Introduction
Liver metastasis occurs in almost 50% of patients
with colon cancer during the course of the disease (1). Confined liver metastasis from colon
cancer may be successfully treated by surgical resection with a
5-year survival ranging from 20 to 40% (2). However, of those patients who undergo
surgical resection for liver metastasis, more than two thirds
experience recurrence (3–5). Therefore, liver metastasis is a
crucial issue for the treatment of colon cancer and it would be
invaluable to develop predictive markers for screening high risk
groups of patients for liver metastasis and prognostic markers for
recurrence following liver resection. Although numerous studies
have reported prognostic factors for recurrence and survival
following hepatectomy and predicive factors for liver metastasis
our current knowledge remains incomplete. Many studies regarding
this issue have been carried out. However, the majority of previous
studies investigated only clinical parameters. Recent studies in
search of prognostic factors for cancer have involved molecular
markers that are known to be associated with the mechanism of the
disease (6,7). Thus, investigating the molecular
markers that are correlated with colon cancer liver metastasis may
improve our understanding of its prognosis. Currently, a limited
number of studies are available that cover not only clinical
parameters but also molecular markers.
The purpose of this study was to develop
predictive/prognostic markers for liver metastasis and recurrence
following liver resection, investigating not only clinical
parameters but also molecular markers that are known to be involved
in the process of liver metastasis.
Materials and methods
Patients, treatment and follow-up
protocol
We prospectively enrolled 70 colon cancer patients
who underwent surgery between August 2004 and June 2006. The
eligibility criteria included age between 20 and 70 years, biopsy
proven adenocarcinoma arising from the colon or rectum,
radiologically confirmed M0 or with resectable synchronous liver
metastasis (M1). Exclusion criteria were: patients with
unresectable liver metastasis or extrahepatic metastasis, history
of cancer within five years, poor medical condition that disallowed
surgery or chemotherapy, and pregnancy. Resectability was
determined by the Colorectal Tumor Board at Severance Hospital,
Yonsei University Health System, Seoul, Korea, which is composed of
surgical and medical oncologists as well as radiologists and
pathologists. Abdominal computed tomography (CT) scans and/or
positron emission tomography (PET) ruled out extrahepatic
metastasis. Enrolled patients were classified into two groups:
group A comprised patients without liver metastasis and group B
comprised patients with synchronous liver metastasis.
The patients received neither chemotherapy nor
radiation therapy prior to surgery. The patients in group A
received curative surgery for the primary tumor (i.e., colon
cancer) only and postoperative adjuvant chemotherapy was
administered on the basis of their pathological reports. In group
B, the patients received surgery for the primary tumor plus liver
resection with curative intent and all were recommended to receive
postoperative chemotherapy. The regimen was decided by the treating
physician. Postoperative follow-up was carried out every three
months. Radiological evaluation with CT and/or PET-CT was carried
out every six months to detect any recurrence.
The study was approved by the Institutional Review
Board, and informed consent was obtained either from the patient or
the patient’s family.
Selection of candidate molecules
To determine potential candidate molecules for this
study, we searched the medical database PubMed using the keywords:
colon neoplasms, liver metastasis and biological markers. We
limited our search scope to studies involving adults above the age
of 19 and human subjects only. We only included English language
publications. Following retrieval of the list of publications we
limited our focus to molecules that could be detected in the blood
and that could be analyzed by enzyme-linked immune specific assay
(ELISA) with commercially available antibodies. We then reviewed
the body of relevant studies published and selected the seven
molecules that were most widely studied and were known to be
involved in each step of the liver metastatic process (Table I).
 | Table ISelected molecular markers: previous
study results and the suggested relationship with colon cancer
liver metastasis. |
Table I
Selected molecular markers: previous
study results and the suggested relationship with colon cancer
liver metastasis.
| Molecule | Previous study
results and suggested mechanism (Refs.) |
|---|
| MMP-7
(matrilysin) | Overexpressed in
liver metastasis (13,14); degrades basement membrane and
activates gelatinases (15,16) |
| TIMP-1 | Higher serum level in
patients with liver metastasis (10,11);
growth stimulation and inhibition of apoptosis (17) |
| E-selectin | Overexpressed in
patients with metastasis (18,19);
anchoring to target organs (20,21) |
| CEA | Stimulates Kupffer
cells to enhance cancer cell adhesion (22) |
| Osteopontin | Correlates with
cancer progression, silencing suppresses metastasis (23,24) |
| VEGF | Correlates with
cancer progression (8,25) |
| HGF | Correlates with
cancer progression (9,17) |
Blood sampling and ELISA
Blood samples (5 ml) were drawn from the peripheral
vein (PV) and tumor drainage vein (DV) and placed in plain tubes.
