Introduction
Several cancer treatment strategies, including
surgery, chemotherapy and radiation therapy, have been developed to
treat cancer and cancer-related diseases. Among these treatments,
chemoprevention and chemotherapy using natural compounds have been
found to effectively prevent the progression of cancer. Although
various types of cancer initially respond to chemotherapy, the
development of chemotherapy resistance continues to be the main
problem in the treatment of cancer. Therefore, new agents
demonstrating chemotherapeutic and chemopreventive activities
should be identified.
Triterpenoids are a large family of natural
compounds commonly found in a diversity of plants (1–3).
Certain natural triterpenoids, including oleanolic acid
(3β-hydroxy-olean-12-en-28-oic acid) and its isomer ursolic acid
(3β-hydroxy-urs-12-en-28-oic acid), possess anti-cancer and
anti-inflammatory activities (4–7).
Moreover, results of previous studies showed that several synthetic
triterpenoid compounds derived from oleanolic acid including
2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) and its methyl
ester (CDDO-Me) derivative possess significant anti-tumorigenic and
anti-inflammatory activity (8–11).
Triterpenoids have also been known to prevent oxidative stress,
inflammation and hypertension (12).
Calenduloside E 6′-methyl ester (oleanolic acid
3-O-β-D-glucuronopyranoside-6′-methyl ester) is a naturally
occurring oleanane-type triterpenoid. Calenduloside E 6′-methyl
ester was isolated from the Brazilian ginseng, Pfaffia
paniculata (13), but can also
be obtained from Acanthopanax sessiliflorus (A. sessiliflorus).
A. sessiliflorus, an Acanthopanax species that occurs in
abundance in Korea, belongs to the herbaceous type of
Araliaceae. The shoots and roots of diverse species of
Acanthopanax have traditionally been used as medicines for a
number of diseases, including diabetes, neuralgia, palsy, gastric
ulcer, learning-behavior difficulties and cancer (14–16).
To the best of our knowledge, neither the biological
activities of calenduloside E 6′-methyl ester nor its effect on
cancer cells have been reported. Thus, we isolated calenduloside E
6′-methyl ester from A. sessiliflorus fruits and examined
the anti-cancer activity in mouse colon carcinoma CT-26 cells. In
addition, the anti-tumor activity of calenduloside E 6′-methyl
ester was evaluated in a CT-26 colon carcinoma animal model.
Materials and methods
Extraction and isolation of calenduloside
E 6′-methyl ester
The air-dried fruit of A.sessiliflorus (10
kg) was powdered and extracted with 36 litres of aqueous 70% EtOH
at room temperature for 3×24 h. After concentration, the EtOH
extract (2,012 g) was suspended in H2O and then
partitioned successively with EtOAc, n-BuOH and H2O to
produce EtOAc (E, 118 g), n-BuOH (B, 284 g) and water fractions,
respectively. Fraction B was chromatographed on a column of highly
porous polymer (Diaion HP-20) and eluted with H2O and
MeOH, respectively, to yield two fractions (B1 and B2). Fraction B2
(73.40 g) was subjected to silica gel column (12×13 cm)
chromatography (c.c.) using a gradient of
CH3Cl3:MeOH:H2O (7:3:1→65:35:10, 4
litres of each) to yield 11 major fractions (B2-1 to B2-11).
Fraction B2-4 [3.50 g, Ve/Vt (elution volume/total volume),
0.41–0.57] was subjected to RP-18 c.c. [(12×13
cm)(MeOH:H2O, 1.5:1→2:1→4:1)] to produce six
subfractions (B2-4-1 to B2-4-6). Subfraction B2-4-6 (1.44 g, Ve/Vt
0.76–0.99) was purified over SiO2 c.c. (4.5×15 cm) and
eluted with CH3Cl3-MeOH-H2O
(13:3:1) to yield calenduloside E 6′-methyl ester [88 mg, TLC
(SiO2 F254) Rf 0.60 in
CH3Cl3:MeOH:H2O (65:35:10)].
Cell line and culture condition
Mouse colon carcinoma CT-26 cells were obtained from
the Korean Cell Line Bank (KCLB; Seoul, Korea) and grown at 37°C
with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM)
with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
Cell culture medium and reagents were purchased from Thermo
Scientific Hyclone (Waltham, MA, USA).
Cytotoxicity assay
The cytotoxicity of calenduloside E 6′-methyl ester
was measured using the MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
(Sigma, St. Louis, MO, USA) colorimetric assay. CT-26 cells were
seeded onto 96-well plates at a density of 1× cells/well in 100 μl
of DMEM supplemented with 10% FBS. After 24 h of incubation at
37°C, cells were treated with serum-free DMEM containing various
concentrations of calenduloside E 6′-methyl ester. After 24 h of
incubation, 50 μl of MTT (5 mg/ml in PBS) was added to each well.
