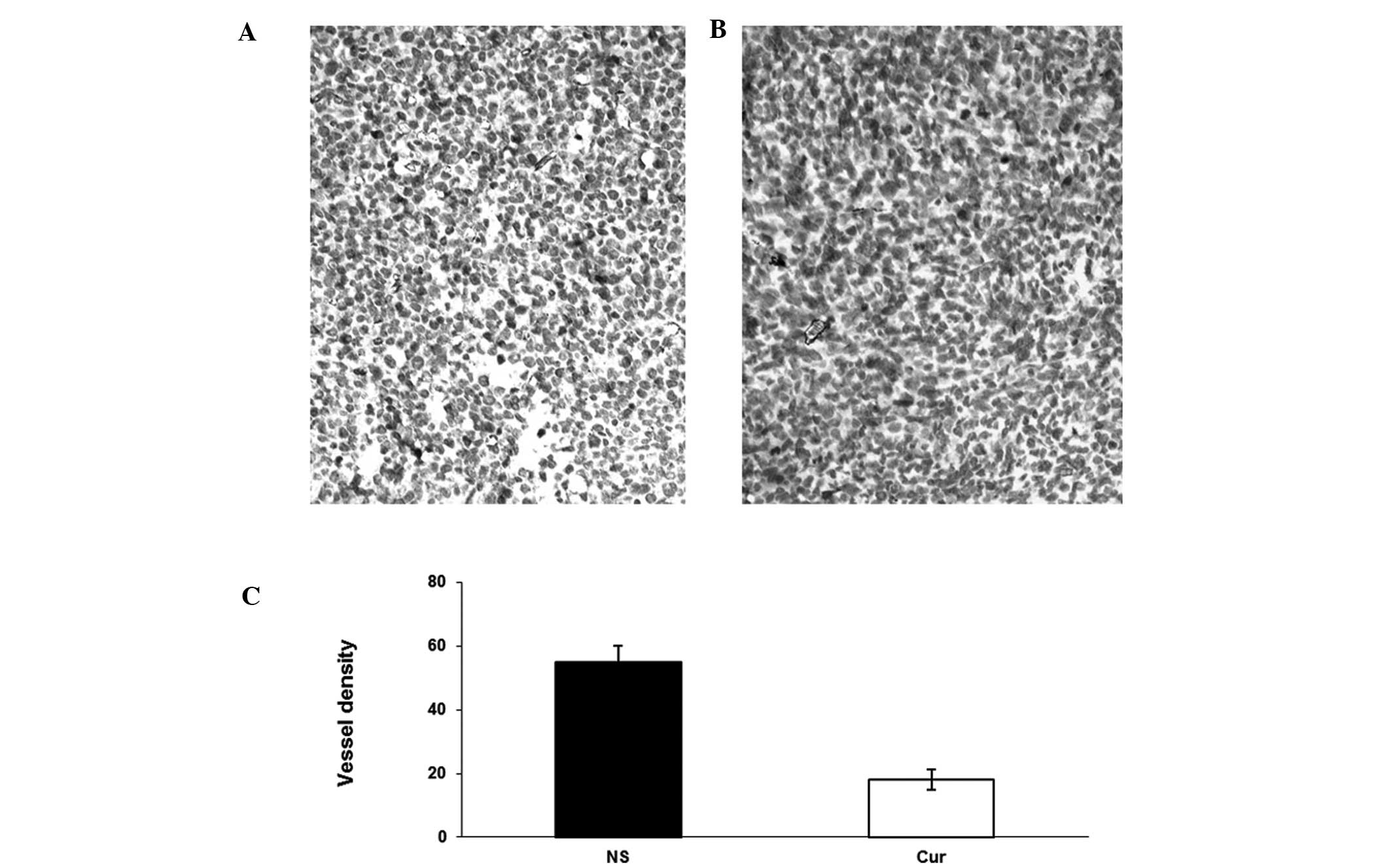
anti-CD31 antibody (Fig. 5).
Liposomal curcumin treatment resulted in the significant inhibition
of angiogenesis in tumors (Fig. 5B)
compared with the controls (Fig.