PV blood was obtained 1 h prior to surgical incision. DV blood was
obtained prior to the ligation of any branch of the superior
mesenteric vein in patients with proximal colon cancer and prior to
the ligation of the inferior mesenteric vein in patients with
distal colon and rectal cancer. Samples were immediately
centrifuged and plasma and serum were separately stored at −70°C
until analysis. ELISA for all seven molecules was performed using
commercially available kits according to the manufacturer’s
instructions (VEGF, HGF, E-selectin, MMP-7, TIMP-1, osteopontin:
R&D Systems, Inc., MN, USA; CEA: IBL-Hamburg GmbH, Hamburg,
Germany). Diluted serum was transferred to wells of plates
pre-coated with primary antibody. Following the recommended
incubation period, plates were washed with buffer solution, and
substrate solution was added and incubated as instructed. The wells
were developed with color-reagent. Stop solution was added to each
well after incubation and the optical density was measured at a
wavelength of 450 nm using automated optical densitometry. Each
sample was run in triplicate, and the mean value was used for
analysis. If the R2 of standard solutions was <0.98,
data from the plate were excluded.
Statistical analysis
Analyzed clinical parameters included age, gender,
the location of the tumor (right versus left colon), TNM stage,
histological grade and lymphovascular invasion. Data on molecular
factor levels are shown as the mean ± standard deviation (SD). The
Mann-Whitney U-test, Fisher’s exact test and McNemar test were used
to compare the differences in molecular factor levels between the
clinical and pathological features. Cox proportional hazards
analysis was used to evaluate the relationship between the
pathological features, plasma molecular factor levels and overall
survival. P<0.05 was considered statistically significant.
Results
Patient characteristics and factors
related to synchronous liver metastasis
The mean age of group B (58.8 years) was higher than
that of group A (56.5 years), but did not reach statistical
significance. In both groups A and B, pT3–4 occurred more
frequently than the other stages and lymph node metastasis was
found equally in both groups A and B (Table II). Overall groups A and B had
statistically identical clinical features with the exception of
lymphovascular invasion, the frequency of which was significantly
higher in group B (p=0.004). No surgery-related mortality was
reported in either group.
 | Table IIPatient characteristics. |
Table II
Patient characteristics.
