1. Introduction
Endometriosis is a common gynecological condition
affecting 10% of women of childbearing age. It is a chronic disease
that is characterized by ectopic endometrial tissue outside the
uterus, typically leading to painful symptoms, dysmenorrhea,
dyspareunia, pelvic pain, infertility, and malignant transformation
(1). It has been reported that
endometriosis can be a precursor of some ovarian cancers. Previous
studies have shown that endometriosis has the potential to induce
several types of premalignant lesions (1–3).
Herein, we reviewed the malignant transformation of
endometriosis and described the mechanisms whereby genetic and
microenvironmental factors contribute to the neoplastic progression
of endometriosis.
2. Review of the literature
A comprehensive review of the literature was
conducted to investigate record numbers of ‘malignant
transformation of endometriosis’ reported worldwide. A Medline
search of the literature was performed using the keywords
endometriosis, cancer, tumour, tumor, carcinoma, adenocarcinoma,
sarcoma, malignancy, neoplasm, ovary, ovarian and extragonadal.
English-language publications in PubMed and references from
relevant articles published between January 1, 1966 and December
31, 2010 were analyzed. References in the studies identified were
also searched, and some unpublished data were obtained.
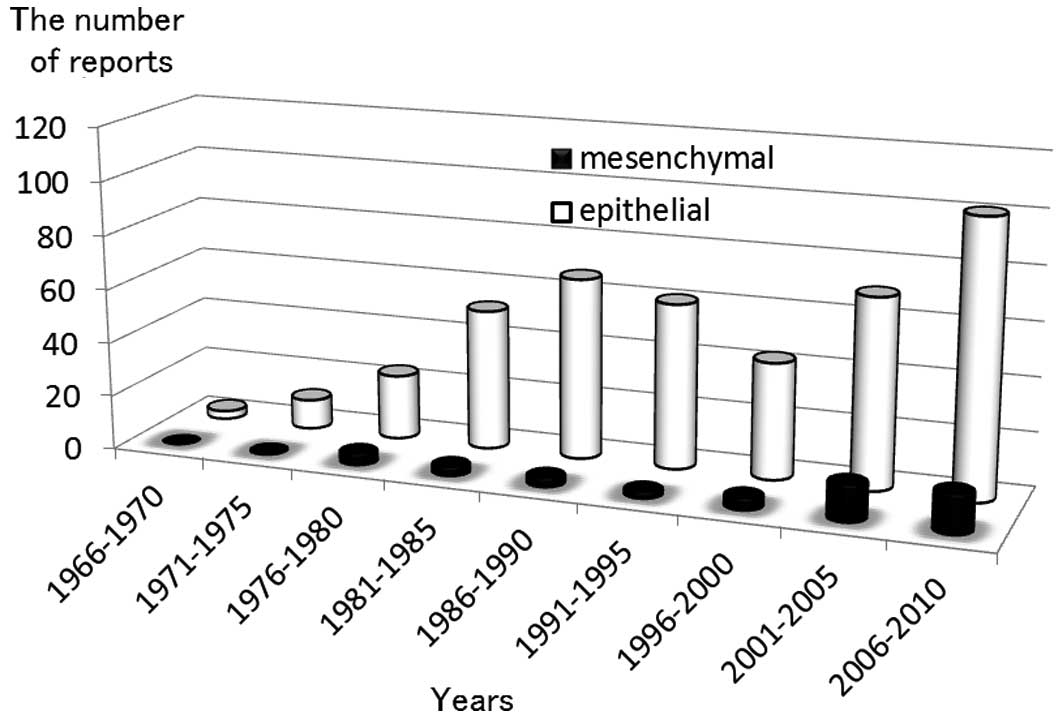
Between 1966 and 2011, the literature search
identified 483 reports, with the incidence of reports increasing
over time (Fig. 1). Of these 483
reports, 432 (89%) described epithelial malignancy and only 51
(11%) described mesenchymal malignancy. Data describing
endometriosis and epithelial malignancy from the PubMed database,
encompassing the years 2001 to 2010, clearly show a marked increase
of reports. The number of reports describing endometriosis and
mesenchymal malignancy has also increased, but less rapidly than
those describing endometriosis and epithelial malignancy.
3. Endometriosis-associated malignant
transformation
Endometriosis is a complex disease with a
multifactorial pathogenesis. Extensive investigation has explored
the role of genetics, epigenetics, environmental factors and
ethnicity in predisposing women to develop endometriosis (1–3). Some
studies reported a higher prevalence of upper respiratory
infection, vaginal infection and pelvic inflammatory disease
compared to the general population (2). Numerous immune factors are modified,
not only within endometriotic lesions, but also within the eutopic
endometrium (3). Thus,
endometriosis is an inflammatory condition, associated with a
dysregulated immune response (2).
Further studies reported a higher prevalence of
melanoma, non-Hodgkin's lymphoma, ovarian cancer, endometrial
cancer and breast cancer in women with endometriosis (2,4–6).
Although endometriosis cannot be termed a premalignant disease,
epidemiological, histopathological and molecular data suggest that
this condition has malignant potential (7). The presence of endometriosis is
associated with an increased risk of synchronous neoplasms in the
ovary and endometrium, so-called endometrial and ovarian cancer
synchronous to endometriosis (8).
Concurrent endometrial pathology has been observed in cases of
malignant transformation of endometriosis (approximately 30% of
cases) (9). In their study, Mabrouk
et al reported a case of mixed clear cell and endometrioid
type adenocarcinoma of the extragonadal, rectovaginal septum,
arising from endometriosis (deep infiltrating endometriosis) and
associated with a differentiated, endometrioid endometrial
carcinoma (10). Since
endometriosis is frequently found in association with malignancies,
it could be viewed as a neoplastic process. Endometriosis might
also be the product of numerous predisposing factors such as
genetic abnormalities and genomic imbalances in specific
chromosomes for the development of neoplasms (11). Accumulating evidence suggests a role
for genetic activation and inactivation mutations. For example,
ovarian cancers and adjacent endometriotic lesions have shown
common genetic alterations, suggesting a possible malignant genetic
transition spectrum (12,13).