5A). Angiogenesis within tumor tissue was estimated by counting
the number of microvessels on the section stained with an antibody
reactive to CD31. Tumors from the liposomal curcumin group
exhibited lower vessel density than those of the NS group (Fig. 5C). In addition, the inhibition of
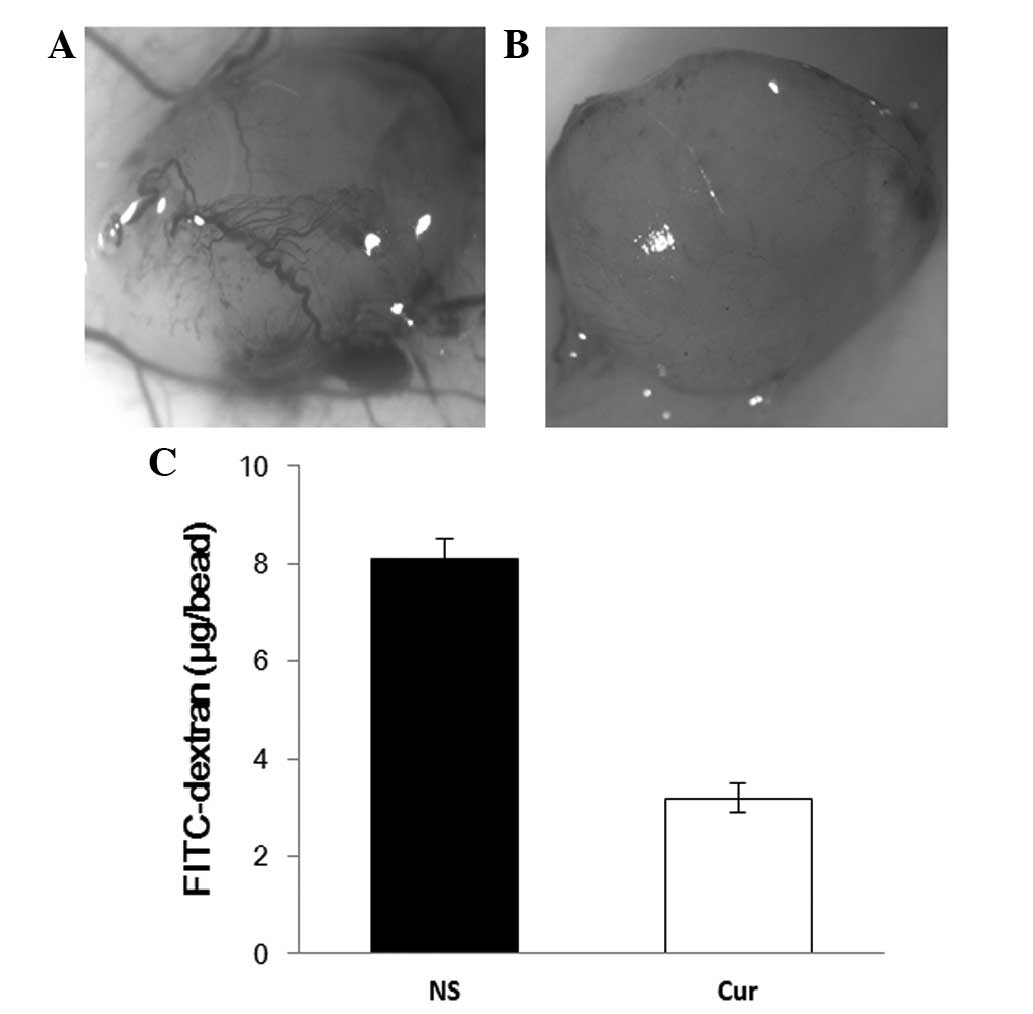
angiogenesis in vivo was observed through alginate
encapsulation assay. Alginate implant angiogenesis was quantitated
by measuring the uptake of FITC-dextran into beads. Vascularization
of the alginate beads was reduced, and FITC-dextran uptake was
decreased in liposomal curcumin-treated mice compared to the
controls (Fig. 6).