| Clinical
variables | Group A (n=37) | Group B (n=33) | P |
|---|
| Mean age (years) | 56.5±10.0 | 58.8±10.2 | 0.903 |
| Male-to-female
ratio | 19:18 | 21:12 | 0.300 |
| pT |
| pT2 | 1 | 0 | >0.999 |
| pT3–pT4 | 36 | 33 | |
| pN |
| pN0 | 12 | 10 | 0.544 |
| pN1 | 9 | 5 | |
| pN2 | 16 | 18 | |
| Location |
| Right colon | 6 | 5 | 0.903 |
| Left
colon/rectum | 31 | 28 | |
| Histology |
| Well/mod. diff. | 30 | 26 | 0.811 |
| Poorly/mucinous | 7 | 7 | |
| Lymphovascular
invasion | 2 | 11 | 0.004 |
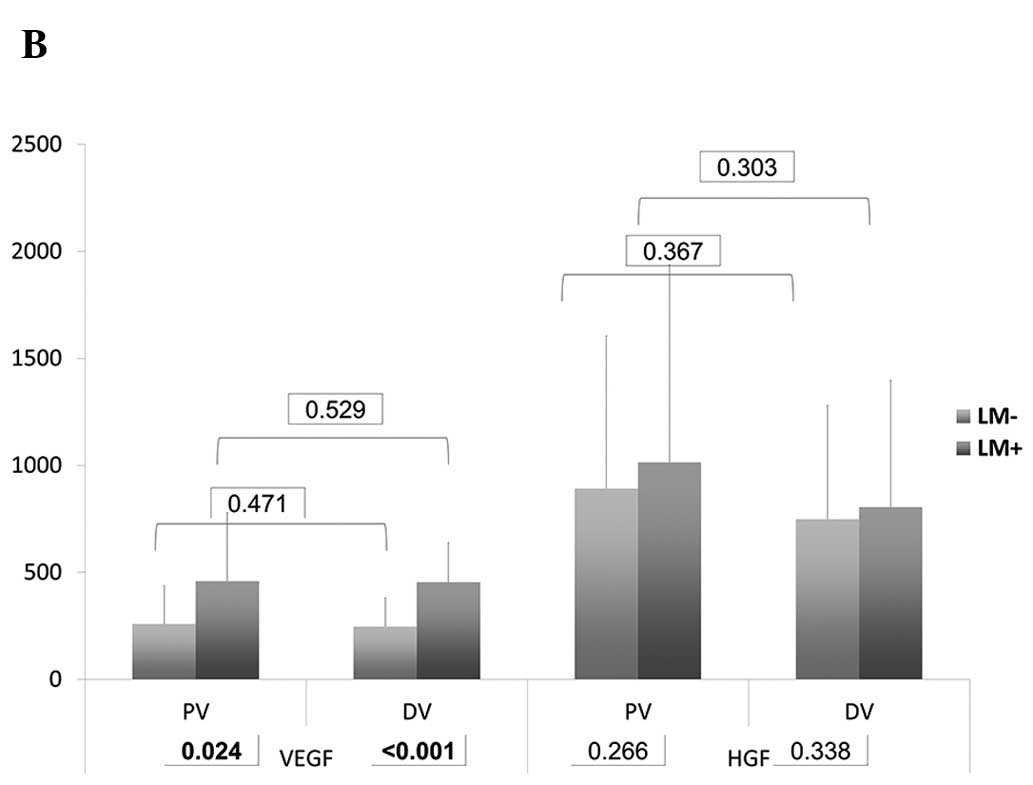
In the DV, the serum levels of VEGF (p<0.001) and
TIMP-1 (p<0.001) in group B were significantly higher than those
in group A. In PV, the serum levels of E-selectin (p=0.049), VEGF
(p=0.024) and TIMP-1 (p<0.001) demonstrated a significant
difference (Table III and
Fig. 1). Of note, the levels of
VEGF and TIMP were higher in group B, whereas the levels of
E-selectin were higher in group A.
 | Table IIIAnalyses of the levels of molecular
markers in tumor drainage and peripheral veins. |
Table III
Analyses of the levels of molecular
markers in tumor drainage and peripheral veins.
| | Group A | Group B | |
|---|
| |
|
| |
|---|
| Markers | | Value | Pa | Value | Pa | Pb |
|---|
| CEA | PV | 45.15±32.19 | <0.001 | 116.70±100.31 | <0.001 | 0.116 |
| DV | 135.46±91.31 | | 350.11±189.12 | | 0.057 |
| E-selectin | PV | 41.54±19.38 | 0.009 | 20.38±12.01 | 0.280 | 0.049 |
| DV | 24.37±10.01 | | 21.16±13.45 | | 0.311 |
| Osteopontin | PV | 27.21±27.12 | 0.344 | 50.17±40.70 | 0.102 | 0.068 |
| DV | 25.44±18.48 | | 31.24±19.78 | | 0.528 |
| VEGF | PV | 258.80±179.33 | 0.471 | 459.15±319.82 | 0.529 | 0.024 |
| DV | 246.58±134.29 | | 454.17±184.18 | | <0.001 |
| HGF | PV | 891.87±712.39 | 0.367 | 1015.1±921.63 | 0.303 | 0.266 |
| DV | 748.91±532.47 | | 805.69±591.17 | | 0.338 |
| MMP-7 | PV | 3.90±1.17 | 0.059 | 4.88±2.61 | 0.075 | 0.229 |
| DV | 4.63±1.21 | | 5.15±2.04 | | 0.517 |
| TIMP-1 | PV | 74.05±31.42 | 0.194 | 233.71±97.71 | 0.010 | <0.001 |
| DV | 101.9±87.27 | | 194.01±90.73 | | <0.001 |
Multivariate analyses were performed to identify
clinicopathological factors and molecular markers associated with
liver metastasis. The level of TIMP-1 from the DV was found to be
the most significantly different factor between groups A and B (HR
4.20; p=0.001) followed by the level of VEGF from DV (HR 4.87;
p=0.004). The levels of TIMP-1 from both the DV and the PV were
significantly different between groups A and B (Table IV). No clinical factors were found
to be significant in the multivariate analysis.