The exact incidence of malignant transformation of
endometriosis is difficult to ascertain, since a strict criterion
is the demonstration of a histologically proven transition from
benign precursors to malignant lesions (14). Nonetheless, malignant transformation
is not a rare complication of endometriosis and is estimated to
occur in 0.6–0.8% of women with ovarian endometriosis (15,16).
The frequency of malignancy in surgically proven endometriosis is
>10% (9).
Cases of surgically proven endometriosis were
reviewed to evaluate the associated types of pelvic cancers.
Adenocarcinoma is the most common (70%), and sarcoma is the second
most common malignancy (12%) (17).
The ovary was the primary site in 79% of the cases with a malignant
tumor arising in endometriosis, whereas extragonadal sites
represented 21% of the cases (17).
In the ovary, endometrioid adenocarcinomas accounted for 69% of
disease, clear cell carcinomas 14%, sarcomas 12%, and rare cell
types 6% (17). By contrast, clear
cell carcinoma and adenosarcoma were most commonly observed in
extraovarian endometriosis (9). A
specific link between endometriosis and endometrioid and/or clear
cell carcinoma of the ovary has been reported, with an odds ratio
ranging between 3.7 and 35.4 (confidence interval 95%) (18). Kobayashi et al evaluated the
risk of ovarian cancer based on varying time periods from the time
of diagnosis of ovarian endometriosis (19). During a follow-up period of up to 17
years, 46 incidental ovarian cancers were identified, translating
into a standardized incidence ratio of 8.95. This risk increased
with age, with an incidence ratio of 13.2 in women over 50 years of
age (19). This study emphasizes
the difficulties associated with a diagnosis of malignancies
arising from endometriosis.
4. Endometrioid adenocarcinoma of the
ovary
Compared with the general population, women with
endometriosis have a higher risk of developing epithelial ovarian
cancer. The association between endometriosis and endometrioid and
clear cell carcinoma of the ovary has been frequently described in
the medical literature and is much stronger than that of serous and
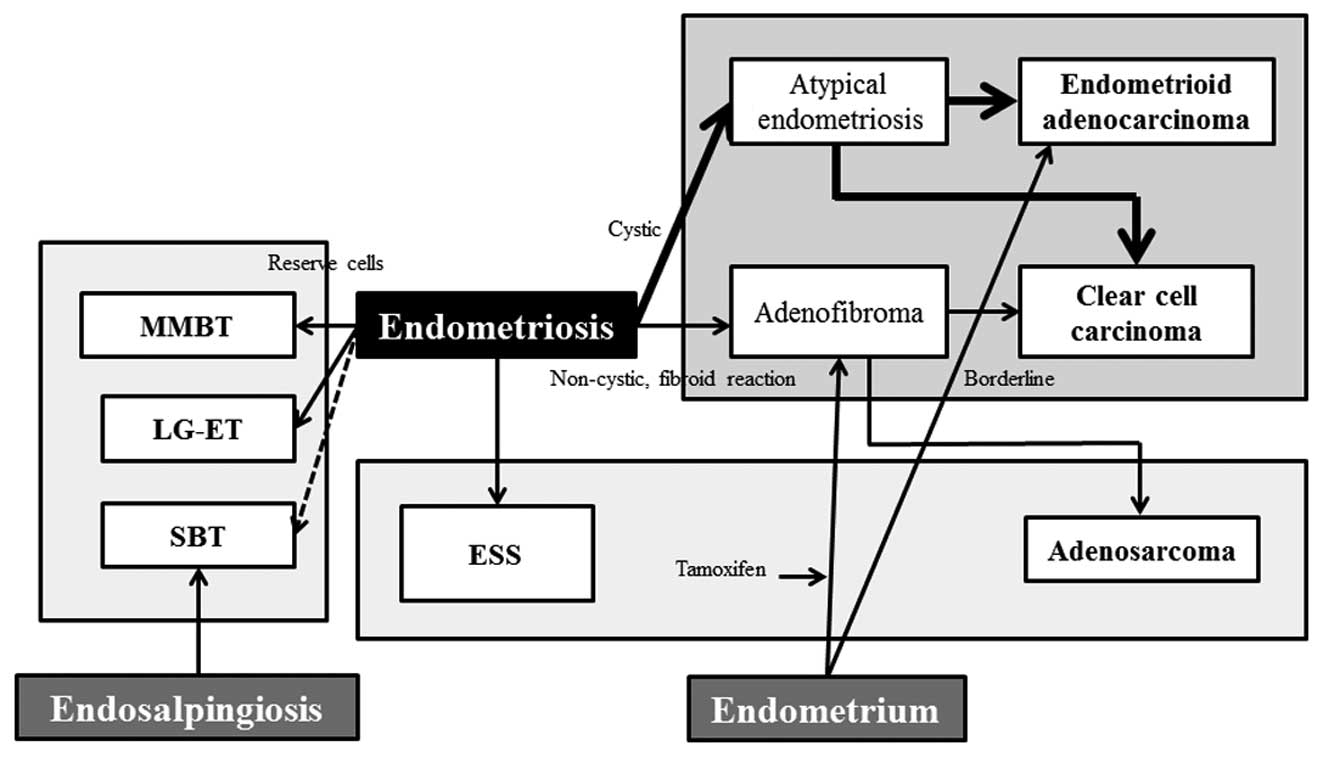
mucinous tumors (Fig. 2) (9). Endometriosis-associated ovarian cancer
generally affects women who are younger, diagnosed in earlier
stages, have lower grade lesions and a better prognosis than those
affected by ovarian cancer without endometriosis (20). The majority of cases of endometrioid
adenocarcinoma typically arise from endometriosis. It is common to
find positivity for estrogen and progesterone receptors, suggesting
that estrogen stimulation is somehow involved in tumor pathogenesis
(9). Estrogen receptor expression
is significantly more common in endometrioid than serous
adenocarcinomas and clear cell carcinoma of the ovary. Tamoxifen,
as a result of its estrogenic effects, may cause malignant changes
in endometriosis (21). Martin also
reported that there may be an increased risk of developing
endometrioid adenocarcinoma in hormone replacement management in
patients with endometriosis (22).