Liposomal curcumin-mediated
anti-angiogenesis in development of the zebrafish embryo
Our data demonstrate that liposomal curcumin
effectively inhibited endothelial cell growth in vitro and
in vivo. The endothelial cell line MS1 is a pancreatic islet
endothelial cell line derived from C57BL/6 mice and may exhibit
physiological and pathological roles in the current C57BL/6 mouse
model. Therefore, our data also revealed that the inhibition of
angiogenesis mediated by liposomal curcumin is non-specific to
tumor angiogenesis. To verify this, the anti-angiogenic effects of
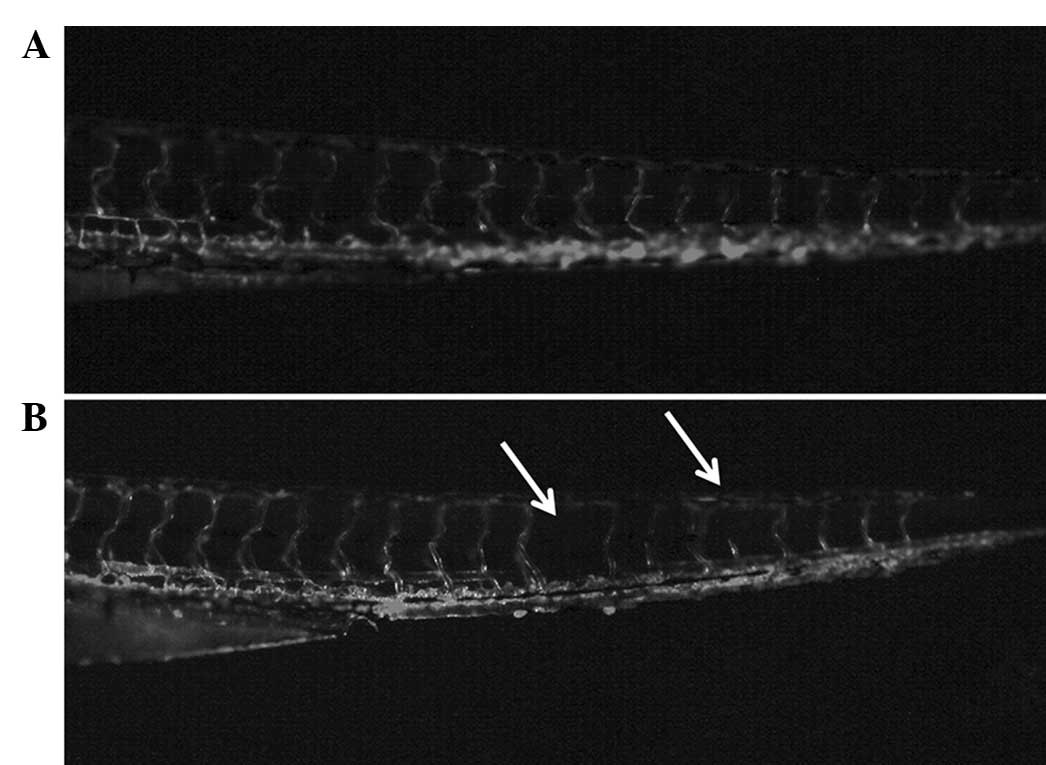
liposomal curcumin on the development of the zebrafish embryo were
investigated. Larvae hatched from fertilized eggs treated with
liposomal curcumin exhibited developmental defects. In the control
fish, vascularization was normal. The angiogenetic defects caused
by liposomal curcumin were evident compared to the control group
(Fig. 7), indicating that
inhibition of angiogenesis mediated by liposomal curcumin is
non-specific and thus may show toxicity in physiological processes,
such as wound healing.
Discussion
Curcumin inhibits tumor growth by targeting tumor
cells and endothelial cells. The current study evaluated the
anti-tumor and anti-angiogenesis activity of liposomal curcumin in
a Lewis lung cancer model. Since a critical step in angiogenesis
involves the proliferation of endothelial cells, we also determined
the effect of liposomal curcumin on the viability of murine
endothelial cells both in vitro and in vivo. Our data
showed that the inhibitory effects of liposomal curcumin on
angiogenesis were more effective. Inhibition of the NF-κB pathway
mediated by curcumin is known to play a key role in its
pharmacological activity (14,15).
Accumulating evidence has shown that both physiological and
pathological angiogenesis rely on the activity of the NF-κB pathway
(16). However, NF-κB signaling has
not been a preferred drug target in current clinical cancer therapy
since the activity of NF-κB signaling is not necessary for tumor
growth.
The significant finding of the current study is that
murine endothelial cells (MS1) were more sensitive than murine lung
tumor cells (LL/2). The cytotoxic activity of curcumin against
these two cell lines was evaluated by MTT assay. Following
treatment with curcumin (5 to 40 μg/ml) for 48 h, the viability of
endothelial cells was markedly inhibited and lung cancer cells by
comparison were only slightly inhibited. Moreover, the difference
is clearer in low concentrations of curcumin (<20 μg/ml). We
further investigated the possible mechanisms of this result.
Curcumin inhibits cell proliferation through diverse mechanisms.