 | Table IVMultivariate analysis for factors
significantly associated with the presence of liver metastasis. |
Table IV
Multivariate analysis for factors
significantly associated with the presence of liver metastasis.
| Factors | HR | 95% CI | P |
|---|
| Depth of invasion
(pT2 vs. pT3–4) | 2.01 | 0.35–5.83 | 0.83 |
| Lymph node
metastasis | 3.42 | 0.44–7.21 | 0.35 |
| Lymphovascular
invasion | 2.51 | 0.87–6.33 | 0.093 |
| Histological grade
(G1–2 vs. G3–4) | 1.74 | 0.18–4.00 | 0.88 |
| E-selectin (PV)
(high vs. low) | 0.61 | 0.25–1.94 | 0.078 |
| VEGF (PV) (high vs.
low) | 4.32 | 0.97–29.07 | 0.054 |
| VEGF (DV) (high vs.
low) | 4.87 | 2.07–34.41 | 0.004 |
| TIMP-1 (PV) (high
vs. low) | 3.15 | 1.66–17.86 | 0.017 |
| TIMP-1 (DV) (high
vs. low) | 4.20 | 1.94–22.73 | 0.001 |
Factors associated with metachronous
liver metastasis
The median follow-up period for group A was 54
months (range, 33–78) and the 5-year disease-free and overall
survival rates were 64.1 and 77.4%, respectively. Of the 37
patients in group A, 4 patients developed local recurrence, 10
developed systemic recurrence and 3 developed both local and
systemic recurrence simultaneously. Of the 13 patients who had
systemic recurrence, 10 patients (27.0%) had metachronous liver
metastasis. The median time to recurrence was 27.3 months. We
investigated factors correlated with metachronous liver metastasis
by univariate and multivariate analyses (Table V), which revealed that only the
levels of VEGF and TIMP-1 from DV were found to be independent
prognostic factors for metachronous liver metastasis.
 | Table VUnivariate and multivariate analyses
for prognostic factors (metachronous liver metastasis) in group
A. |
Table V
Univariate and multivariate analyses
for prognostic factors (metachronous liver metastasis) in group
A.
| Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|---|
| Factors | P | P | HR (95% CI) |
|---|
| pT2 vs. pT3–4 | 0.209 | 0.567 | 2.03
(0.212–24.011) |
| pN0 vs. pN1–2 | 0.028 | 0.176 | 24.41
(0.039–72.029) |
| LVI (+ vs. −) | 0.433 | 0.629 | 10.42
(0.486–48.001) |
| Disease-free
interval (<1 yr vs. >1 yr) | 0.040 | 0.133 | 15.76
(0.203–62.761) |
| Adjuvant CTx (yes
vs. no) | 0.108 | 0.872 | 24.03
(0.739–104.321) |
| CEA (PV) | 0.107 | 0.239 | 5.11
(0.623–31.340) |
| CEA (DV) | 0.032 | 0.118 | 7.32
(0.845–27.328) |
| VEGF (PV) | 0.059 | 0.200 | 6.09
(0.742–25.823) |
| VEGF (DV) | <0.001 | 0.002 | 6.21
(1.241–30.048) |
| TIMP-1 (PV) | 0.013 | 0.056 | 3.12
(0.893–18.295) |
| TIMP-1 (DV) | 0.008 | 0.048 | 4.01
(1.003–20.180) |
Factors associated with hepatic
recurrence following the simultaneous resection of colon cancer and
synchronous liver metastasis
In group B, 7 patients had a solitary lesion and the
remaining 26 patients had multiple lesions. The median follow-up
period was 45 months (range, 27–69). The 5-year disease-free and
overall survival rates were 19.2 and 36.8%, respectively. A total
of 27 patients had recurrence following their initial surgery,
among which 20 patients (60.6%) developed intrahepatic recurrence.