Cells were incubated at 37°C for 2 h. After removal of the medium,
cells were treated with 100 μl of dimethyl sulfoxide (DMSO) for 5
min, and then the optical density was measured using a microplate
reader (Bio-Tek, Winooski, VT, USA) at 550 nm. Cell viability was
calculated as the percentage of viable cells in the calenduloside E
6′-methyl ester-treated group (2.5, 5, 10, 15, 20 and 25 μM) versus
the control group using the equation: Cell viability (%) =
[(ODCompound − ODBlank)/(ODContol
− ODBlank)] × 100.
Cell cycle analysis
CT-26 cells were seeded onto 6-well plates at a
density of 3×105 cells/well in 2 ml of DMEM supplemented
with 10% FBS. After 24 h of incubation at 37°C, cells were treated
with serum-free DMEM containing different concentrations of
calenduloside E 6′-methyl ester. After 12 h of incubation, cells
were collected and washed twice with ice-cold PBS. Cell pellets
were fixed in 70% cold ethanol overnight at −20°C. Fixed cells were
centrifuged, washed and resuspended in 100 μl of PBS, then mixed
with 100 μl of RNase A (1 mg/ml; Sigma) and incubated for 30 min at
37°C. The cells were stained by adding 400 μl of propidium iodide
(PI, 50 μg/ml; Sigma). After filtering through a nylon mesh (40
μm), the DNA content of the stained cells was analyzed using the
FACSVantage SE and CellQuest program (BD Biosciences, San Jose, CA,
USA).
Annexin V staining assay
Modulation of phosphatidylserine externalization
during apoptosis was assessed using annexin V conjugated with the
fluorescent dye fluorescein isothiocyanate (FITC). CT-26 cells were
seeded onto 6-well plates at a density of 3×105
cells/well and incubated for 24 h. After treatment with serum-free
DMEM containing 10 μM calenduloside E 6′-methyl ester for 6 h,
cells were stained with the annexin V-FITC conjugate, and images
were captured at an objective magnification of ×40 using a confocal
laser scanning microscope (LSM 510 Meta, Carl Zeiss, Oberkochen,
Germany).
Terminal deoxynucleotidyl transferase
mediated dUTP nick end labeling (TUNEL) assay
TUNEL staining was performed on calenduloside E
6′-methyl ester-treated CT-26 cells using an in situ cell
death detection kit (Roche, Basel, Switzerland). Briefly, cells
were fixed with 4% paraformaldehyde and permeabilized with 0.1%
Triton X-100 in 0.1% sodium citrate buffer. Fixed and permeabilized
cells were stained with enzyme buffer containing terminal
deoxynucleotidyl transferase (TdT) and fluorescein-dUTP. Following
washes, the nuclei were stained with a PI (2 μg/ml) solution for 1
min. Cells were imaged under ×40 objective magnification using a
confocal laser scanning microscope.
DNA fragmentation assay
Genomic DNA was isolated from calenduloside E
6′-methyl ester-treated CT-26 cells using a DNA Mini kit (Qiagen,
Hilden, Germany) according to the manufacturer’s instructions. The
isolated genomic DNA samples were electrophoresed on a 1.5% agarose
gel at 50 V for 1 h. The gel was stained with ethidium bromide
(EtBr; Sigma) and visualized using a UV transilluminator (Wealtech,
Reno, NV, USA).
Western blot analysis
After seeding onto 6-well plates at a density of
3×105 cells/well and incubating for 24 h, cells were
treated with 10 μM calenduloside E 6′-methyl ester for various
times up to 12 h. The cells were then lysed with RIPA buffer
(Thermo Fisher Scientific Inc., Rockford, IL, USA) supplemented
with a protease inhibitor cocktail (Roche, Mannheim, Germany).
Protein concentrations were determined using an RC/DC Bio-Rad assay
kit (Bio-Rad, Hercules, CA, USA) following the manufacturer’s
instructions. Protein samples were separated by electrophoresis on
10–15% sodium dodecyl sulfate (SDS)-polyacrylamide gels. The
proteins on the gel were transferred onto a polyvinylidene fluoride
(PDVF) membrane (PALL Life Science, Port Washington, NY, USA),
blocked with 5% skimmed milk (BD Biosciences), incubated with an
anti-mouse caspase-3, -8, -9 and poly ADP-ribose polymerase (PARP)
(Cell Signaling Technology Inc., Danvers, MA, USA), and anti-mouse
actin (Sigma). The membrane was then probed with the horseradish
peroxidase-conjugated anti-rabbit IgG (GE Healthcare Life Sciences,
Stockholm, Sweden). Protein bands were detected using an enhanced
chemiluminescent western blotting detection system (GE Healthcare
Life Sciences).