Endometrioid adenocarcinoma of the ovary exhibits
activation of Wnt signaling and somatic mutations of CTNNB1
[encoding β-catenin [cadherin-associated protein)], PTEN
(phosphatase and tensin homolog) and PIK3CA
(phosphoinositide-3-kinase, catalytic, α polypeptide) (23). Wnt/β-catenin pathway defects are key
contributing factors in the cancer phenotype. Recently, Wiegand
et al (12) and Jones et
al (24) published new data
suggesting AT-rich interactive domain-containing protein 1A
(ARID1A) as a tumor-suppressor gene disrupted in ovarian clear cell
and endometrioid adenocarcinomas. Other authors also showed that
the ARID1A complex interacts with p53 (25). Therefore, ARID1A is a tumor
suppressor gene that collaborates with p53 to regulate tumor growth
and the cell cycle.
5. Clear cell carcinoma of the ovary
The clear cell carcinoma evolution from ovarian
endometriosis is similar to the process that occurs in endometrioid
adenocarcinoma, a type I tumor (26). In their study, Mandai et al
have shown that a stressful microenvironment within the
endometrioma may lead to cancer development by inducing unique gene
expressions, the so-called ‘Clear Cell Carcinoma Signature’
(27). Several investigators have
hypothesized that collection of blood inside endometriomas would
lead to iron-induced oxidative stress on endometriotic cells
(28,29). This would explain the reason for
endometriosis-associated cancers developing more frequently in the
ovarian than the extragonadal sites.
Two types of clear cell carcinomas, non-cystic
(adenofibroma) and cystic (endometrioma), appear to be derived from
endometriosis (Fig. 2) (26). The former involves the step-wise
progression sequence of adenofibromas, atypical proliferative
(borderline) tumors, and clear cell carcinoma (26). This adenofibroma-clear cell
carcinoma sequence develops from non-cystic endometriosis and is
associated with adenofibromatous components (26). The latter arises from ovarian
endometrioma and is not associated with an adenofibromatous
background (26). The two pathways
can overlap. Both forms of clear cell carcinoma development are
closely associated with type I tumors. In certain cases, there were
foci of benign and borderline endometrioid adenofibroma in the same
ovary (21). A synchronous
endometrioid endometrial adenocarcinoma has sometimes been observed
in the uterus (21).
Endometriosis-associated clear cell carcinoma has a
high percentage of PIK3CA-activating mutations and ARID1A
inactivation mutations (30).
PIK3CA and ARID1A are early events in carcinogenesis, probably
initiating the malignant transformation of endometriosis. In
addition, the platelet-derived growth factor (PDGF) pathway was
activated in the adenofibroma-clear cell carcinoma sequence
(31). This suggests biological
differences between clear cell carcinomas that arise in association
with adenofibroma vs. endometriosis. Recent genome-wide expression
analyses have demonstrated the specific expression of hepatocyte
nuclear factor (HNF)-1β in endometriosis and clear cell carcinoma,
but not endometrioid adenocarcinoma, suggesting that early
differentiation into the clear cell lineage occurs in the
endometriosis with HNF-1β overexpression (32). The HNF-1β-dependent pathway provides
new insights into the regulation of apoptosis, the cell cycle,
glycogen synthesis and chemoresistance (32). It has been reported that HNF-1β gene
might play a role in carcinogenesis, pathogenesis, and the
pathophysiology of clear cell carcinoma of the ovary (32).
6. Serous borderline tumor
Atypical endometriosis was rarely found in serous
borderline tumor (SBT) (33,34).
Patients with peritoneal SBT also had typical endosalpingiosis
(35). Endosalpingiosis is a benign
condition and describes the ectopic growth of ciliated fallopian
tubal-type epithelium (Fig. 2).
Although peritoneal SBT is a rare entity, it is of note since this
disorder shows close macroscopic similarity to endometriosis and
pathologically microscopic similarity to other Müllerian
proliferations with malignant potential (36).
Several hypotheses have been proposed regarding the
development of SBT. The different models can be traced back to at
least two pathological theories: implantation theory and
metaplasia/embryonic theory. A popular hypothesis concerning the
pathogenesis of this rare condition is that endometrial cells or
tubal cells are transported by various routes (transtubal,
hematogenous, lymphogenous or by direct apposition) and implanted
in the affected organ and subsequently have a malignant potential.
Another hypothesis is that a secondary Müllerian system exists,
thus Müllerian ectopic lesions are the result of metaplastic
processes in the target organ or from scattered embryonic rest.
Previous studies have demonstrated that the origin of the majority
of low-grade serous tumors or SBT is presumed to be ectopic
Müllerian epithelium (37). Thus,
the epithelium of SBT may derive from the salpinx, endometriosis,
or ovarian surface epithelium that is capable of Müllerian
metaplasia (37). Endosalpingiosis,
endometriosis and endocervicosis constitute the triad of
non-neoplastic disorders of the Müllerian system (38). Non-neoplastic glandular
proliferation showing spontaneous Müllerian differentiation has
been described in numerous sites including the vagina, uterine
cervix, urinary bladder, appendix, peritoneum, abdominal wall
(inguinal channel, umbilicus) and the lymph nodes (38,39).