Eight patients had liver-only recurrence and 12 patients had liver
and other organ metastasis simultaneously. The median time to
recurrence was 19 months. For adjuvant chemotherapy following
initial surgery, 25 patients received FOLFOX, 1 FOLFOX +
bevacizumab, 6 FOLFIRI, and 1 FOLFIRI + cetuximab. We performed
univariate and multivariate analyses for factors associated with
intrahepatic recurrence. A significant correlation was found
between the levels of VEGF from the DV and TIMP-1 from both the PV
and DV (Table VI).
 | Table VIUnivariate and multivariate analyses
for prognostic factors (intrahepatic recurrence) in group B. |
Table VI
Univariate and multivariate analyses
for prognostic factors (intrahepatic recurrence) in group B.
| Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|---|
| Factors | P | P | HR (95% CI) |
|---|
| pT2 vs. pT3–4 | 0.809 | 0.991 | 12.03
(0.011–62.074) |
| pN0 vs. pN1–2 | 0.337 | 0.728 | 9.84
(0.339–73.279) |
| LVI (+ vs. −) | 0.043 | 0.278 | 5.20
(0.641–28.311) |
| Disease-free
interval (<1 yr vs. >1 yr) | 0.038 | 0.055 | 8.06
(0.403–37.461) |
| Postop. CTx.
(oxaliplatin vs. irinotecan) | 0.847 | 0.895 | 33.92
(0.123–100.419) |
| Use of biological
agents (yes vs. no) | 0.764 | 0.925 | 40.34
(0.250–193.004) |
| CEA (PV) | 0.711 | 0.886 | 35.61
(0.213–119.407) |
| CEA (DV) | 0.099 | 0.280 | 17.32
(0.801–47.822) |
| E-selectin
(PV) | 0.069 | 0.374 | 9.07
(0.724–36.770) |
| E-selectin
(DV) | 0.040 | 0.195 | 9.72
(0.857–40.226) |
| VEGF (PV) | 0.055 | 0.252 | 16.09
(0.472–52.238) |
| VEGF (DV) | <0.001 | <0.001 | 12.16
(1.431–58.348) |
| TIMP-1 (PV) | 0.009 | 0.042 | 8.12
(1.983–60.924) |
| TIMP-1 (DV) | <0.001 | 0.026 | 7.40
(1.020–28.653) |
Prognostic factors following liver
resection
In group A, 10 patients developed metachronous liver
metastasis, of which 7 patients received surgical resection or
radiofrequency ablation (RFA). In group B, 33 patients developed
synchronous liver metastasis, and the prognosis following resection
of liver metastasis was analyzed. The median follow-up period was
18 months. The 5-year disease-free and overall survival rates for
groups A and B were 15.9 and 33.2%, respectively. Of 7 patients
from group A who had received surgical resection or RFA with
recurrence, four had solitary lesions and the other three had
multiple lesions. All 7 patients had recurrence following liver
resection. Five patients had hepatic-only recurrence and the
remaining 2 had other systemic recurrences in lung and paraaortic
lymph nodes.