However, as yet, the exact mechanism is not clear since various
mechanisms act on different cells. Two possible mechanisms may have
been involved in generating the results of this study. The first is
induction of apoptosis: Our study showed that liposomal curcumin
induces more cells to apoptosis in MS1 murine endothelial cells
than in LL/2 murine lung tumor cells. It is also noteworthy that it
had almost no effect on LL/2 at a concentration of 20 μg/ml. The
possible mechanisms underlying the induction of apoptosis by
liposomal curcumin may differ in the two cell lines; a more
mechanistic study is therefore required in this area. The second
possible mechanism is cell cycle arrest: We report in this study
that liposomal curcumin treatment leads to G2/M arrest in MS1 and
LL/2 cell lines. However, the effect on MS1 is more obvious than
that on LL/2. In recent studies, other authors have reported that
certain tumor cells are resistant to curcumin (17,18),
which may correspond with the different genetics and biologies of
individual tumors. The lack of effect of curcumin on LL/2 shown in
our current study is consistent with their reports. Therefore, the
inhibition of tumor angiogenesis is thought to play a more
significant role in its anti-tumor effects. In addition, it is
known that endothelial cells are genetically stable, and therefore
less likely to rapidly develop drug resistance. Taken together,
these results indicate that liposomal curcumin targeting
angiogenesis that supports tumor growth rather than the tumors
themselves is a promising therapeutic approach for certain cancer
types.
Various angiogenetic inhibitors have been developed
to target vascular endothelial cells and block tumor growth. The
majority of these inhibitors have limited potential since they are
extremely toxic or highly expensive, and are therefore beyond the
reach of most patients. Curcumin, a plant-derived compound, has
been used safely as a food additive for centuries without reports
of significant toxicity. In addition, curcumin is affordable and
has been found to suppress angiogenesis through multiple mechanisms
(18). Most of the studies used
free curcumin, which is poorly water-soluble and has low
bioavailability, and is therefore limited in its clinical efficacy.
We prepared liposomal curcumin solution via the ethanol injection
method. This solution is well dispersed and shows an average size
of 125.7 nm, determined using a nano-particle size analyzer (data
not shown). Liposome encapsulation of curcumin renders this agent
amenable to intravenous administration.
A particularly encouraging aspect of this study is
the observation that liposomal curcumin significantly suppressed
LL/2 tumor growth in vivo, although it was almost
ineffective on LL/2 cells in vitro. This result indicates
that liposomal curcumin can be used for cancer therapy in
curcumin-sensitive and -resistant tumor cells. The approach of
anti-angiogenesis for the curcumin treatment of cancer appears
promising. Our results also indicate that curcumin has the ability
to block angiogenesis in vivo. This result may be associated
with the inhibition of proliferation and the induction of apoptosis
and cell cycle arrest in endothelial cells in vitro.
Liposomal curcumin has been reported to exhibit anti-cancer
activity on colorectal cancer (19), prostate cancer (20), head and neck squamous cell carcinoma
(21), and pancreatic (22) and cervical cancer (23). In this study, we revealed that
liposomal curcumin inhibits tumor growth in the Lewis lung cancer
mouse model primarily by targeting tumor angiogenesis.
The effect of liposomal curcumin on physiological
angiogenesis was also investigated in this study. We used zebrafish
as vertebrate model organisms to investigate the anti-angiogenic
effect of curcumin in the development of the embryo. Advantages of
using zebrafish as model organisms include their fecundity, optical
clarity, and genetic similarity to mammals. Research using rats as
animal models revealed that orally administered curcumin had no
toxic effects on fertility or pregnancy (24). Our findings have shown that, unlike
in rats, liposomal curcumin had embryotoxic and anti-angiogenic
effects on the development of zebrafish embryos. Therefore, our
study indicates that liposomal curcumin-mediated anti-angiogenic
effects are not tumor-specific but broad-spectrum and may be used
to treat angiogenesis-related diseases. However, further
investigation into the toxicity of liposomal curcumin is
required.
Acknowledgements
This study was financially supported by the Chinese
National Natural Science Foundation.
References
|
1
|
Pongrakhananon V, Nimmannit U, Luanpitpong
S, Rojanasakul Y and Chanvorachote P: Curcumin sensitizes non-small
cell lung cancer cell anoikis through reactive oxygen
species-mediated Bcl-2 downregulation. Apoptosis. 15:574–585. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bharti AC, Donato N, Singh S and Aggarwal
BB: Curcumin (diferuloylmethane) down-regulates the constitutive
activation of nuclear factor-kappa B and IkappaBalpha kinase in
human multiple myeloma cells, leading to suppression of
proliferation and induction of apoptosis. Blood. 101:1053–1062.