Gene expression profiling studies suggest that SBT
is genetically distinct from high-grade serous cancers (40). Ethnic and geographical variation may
exist in the process of SBT carcinogenesis. Extensive molecular
work-up demonstrates an increased frequency of KRAS (v-Ki-ras2
Kirsten rat sarcoma viral oncogene homolog), BRAF (v-raf murine
sarcoma viral oncogene homolog B1), or ERBB2 (v-erb-b2
erythroblastic leukemia viral oncogene homolog 2) mutations in a
subset of low-grade serous carcinomas, and a lack of TP53 (tumor
protein p53) mutations (40,41).
7. Müllerian mucinous borderline tumor
The mucinous borderline tumors are of intestinal
type or Müllerian (endocervical-like) type. The intestinal-type
tumors (intestinal-type mucinous borderline tumor, IMBT) are the
most common. Although Müllerian metaplasia is well recognized at
different sites within the female genital tract, the ovarian
mucinous borderline tumor of Müllerian type (MMBT) and mixed
epithelial borderline tumor of Müllerian type (MEBT) are uncommon
subtypes of ovarian tumors (42).
MMBT is composed of endocervical gland-like mucinous cells and
ciliated columnar epithelium reminiscent of the fallopian tube
(43). MMBT likely arises from
endometriosis, possibly through its precursor (42,44),
while IMBT is not associated with endometriosis (Fig. 2).
MMBT occurs with a relatively high frequency in
women of Japanese descent (43).
Compared with IMBT, the characteristic features of MMBT are as
follows: more frequent bilateral occurrence, paucilocular cysts,
association with endometriosis, absence of pseudomyxoma but a
possible association of peritoneal implants and lymph node
metastases, and a lower mortality rate (43). Other malignancies are also
associated with MMBT. D'Angelo et al reported a female
individual in whom a keratinizing squamous cell carcinoma of the
ovary had arisen from MMBT (45).
Hamada et al draw attention to the existence
of the reserve cell-like cells (RCLCs) in MMBT (42). The similarity to the cervical
reserve cells may indicate their potential role as precursor cells
that may subsequently differentiate into various Müllerian cell
types (42). Immunohistochemically,
all cases were reactive for estrogen receptor and progesterone
receptor, with no nuclear expression of β-catenin (46). There was reactivity for cytokeratin
(CK)7, but not CK20 (47). KRAS
mutations were detected in exon 1 and codon 12 in 69% of cases
(46,48). No PTEN mutation was identified in
any of the nine exons and PTEN immunoreactivity was diffuse in the
nuclei of epithelial and underlying stromal cells (46).
8. Adenosarcoma
Müllerian adenosarcoma is an uncommon, but not rare,
tumor which is characterized by an admixture of benign, but
occasionally atypical, epithelial and a malignant, usually
low-grade, stromal component (49).
The most common site is the uterine corpus endometrium (50). Adenosarcoma sometimes includes the
lower uterine segment and endocervix. There are also reports on
adenosarcoma arising from endometriosis (Fig. 2) (51,52).
Of note is the association between adenosarcoma and endometriosis,
especially in extrauterine forms (9).
Risk factors include pelvic irradiation,
hyperestrogenism, such as after prolonged estrogen stimulation or
long-term oral contraceptive use, and the use of tamoxifen and
tremifen for breast cancer (53).
Toremifene may be effective in cases resistant to tamoxifen. Among
the tamoxifen-induced uterine malignancies, the most common uterine
malignancy is endometrial adenocarcinoma. Chung et al
reported a case of an adenosarcoma after toremifene treatment in a
breast cancer patient (54). After
one year of toremifene treatment, the patient developed
adenofibroma. After an additional four years of treatment, this
lesion was transformed into adenosarcoma (54).
Adenosarcoma rarely occurs in the ovary, vagina or
fallopian tubes, arising from peritoneal surfaces, or outside the
female genital tract, for example in the intestine (50). However, a rare case of a clear cell
adenocarcinoma and an adenosarcoma coexisting with a heterologous
rhabdomyosarcoma in ovarian endometrioma has been reported
(55). Adenosarcoma also arises
within the myometrium from adenomyosis.
Uterine sarcomas are relatively rare tumors that
account for 1–3% of female genital tract malignancies and between
4–9% of uterine neoplasms (49).
Uterine sarcomas are divided into the following pathological types:
carcinosarcomas (50%), leiomyosarcomas (30%), endometrial stromal
sarcomas (10–15%), and adenosarcomas (5–10%). The increased
incidence rate of uterine sarcomas is due to an increase of
carcinosarcomas. These neoplasms may be divided into two major
groups: i) sarcomas showing specific genetic alterations (the
EWSR1-FLI1 gene fusion in Ewing's sarcoma), and ii) sarcomas
showing multiple and variable gene alterations without a specific
pattern (leiomyosarcoma and osteosarcoma).
The biological behaviors of adenosarcoma show low
malignant potential (56). Some of
the tumors currently classified as adenofibromas are, in fact,
well-differentiated adenosarcomas (57). The prognosis in
endometriosis-associated tumors is far better than in unassociated
mixed Müllerian tumors (9).
However, Müllerian adenosarcoma with sarcomatous overgrowth is an
extremely aggressive variant, even when diagnosed in its early
stage (49). The prognosis in
typical adenosarcomas without sarcomatous overgrowth is similar to
that of adenofibromas associated with favorable outcome (57). Müllerian adenosarcomas with
sarcomatous overgrowth showed strong immunoreactivity for Ki-67 and
p53 and loss of CD10 and progesterone receptor immunostaining.
9. Endometrial stromal sarcoma
Endometrial stromal tumors are rare uterine
neoplasms including benign stromal nodules, low-grade endometrial
stromal sarcomas (ESS), and undifferentiated endometrial sarcomas
(58). The origin of ESS is
explained by at least two hypotheses. One is the malignant
transformation of endometriosis (Fig.