Results of the univariate and multivariate analyses
of prognostic factors revealed that the levels of VEGF and TIMP-1
from DV as well as the presence of lymph node metastasis from the
primary tumor, synchronous metastasis and R1 resection were
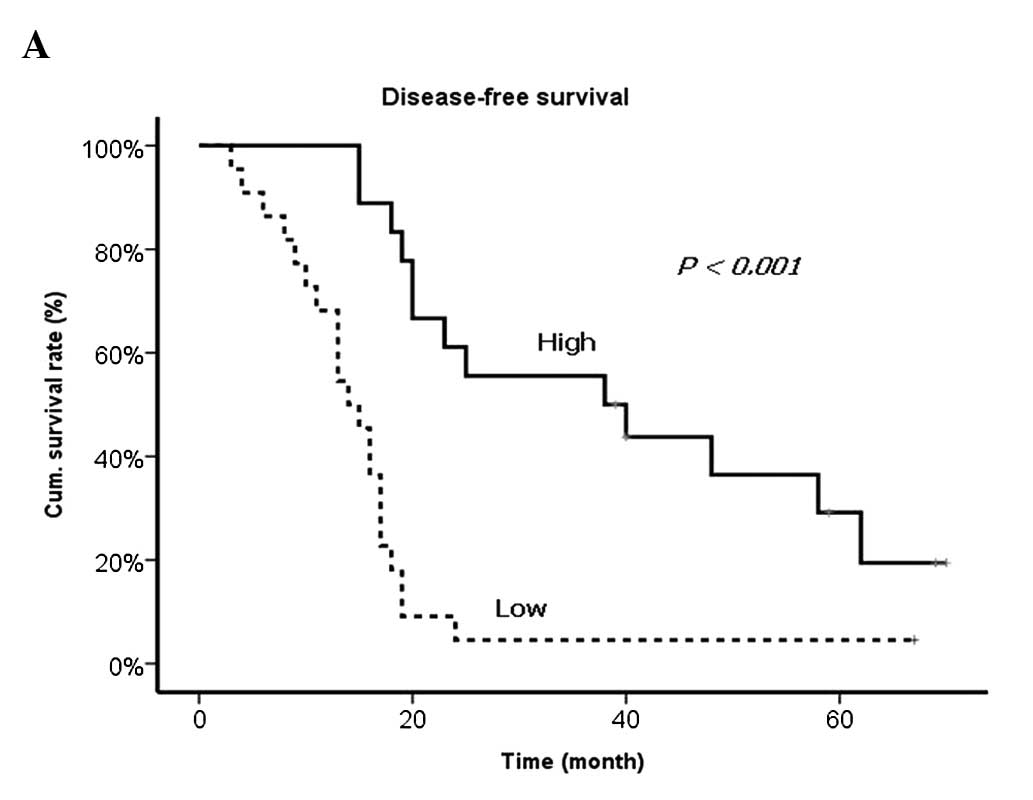
significantly correlated with worse prognosis (Table VII and Fig. 2).
 | Table VIIUnivariate and multivariate analyses
for prognostic factor (disease-free survival) after resection of
liver metastasis. |
Table VII
Univariate and multivariate analyses
for prognostic factor (disease-free survival) after resection of
liver metastasis.
| Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|---|
| Factors | P | P | HR (95% CI) |
|---|
| pT2 vs. pT3–4 | 0.632 | 0.811 | 10.22
(0.421–87.447) |
| pN0 vs. pN1–2 | <0.001 | 0.012 | 3.44
(1.003–17.019) |
| LVI (+ vs. −) | 0.043 | 0.398 | 8.90
(0.422–19.882) |
| Synchronous vs.
metachronous | <0.001 | 0.003 | 5.61
(1.411–24.376) |
| Postop. CTx. (yes
vs. no) | 0.729 | 0.995 | 26.43
(0.092–112.921) |
| Solitary vs.
multiple metastasis | 0.037 | 0.123 | 20.540
(0.807–89.424) |
| R0 vs. R1 | <0.001 | 0.003 | 15.001
(1.596–102.531) |
| CEA (PV) | 0.612 | 0.808 | 54.72
(0.093–341.740) |
| CEA (DV) | 0.211 | 0.420 | 32.75
(0.059–199.094) |
| E-selectin
(PV) | 0.091 | 0.284 | 18.78
(0.124–72.810) |
| E-selectin
(DV) | 0.100 | 0.282 | 23.04
(0.097–152.376) |
| VEGF (PV) | 0.067 | 0.142 | 37.09
(0.852–212.52) |
| VEGF (DV) | <0.001 | <0.001 | 20.72
(1.781–152.377) |
| TIMP-1 (PV) | 0.044 | 0.101 | 12.12
(0.512–243.029) |
| TIMP-1 (DV) | <0.001 | <0.001 | 11.35
(1.110–57.442) |
Discussion
Findings of the present study have shown that the
levels of VEGF and TIMP-1, detected in the blood from the vein that
directly drains from the primary tumor, were correlated with
synchronous and metachronous liver metastasis and hepatic
recurrence following R0 resection of liver metastasis more
significantly than any other clinical parameters. VEGF is a
well-known angiogenic molecule that is overexpressed in the
majority of solid organ cancers. It is known to act specifically on
endothelial cells to promote new vessel formation and is uniformly
reported to be associated with cancer progression (8,9).
TIMP-1 is the primary inhibitor of MMP-9 and an imbalance in the
MMP-9/TIMP-1 ratio has been proposed to be a potential reason for
progression of adenoma to carcinoma (10). Several studies have shown that
TIMP-1 level was increased in colon cancer patients and correlated
with poor prognosis (10,11).