2003. View Article : Google Scholar
|
|
3
|
O’Sullivan-Coyne G, O’Sullivan GC,
O’Donovan TR, Piwocka K and McKenna SL: Curcumin induces
apoptosis-independent death in oesophageal cancer cells. Br J
Cancer. 101:1585–1595. 2009.
|
|
4
|
Bhandarkar SS and Arbiser JL: Curcumin as
an inhibitor of angiogenesis. Adv Exp Med Biol. 595:185–195. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng AL, Hsu CH, Lin JK, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
6
|
Dhillon N, Aggarwal BB, Newman RA, et al:
Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi M, Uechi S, Takara K, Asikin Y
and Wada K: Evaluation of an oral carrier system in rats:
bioavailability and antioxidant properties of liposome-encapsulated
curcumin. J Agric Food Chem. 57:9141–9146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allen TM and Cullis PR: Drug delivery
systems: entering the mainstream. Science. 303:1818–1822. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bejjani RA, Jeanny JC, Bochot A and
Behar-Cohen F: The use of liposomes as intravitreal drug delivery
system. J Fr Ophtalmol. 26:981–985. 2003.(In French).
|
|
10
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gasparini G, Longo R, Toi M and Ferrara N:
Angiogenic inhibitors: a new therapeutic strategy in oncology. Nat
Clin Pract Oncol. 2:562–577. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai RZ, Wu Y, Liu Q, et al: Suppression of
lung cancer in murine model: treated by combination of recombinant
human endostsatin adenovirus with low-dose cisplatin. J Exp Clin
Cancer Res. 28:312009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He QM: Inhibition of tumor growth with a
vaccine based on xenogeneic homologous fibroblast growth factor
receptor-1 in mice. J Biol Chem. 278:21831–21836. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng M, Ekmekcioglu S, Walch ET, Tang CH
and Grimm EA: Inhibition of nuclear factor-kappaB and nitric oxide
by curcumin induces G2/M cell cycle arrest and apoptosis in human
melanoma cells. Melanoma Res. 14:165–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liao S, Xia J, Chen Z, et al: Inhibitory
effect of curcumin on oral carcinoma CAL-27 cells via suppression
of Notch-1 and NF-κB signaling pathways. J Cell Biochem.
112:1055–1065. 2011.PubMed/NCBI
|
|
16
|
Tabruyn SP and Griffioen AW: A new role
for NF-κB in angiogenesis inhibition. Cell Death Differ.
14:1393–1397. 2007.
|
|
17
|
Wang WZ, Cheng J, Luo J and Zhuang SM:
Abrogation of G2/M arrest sensitizes curcumin-resistant hepatoma
cells to apoptosis. FEBS Lett. 582:2689–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yadav VR and Aggarwal BB: Curcumin: a
component of the golden spice, targets multiple angiogenic
pathways. Cancer Biol Ther. 11:236–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Bernard K, Li G and Kirk KL:
Curcumin opens cystic fibrosis transmembrane conductance regulator
channels by a novel mechanism that requires neither ATP binding nor
dimerization of the nucleotide-binding domains. J Biol Chem.
282:4533–4544. 2007. View Article : Google Scholar
|
|
20
|
Thangapazham RL, Puri A, Tele S,
Blumenthal R and Maheshwari RK: Evaluation of a
nanotechnology-based carrier for delivery of curcumin in prostate
cancer cells. Int J Oncol. 32:1119–1123. 2008.PubMed/NCBI
|
|
21
|
Wang D, Veena MS, Stevenson K, et al:
Liposome-encapsulated curcumin suppresses growth of head and neck
squamous cell carcinoma in vitro and in xenografts through the
inhibition of nuclear factor kappaB by an AKT-independent pathway.
Clin Cancer Res. 14:6228–6236. 2008. View Article : Google Scholar
|
|
22
|
Mach CM, Mathew L, Mosley SA, Kurzrock R
and Smith JA: Determination of minimum effective dose and optimal
dosing schedule for liposomal curcumin in a xenograft human
pancreatic cancer model. Anticancer Res. 29:1895–1899.
2009.PubMed/NCBI
|
|
23
|
Sreekanth CN, Bava SV, Sreekumar E and
Anto RJ: Molecular evidences for the chemosensitizing efficacy of
liposomal curcumin in paclitaxel chemotherapy in mouse models of
cervical cancer. Oncogene. 30:3139–3152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ganiger S, Malleshappa HN, Krishnappa H,
Rajashekhar G, Ramakrishna Rao V and Sullivan F: A two generation
reproductive toxicity study with curcumin, turmeric yellow, in
Wistar rats. Food Chem Toxicol. 45:64–69. 2007. View Article : Google Scholar : PubMed/NCBI
|