2) (59). There have been some
reports in which ESS is considered to be associated with
endometriosis (59). However, the
exact incidence of ESS in endometriosis is not well described. The
nodules were composed of foci of adenomyosis and endometriosis
(with focal atypical complex hyperplasia) associated with a stromal
spindle cell population immunoreactive for estrogen and
progesterone receptors (60).
The other theory is a de novo tumor,
potentially derived from Müllerian cells (59). A report suggests that ESS is derived
from submesothelial pluripotential cells (61). The pluripotential Müllerian
epithelium is considered to be widely distributed in the abdominal
and pelvic cavities. Therefore, ESS may originate from
endometriosis or from metaplasia of the pelvic Müllerian cells
(62). Among patients with ESS of
ovarian origin, 11% were reported to have succumbed to disease,
compared to 38% of patients with ESS of extraovarian origin,
suggesting that extraovarian origin indicates a poor prognosis
(63).
Immunohistochemical features of ESS include
reactivity to estrogen receptors, progesterone receptors, aromatase
and CD10 (21,61,64).
Immunohistochemical characteristics were not consistent with
Müllerian adenocarcinoma with sarcomatous overgrowth. In a previous
study, a gene fusion on chromosome 7 that includes two zinc-finger
genes (JAZF1 and JJAZ1) was identified in ESS (58). This genetic abnormality is specific
to ESS (58). Furthermore, loss of
heterozygosity of tumor suppressor genes and deregulation of the
Wnt signaling pathway have also been found to be involved in ESS
tumorigenesis (65). Notably, the
Wnt signaling inhibitor might play a role in the development of
endometriosis (66).
10. Discussion
Malignant transformation of endometriosis has been
described in numerous case reports and reviews of the literature.
Endometriosis-associated tumors that involve the female pelvic
cavity, i.e., the peritoneum, ovary and fallopian tubes are a
diverse group of disorders that range in biological behavior from
benign to malignant. Approximately 1.0% of women with endometriosis
have lesions that undergo malignant transformation (19,67).
Anatomically, lesions are divided into gonadal (80%) or
extragonadal (20%) lesions (17,67).
Endometriosis-associated epithelial ovarian cancers,
endometrioid adenocarcinoma and clear cell carcinoma, are the most
common tumors. Furthermore, other Müllerian-type borderline tumors
[MMBT, SBT and low-grade endometrial tumors (LG-ET)] may be
associated with endometriosis. Studies regarding the association of
sarcoma (adenosarcoma and ESS) with endometriosis have been
conducted. However, the relationship between endometriosis and
extraovarian malignancy, although noteworthy, has yet to be
investigated.
Knowledge of clinicopathological features of
secondary lesions is essential for the identification, differential
diagnosis and treatment strategies of endometriosis-associated
malignancies. When these tumors arise from endometriosis, they are
usually identified at an early stage and are often of low grade,
compared with tumors without endometriosis. Furthermore, a better
prognosis and lower mortality rate are the characteristic features.
In some patients, the diagnosis is relatively obvious since there
is clinical, imaging, or pathological evidence of endometriosis.
However, on occasion it may be necessary to distinguish this
disease from primary pelvic lesions such as malignant mesothelioma,
primary epithelial and mesenchymal tumors, or primary ovarian and
peritoneal high-grade serous carcinoma, as well as metastatic
tumors. Knowledge of histology, pathology and molecular genetics is
essential in the differential diagnosis of endometriosis-associated
malignancies.
Individual glands of the endometriotic lesions are
genetically derived from single precursor cells, suggesting that
endometriosis essentially expands monoclonally (68). Endometriotic lesions would harbor
somatic genetic changes in chromosomal regions that supposedly
contain genes involved in tumorigenesis. A molecular continuum and
lineage between the benign precursor and the malignant entity
require strong evidence of common mutational events. Endometriosis
and cancer may share similar risk factors. These findings would be
consistent with the progression model for tumorigenesis from the
benign precursor to malignant lesions.
Genome-wide gene expression profiling studies have
provided a first step for identifying molecules key to the
characteristics of endometriosis-associated neoplasms. Several
investigators have also generated mouse models that spontaneously
develop carcinoma similar to human ovarian cancer to understand the
molecular genetic events underlying carcinogenesis. Multiple
genetic alterations play a role in the pathogenesis of
endometriosis-associated neoplams. For example, a variety of
molecular events, such as p53 alteration, PTEN silencing, KRAS
activation mutation, ARID1A inactivation mutation, PIK3CA
activation mutation and HNF-1β activation, have been identified in
endometriosis-associated epithelial ovarian cancers (27). KRAS, BRAF, or ERBB2 mutations have
been reported in SBT (40,41). MMBT also showed KRAS mutations
(46,48). Wnt signaling dysregulation has been
suggested to play a role in ESS tumorigenesis (65).
Although a number of crucial pathways involved in
carcinogenesis have been identified, knowledge of the early steps
remains poorly understood. Mandai et al found that
microenvironmental factors, including oxidative stress and
inflammation, are crucial in endometriosis-associated ovarian
carcinogenesis (27). Retrograde
menstruation or ovarian hemorrhage carries highly pro-oxidant
factors, such as heme and iron, into the peritoneal cavity or
ovarian endometrioma (29). A
histologically normal ectopic endometrium may bear genetic damage
caused by iron-dependent oxidative stress (29). Persistent oxidative stress has been
associated with carcinogenesis (69,70).
Iron overload is considered as one such condition that causes
oxidative stress via the Fenton reaction, thus promoting the
generation of the undesirable free radical molecule, •OH
(69). Abnormal iron uptake leads
to lipids, proteins and DNA damage derived from free radical
toxicity. In general, hemochromatosis, chronic viral hepatitis,
inflammatory bowel diseases such as Crohn's disease and ulcerative
colitis or asbestosis induce iron overload, which may lead to
hepatocellular carcinoma, colon cancer or mesothelioma in humans
(69). Several investigators have
demonstrated that iron-induced oxidative stress is also able to
induce specific gene modifications in endometriosis-associated
ovarian cancer (27,28,71–74).