To the best of our knowledge, this may be the first
study to address the clinical importance of molecular levels from
the tumor drainage vein (DV), rather than the peripheral vein (PV)
in liver metastasis. The rationale behind this finding is that
molecules that are expressed and secreted by the primary tumor
circulate through the capillaries of the liver, lungs and the rest
of the body prior to reaching the peripheral veins and during that
circulation a large part of the molecules may be metabolized or
decayed. Our hypothesis was that the level of these molecules
detected in the DV might carry more accurate information regarding
the tumor status and prognosis than in the PV. Tien et al
(12) analyzed the relationship
between the level of angiogenic factors in both the DV and the PV
and prognosis in colon cancer patients. These authors found that
the level of VEGF in the DV was an independent prognostic factor.
Results of the present study are in concordance with those of Tien
et al (12).
A previous study by Yoon et al (9) successfully demonstrated the clinical
implication of angiogenic molecular markers in colon cancer
patients with liver metastasis. These authors measured the
circulating levels of angiogenic molecules such as VEGF, bFGF, EGF
and HGF preoperatively and on the third day, first and third month
postoperatively. They also found that the preoperative VEGF and HGF
levels were significant prognostic factors for recurrence-free
survival. High levels of either of these molecular markers were
prognostic of poor survival. The fact that a high VEGF level was
correlated with poor prognosis is in concordance with our results,
whereas the VEGF level found in the study by Yoon et al
(9) was measured only in the
peripheral vein. In our study, univariate analysis revealed that
the VEGF level from both the tumor drainage vein and peripheral
vein were significantly correlated with the prognosis. However,
multivariate analysis revealed that the VEGF level from only the
tumor drainage vein was an independently significant prognostic
factor. This might result from the fact that the peripheral VEGF
level was significantly influenced by the tumor drainage vein VEGF
level. However, the correlation between peripheral and tumor
drainage vein VEGF levels remains to be determined, as well as
whether we can predict VEGF level in the tumor drainage vein from
that in the peripheral vein, which is a crucial issue in terms of
clinical practice.
This study has limitations. Although carried out
prospectively, only a small number of patients were enrolled. The
ELISA technique for the detection of circulating molecular marker
is the gold standard. However, the paradigm has now shifted in such
a way that biological processes are not expected to be explicated
through the investigation of individual molecules. Rather it is the
complex interplay between molecules as addressed by high-throughput
technologies that is expected to yield complex results. Although we
found that the VEGF and TIMP-1 levels in the tumor drainage vein
have a significant relationship with synchronous and metachronous
liver metastasis and hepatic recurrence following liver resection
more than any other clinical parameters, blood from the tumor
drainage vein is difficult to obtain, especially in non-surgical
patients.
Nonetheless, despite its limitations, the strong
point of this study might be the well-defined homogenous patient
subsets and long-term follow-up results. This study may also be one
of few to cover not only clinical parameters but also molecular
markers in developing predictive/prognostic factors for colon
cancer liver metastasis.
In conclusion, on the basis of the results from the
current study, colon cancer patients who had a high level of VEGF
and TIMP-1 detected from the tumor drainage vein at their initial
surgery were found to have a high risk of metachronous liver
metastasis and hepatic recurrence following the resection of
synchronous liver metastasis. The high levels of VEGF and TIMP-1
were also found to be significant prognostic factors that predict
poor prognosis following liver resection. These results require
validation but may pave the way for future transitional or clinical
studies that may further elucidate colon cancer liver
metastasis.
Acknowledgements
This study was supported by a National Research
Foundation of Korea grant funded by the Korean Government
(KRF-E00148).
References
|
1
|
Ballantyne GH and Quin J: Surgical
treatment of liver metastases in patients with colorectal cancer.
Cancer. 71:4252–4266. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi HJ, Hyun MS, Jung GJ, Kim SS and Hong
SH: Tumor angiogenesis as a prognostic predictor in colorectal
carcinoma with special reference to mode of metastasis and
recurrence. Oncology. 55:575–581. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fong Y, Cohen AM, Fortner JG, et al: Liver
resection for colorectal metastases. J Clin Oncol. 15:938–946.
1997.PubMed/NCBI
|
|
4
|
Bozzetti F, Doci R, Bignami P, Morabito A
and Gennari L: Patterns of failure following surgical resection of
colorectal cancer liver metastases. Rationale for a multimodal
approach. Ann Surg. 205:264–270. 1987. View Article : Google Scholar
|
|
5
|
Ueno H, Mochizuki H, Hashiguchi Y, Hatsuse
K, Fujimoto H and Hase K: Predictors of extrahepatic recurrence
after resection of colorectal liver metastases. Br J Surg.