Undefined molecular events occurring in endometriosis that has
undergone Müllerian metaplasia may initiate neoplastic change
towards not only carcinoma, but also sarcoma. Although the
molecular changes in other Müllerian-type tumors and sarcomas
remain largely unknown, DNA damage caused by oxidative stress may
be a critical factor in the early event of the malignant
transformation process of endometriosis.
In conclusion, the malignant processes that are
associated with endometriosis may be classified into: i) epithelial
ovarian cancers (endometrioid adenocarcinoma and clear cell
carcinoma), ii) other Müllerian-type tumors (MMBT, SBT and LG-ET),
as well as iii) sarcomas (adenosarcoma and ESS) in the female
pelvic cavity (Fig. 2). The
molecular pathology of endometriosis-associated tumorigenesis is
heterogeneous and may involve various putative precursor lesions
and multiple pathways of development, possibly via genetic
alteration by oxidative stress.
Acknowledgements
This study was supported by a grant [Grant-in-aid
for Scientific Research (H. Kobayashi)] from the Ministry of
Education, Science, and Culture of Japan.
References
|
1
|
Rogers PA, D'Hooghe TM, Fazleabas A, et
al: Priorities for endometriosis research: recommendations from an
international consensus workshop. Reprod Sci. 16:335–346. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gemmill JA, Stratton P, Cleary SD, Ballweg
ML and Sinaii N: Cancers, infections, and endocrine diseases in
women with endometriosis. Fertil Steril. 94:1627–1631. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Jefout M, Tokushige N, Hey-Cunningham
AJ, et al: Microanatomy and function of the eutopic endometrium in
endometriosis. Expert Rev Obstet Gynecol. 4:61–79. 2009. View Article : Google Scholar
|
|
4
|
Brinton LA, Gridley G, Persson I, Baron J
and Bergqvist A: Cancer risk after a hospital discharge diagnosis
of endometriosis. Am J Obstet Gynecol. 176:572–579. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brinton LA, Lamb EJ, Moghissi KS, et al:
Ovarian cancer risk associated with varying causes of infertility.
Fertil Steril. 82:405–414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kokcu A: Relationship between
endometriosis and cancer from current perspective. Arch Gynecol
Obstet. 284:1473–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nezhat F, Datta MS, Hanson V, Pejovic T
and Nezhat C and Nezhat C: The relationship of endometriosis and
ovarian malignancy: a review. Fertil Steril. 90:1559–15570. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grammatikakis I, Zervoudis S,
Evangelinakis N and Tziortzioti V: Endometrium and ovarian cancer
synchronous to endometriosis - a retrospective study of our
experience of 7 years. J Med Life. 3:76–79. 2010.PubMed/NCBI
|
|
9
|
Stern RC, Dash R, Bentley RC, Snyder MJ,
Haney AF and Robboy SJ: Malignancy in endometriosis: frequency and
comparison of ovarian and extraovarian types. Int J Gynecol Pathol.
20:133–139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mabrouk M, Vicenzi C, Ferrini G, et al:
Mixed adenocarcinoma of the rectovaginal septum associated with
endometriosis and endometrial carcinoma: a case report. Case Rep
Oncol. 4:149–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Veiga-Castelli LC, Silva JC, Meola J, et
al: Genomic alterations detected by comparative genomic
hybridization in ovarian endometriomas. Braz J Med Biol Res.
43:799–805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wiegand KC, Shah SP, Al-Agha OM, et al:
ARID1A mutations in endometriosis-associated ovarian carcinomas. N
Engl J Med. 363:1532–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto S, Tsuda H, Takano M, Iwaya K,
Tamai S and Matsubara O: PIK3CA mutation is an early event in the
development of endometriosis-associated ovarian clear cell
adenocarcinoma. J Pathol. 225:189–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scott RB: Malignant changes in
endometriosis. Obstet Gynecol. 2:283–289. 1953.
|
|
15
|
Scully RE, Richardson GS and Barlow JF:
The development of malignancy in endometriosis. Clin Obstet
Gynecol. 9:384–411. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omranipour R and Najafi M: Papillary
serous carcinoma arising in abdominal wall endometriosis treated
with neoadjuvant chemotherapy and surgery. Fertil Steril.
93:1347.e17–e18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heaps JM, Nieberg RK and Berek JS:
Malignant neoplasms arising in endometriosis. Obstet Gynecol.
75:1023–1028. 1990.PubMed/NCBI
|
|
18
|
Sayasneh A, Tsivos D and Crawford R:
Endometriosis and ovarian cancer: a systematic review. ISRN Obstet
Gynecol. 2011:1403102011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi H, Sumimoto K, Moniwa N, et al:
Risk of developing ovarian cancer among women with ovarian
endometrioma: a cohort study in Shizuoka, Japan. Int J Gynecol
Cancer. 17:37–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takeuchi M, Matsuzaki K, Uehara H and
Nishitani H: Malignant transformation of pelvic endometriosis: MR
imaging findings and pathologic correlation. Radiographics.
26:407–417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCluggage WG, Bryson C, Lamki H and Boyle
DD: Benign, borderline, and malignant endometrioid neoplasia
arising in endometriosis in association with tamoxifen therapy. Int
J Gynecol Pathol. 19:276–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin DC: Cancer and endometriosis: do we
need to be concerned? Semin Reprod Endocrinol. 15:319–324. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar
|
|
24
|
Jones S, Wang TL, Shih IeM, et al:
Frequent mutations of chromatin remodeling gene ARID1A in ovarian
clear cell carcinoma. Science. 330:228–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guan B, Wang TL and Shih IM: ARID1A, a
factor that promotes formation of SWI/SNF-mediated chromatin
remodeling, is a tumor suppressor in gynecologic cancers. Cancer
Res. 71:6718–6727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao C, Wu LS and Barner R: Pathogenesis
of ovarian clear cell adenofibroma, atypical proliferative
(borderline) tumor, and carcinoma: clinicopathologic features of
tumors with endometriosis or adenofibromatous components support
two related pathways of tumor development. J Cancer. 2:94–106.