91:327–333. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geiger TR and Peeper DS: Metastasis
mechanisms. Biochim Biophys Acta. 1796:293–308. 2009.PubMed/NCBI
|
|
7
|
Barozzi C, Ravaioli M, D’Errico A, et al:
Relevance of biologic markers in colorectal carcinoma: a
comparative study of a broad panel. Cancer. 94:647–657. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baker EA, Bergin FG and Leaper DJ:
Plasminogen activator system, vascular endothelial growth factor,
and colorectal cancer progression. Mol Pathol. 53:307–312. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoon SS, Kim SH, Gonen M, et al: Profile
of plasma angiogenic factors before and after hepatectomy for
colorectal cancer liver metastases. Ann Surg Oncol. 13:353–362.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waas ET, Wobbes T, Ruers T, Lomme RM and
Hendriks T: Circulating gelatinases and tissue inhibitor of
metalloproteinase-1 in colorectal cancer metastatic liver disease.
Eur J Surg Oncol. 32:756–763. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oberg A, Hoyhtya M, Tavelin B, Stenling R
and Lindmark G: Limited value of preoperative serum analyses of
matrix metalloproteinases (MMP-2, MMP-9) and tissue inhibitors of
matrix metalloproteinases (TIMP-1, TIMP-2) in colorectal cancer.
Anticancer Res. 20:1085–1091. 2000.PubMed/NCBI
|
|
12
|
Tien YW, Chang KJ, Chiu YF, Huang KW and
Lee PH: Comparison of angiogenic factor levels in tumor drainage
and peripheral venous blood from colorectal cancer patients. Ann
Surg Oncol. 13:1357–1363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adachi Y, Yamamoto H, Itoh F, Hinoda Y,
Okada Y and Imai K: Contribution of matrilysin (MMP-7) to the
metastatic pathway of human colorectal cancers. Gut. 45:252–258.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masaki T, Matsuoka H, Sugiyama M, et al:
Matrilysin (MMP-7) as a significant determinant of malignant
potential of early invasive colorectal carcinomas. Br J Cancer.
84:1317–1321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng ZS, Shu WP, Cohen AM and Guillem JG:
Matrix metalloproteinase-7 expression in colorectal cancer liver
metastases: evidence for involvement of MMP-7 activation in human
cancer metastases. Clin Cancer Res. 8:144–148. 2002.PubMed/NCBI
|
|
17
|
Bueno M, Salgado S, Beas-Zarate C and
Armendariz-Borunda J: Urokinase-type plasminogen activator gene
therapy in liver cirrhosis is mediated by collagens gene expression
down-regulation and up-regulation of MMPs, HGF and VEGF. J Gene
Med. 8:1291–1299. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alexiou D, Karayiannakis AJ, Syrigos KN,
et al: Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal
cancer patients: correlations with clinicopathological features,
patient survival and tumour surgery. Eur J Cancer. 37:2392–2397.
2001. View Article : Google Scholar
|
|
19
|
Uner A, Akcali Z and Unsal D: Serum levels
of soluble E-selectin in colorectal cancer. Neoplasma. 51:269–274.
2004.PubMed/NCBI
|
|
20
|
Wittig BM, Kaulen H, Thees R, et al:
Elevated serum E-selectin in patients with liver metastases of
colorectal cancer. Eur J Cancer. 32A:1215–1218. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roselli M, Guadagni F, Martini F, et al:
Association between serum carcinoembryonic antigen and endothelial
cell adhesion molecules in colorectal cancer. Oncology. 65:132–138.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gangopadhyay A, Lazure DA and Thomas P:
Adhesion of colorectal carcinoma cells to the endothelium is
mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp
Metastasis. 16:703–712. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agrawal D, Chen T, Irby R, et al:
Osteopontin identified as colon cancer tumor progression marker. CR
Biol. 326:1041–1043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wai PY, Mi Z, Guo H, et al: Osteopontin
silencing by small interfering RNA suppresses in vitro and in vivo
CT26 murine colon adenocarcinoma metastasis. Carcinogenesis.
26:741–751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Behzadian MA, Windsor LJ, Ghaly N, Liou G,
Tsai NT and Caldwell RB: VEGF-induced paracellular permeability in
cultured endothelial cells involves urokinase and its receptor.
FASEB J. 17:752–754. 2003.PubMed/NCBI
|