2011. View
Article : Google Scholar
|
|
27
|
Mandai M, Matsumura N, Baba T, Yamaguchi
K, Hamanishi J and Konishi I: Ovarian clear cell carcinoma as a
stress-responsive cancer: influence of the microenvironment on the
carcinogenesis and cancer phenotype. Cancer Lett. 310:129–133.
2011. View Article : Google Scholar
|
|
28
|
Yamaguchi K, Mandai M, Toyokuni S, et al:
Contents of endometriotic cysts, especially the high concentration
of free iron, are a possible cause of carcinogenesis in the cysts
through the iron-induced persistent oxidative stress. Clin Cancer
Res. 4:32–40. 2008. View Article : Google Scholar
|
|
29
|
Kobayashi H, Kajiwara H, Kanayama S, et
al: Molecular pathogenesis of endometriosis-associated clear cell
carcinoma of the ovary (Review). Oncol Rep. 22:233–240.
2009.PubMed/NCBI
|
|
30
|
Kuo KT, Mao TL, Jones S, et al: Frequent
activating mutations of PIK3CA in ovarian clear cell carcinoma. Am
J Pathol. 174:1597–1601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamamoto S, Tsuda H, Takano M, et al:
Expression of platelet-derived growth factors and their receptors
in ovarian clear-cell carcinoma and its putative precursors. Mod
Pathol. 21:115–124. 2008.PubMed/NCBI
|
|
32
|
Kobayashi H, Yamada Y, Kanayama S, et al:
The role of hepatocyte nuclear factor-1β in the pathogenesis of
clear cell carcinoma of the ovary. Int J Gynecol Cancer.
19:471–479. 2009.
|
|
33
|
Fukunaga M, Nomura K, Ishikawa E and
Ushigome S: Ovarian atypical endometriosis: its close association
with malignant epithelial tumours. Histopathology. 30:249–255.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Srinivasan R, Gupta N and Gupta I:
Recurrent endocervical-like mucinous borderline tumor (ELMBT)
arising in a case of long-standing endometriosis - a case report.
Indian J Pathol Microbiol. 49:277–278. 2006.PubMed/NCBI
|
|
35
|
Biscotti CV and Hart WR: Peritoneal serous
micropapillomatosis of low malignant potential (serous borderline
tumors of the peritoneum). A clinicopathologic study of 17 cases.
Am J Surg Pathol. 16:467–745. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tinelli A, Malvasi A and Pellegrino M: An
incidental peritoneal serous borderline tumor during laparoscopy
for endometriosis. Eur J Gynaecol Oncol. 30:579–582.
2009.PubMed/NCBI
|
|
37
|
Carlson J, Roh MH, Chang MC and Crum CP:
Recent advances in the understanding of the pathogenesis of serous
carcinoma: the concept of low- and high-grade disease and the role
of the fallopian tube. Diagn Histopathol (Oxford). 14:352–365.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McCoubrey A, Houghton O, McCallion K and
McCluggage WG: Serous adenocarcinoma of the sigmoid mesentery
arising in cystic endosalpingiosis. J Clin Pathol. 58:1221–1223.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cil AP, Atasoy P and Kara SA: Myometrial
involvement of tumor-like cystic endosalpingiosis: a rare entity.
Ultrasound Obstet Gynecol. 32:106–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kurman RJ and Shih IeM: The origin and
pathogenesis of epithelial ovarian cancer: a proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mayr D, Hirschmann A, Löhrs U and Diebold
J: KRAS and BRAF mutations in ovarian tumors: a comprehensive study
of invasive carcinomas, borderline tumors and extraovarian
implants. Gynecol Oncol. 103:883–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hamada T, Kiyokawa T, Nomura K and Hano H:
Immunohistochemical analysis of reserve cell-like cells of ovarian
müllerian mucinous/mixed epithelial borderline tumor. Int J Gynecol
Pathol. 27:199–206. 2008.PubMed/NCBI
|
|
43
|
Moriya T, Mikami Y, Sakamoto K, et al:
Endocervical-like mucinous borderline tumors of the ovary:
clinicopathological features and electron microscopic findings. Med
Electron Microsc. 36:240–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee KR and Nucci MR: Ovarian mucinous and
mixed epithelial carcinomas of mullerian (endocervical-like) type:
a clinicopathologic analysis of four cases of an uncommon variant
associated with endometriosis. Int J Gynecol Pathol. 22:42–51.
2003. View Article : Google Scholar
|
|
45
|
D'Angelo E, Dadmanesh F, Pecorelli S and
Prat J: Squamous cell carcinoma of the ovary arising from a
mucinous cystic tumor of endocervical (müllerian) type. Int J
Gynecol Pathol. 29:529–532. 2010.PubMed/NCBI
|
|
46
|
Kim KR, Choi J, Hwang JE, et al:
Endocervical-like (Müllerian) mucinous borderline tumours of the
ovary are frequently associated with the KRAS mutation.
Histopathology. 57:587–596. 2010.
|
|
47
|
Shin JH, Bae JH, Lee A, et al: CK7, CK20,
CDX2 and MUC2 Immunohistochemical staining used to distinguish
metastatic colorectal carcinoma involving ovary from primary
ovarian mucinous adenocarcinoma. Jpn J Clin Oncol. 40:208–213.
2010. View Article : Google Scholar
|
|
48
|
Auner V, Kriegshäuser G, Tong D, et al:
KRAS mutation analysis in ovarian samples using a high sensitivity
biochip assay. BMC Cancer. 9:1112009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Patrelli TS, Gizzo S, Di Gangi S, Guidi G,
Rondinelli M and Nardelli GB: Cervical Mullerian adenosarcoma with
heterologous sarcomatous overgrowth: a fourth case and review of
literature. BMC Cancer. 11:2362011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McCluggage WG: Mullerian adenosarcoma of
the female genital tract. Adv Anat Pathol. 17:122–129. 2010.
View Article : Google Scholar
|
|
51
|
Han X, Leng J, Guo L, Xiang Y and Lang J:
Vaginal adenosarcoma arising from refractory endometriosis: a case
report. Aust N Z J Obstet Gynaecol. 50:574–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Patrelli TS, Silini EM, Gizzo S, et al:
Extragenital Müllerian adenosarcoma with pouch of Douglas location.
BMC Cancer. 11:1712011.
|
|
53
|
Singh R, Shameema S, Vijaya K and Kumar P:
Mullerian adenosarcoma of the uterus with sarcomatous overgrowth.
Clin Med Insights Case Rep. 3:27–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chung YW, Bae HS, Han SI, Song JY, Kim IS
and Kang JS: Endometrial mullerian adenosarcoma after toremifene
treatment in breast cancer patients: a case report. J Gynecol
Oncol. 21:269–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yasuoka H, Tsujimoto M, Fujita S, et al:
Coexistence of a clear cell adenocarcinoma and an adenosarcoma with
a heterologous rhabdomyosarcoma in an endometriotic cyst of the
ovary: a case study. Int J Gynecol Pathol. 28:362–366. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Clement PB and Scully RE: Mullerian
adenosarcoma of the uterus. A clinicopathologic analysis of ten
cases of a distinctive type of mullerian mixed tumor. Cancer.
34:1138–1149. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gallardo A and Prat J: Mullerian
adenosarcoma: a clinicopathologic and immunohistochemical study of
55 cases challenging the existence of adenofibroma. Am J Surg
Pathol. 33:278–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hrzenjak A, Moinfar F, Tavassoli FA, et
al: JAZF1/JJAZ1 gene fusion in endometrial stromal sarcomas:
molecular analysis by reverse transcriptase-polymerase chain
reaction optimized for paraffin-embedded tissue. J Mol Diagn.
7:388–395. 2005. View Article : Google Scholar
|
|
59
|
Jung SI, Shin SS, Choi C, Hwang EC, Kim SO
and Kang TW: Endometrial stromal sarcoma presenting as prevesical
mass mimicking urachal tumor. J Korean Med Sci. 24:529–531. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Froio E, Piana S, Cavazza A, Valli R,
Abrate M and Gardini G: Multifocal PEComa (PEComatosis) of the
female genital tract associated with endometriosis, diffuse
adenomyosis, and endometrial atypical hyperplasia. Int J Surg
Pathol. 16:443–446. 2008. View Article : Google Scholar
|
|
61
|
Kim JY, Hong SY, Sung HJ, Oh HK and Koh
SB: A case of multiple metastatic low-grade endometrial stromal
sarcoma arising from an ovarian endometriotic lesion. J Gynecol
Oncol. 20:122–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Khan AW, Craig M, Jarmulowicz M and
Davidson BR: Liver tumours due to endometriosis and endometrial
stromal sarcoma. HPB (Oxford). 4:43–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Baiocchi G, Kavanagh JJ and Wharton JT:
Endometrioid stromal sarcomas arising from ovarian and extraovarian
endometriosis: report of two cases and review of the literature.
Gynecol Oncol. 36:147–151. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Roşca E, Venter A, Muţiu G, Drăgan A,
Coroi M and Roşca DM: Endometrial stromal sarcoma developed on
outer endometriosis foci. Rom J Morphol Embryol. 52:489–492.
2011.PubMed/NCBI
|
|
65
|
Chiang S and Oliva E: Cytogenetic and
molecular aberrations in endometrial stromal tumors. Hum Pathol.
42:609–617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cheng CW, Smith SK and Charnock-Jones DS:
Transcript profile and localization of Wnt signaling-related
molecules in human endometrium. Fertil Steril. 90:201–204. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Irvin W, Pelkey T, Rice L and Andersen W:
Endometrial stromal sarcoma of the vulva arising in extraovarian
endometriosis: a case report and literature review. Gynecol Oncol.
71:313–316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Viganó P, Somigliana E, Chiodo I, Abbiati
A and Vercellini P: Molecular mechanisms and biological
plausibility underlying the malignant transformation of
endometriosis: a critical analysis. Hum Reprod Update. 12:77–89.
2006.PubMed/NCBI
|
|
69
|
Toyokuni S: Mysterious link between iron
overload and CDKN2A/2B. J Clin Biochem Nutr. 48:46–49. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Toyokuni S: Molecular mechanisms of
oxidative stress-induced carcinogenesis: from epidemiology to
oxygenomics. IUBMB Life. 60:441–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kajihara H, Yamada Y, Kanayama S, et al:
Clear cell carcinoma of the ovary: Potential pathogenic mechanisms.
Oncol Rep. 23:1193–1203. 2010.PubMed/NCBI
|
|
72
|
Yamada Y, Shigetomi H, Onogi A, et al:
Redox-active iron-induced oxidative stress in the pathogenesis of
clear cell carcinoma of the ovary. Int J Gynecol Cancer.
21:1200–1207. 2011.PubMed/NCBI
|
|
73
|
Mandai M, Yamaguchi K, Matsumura N, Baba T
and Konishi I: Ovarian cancer in endometriosis: molecular biology,
pathology, and clinical management. Int J Clin Oncol. 14:383–391.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yamaguchi K, Mandai M, Oura T, et al:
Identification of an ovarian clear cell carcinoma gene signature
that reflects inherent disease biology and the carcinogenic
processes. Oncogene. 29:1741–1752. 2010. View Article : Google Scholar : PubMed/NCBI
|