Tumor growth in CT-26 allograft bearing
mice
Five-week-old female BALB/c mice were purchased from
Orient Bio Inc. (Seongnam, Korea). The mice were provided with
water and food ad libitum, and were quarantined in a
specific pathogen-free environment with a 12 h light/dark
photoperiod in an animal care facility accredited by the Kyung Hee
University Institutional Animal Care and Use Committee. Animal care
and experimental procedures followed the Kyung Hee University
guidelines for the care and use of laboratory animals.
To establish an allograft colon carcinoma animal
model, 5×105 CT-26 cells in 200 μl PBS were injected
into the right flank of BALB/c mice. The tumors were allowed to
grow into visible masses for 7 days, after which animals were
divided into groups of 5 mice each. Each group was treated daily
with a peritumor injection of either calenduloside E 6′-methyl
ester [0.6, 6 mg/kg/day in PBS with 0.2% DMSO (v/v)] or control
(PBS with 0.2% DMSO) for 8 days. Tumor volumes were measured every
other day with a caliper and calculated according to the formula:
Length × width2 × 0.5, where length is the largest tumor
diameter and width the smallest tumor diameter (17). The mice were sacrificed 15 days
after tumor inoculation, and the tumors were excised and
weighted.
Statistical analysis
Data are shown as the mean ± standard deviation (SD)
or standard error (SE). The Student’s t-test was used to compare
different data groups (p<0.05, p<0.01, p<0.001).
Results
Calenduloside E 6′-methyl ester
demonstrated dose-dependent cytotoxic effects
We isolated calenduloside E 6′-methyl ester from
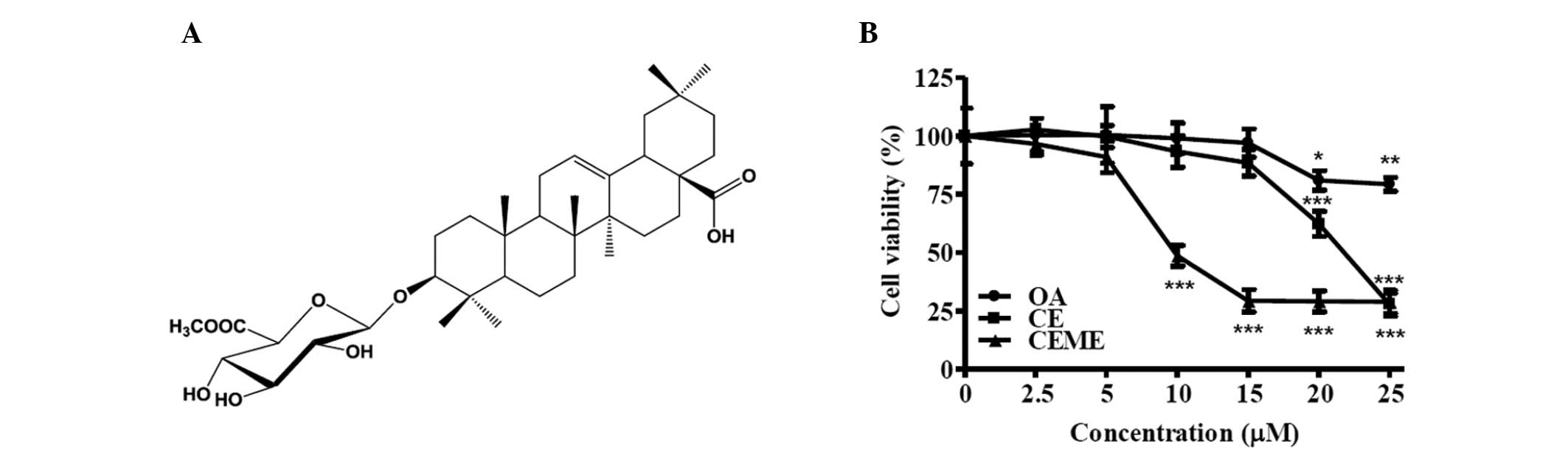
A. sessiliflorus fruits (Fig.
1A). Identification of the structure was confirmed on the basis
of several spectroscopic analyses, including IR, 1H- and
13C-NMR and 2D-NMR (COSY, HSQC, and HMBC) (data not
shown). To determine the cytotoxic effect of calenduloside E
6′-methyl ester on CT-26 cells, cells were treated with different
concentrations (2.5, 5, 10, 15, 20 and 25 μM) of calenduloside E
6′-methyl ester for 24 h and cell viabilities were measured using
an MTT assay. For comparison to its aglycone structure, oleanolic
acid, and its parental structure, calenduloside E, CT-26 cells were
also treated with oleanolic acid and calenduloside E at different
concentrations (2.5, 5, 10, 15, 20 and 25 μM) for 24 h.
Calenduloside E 6′-methyl ester dose-dependently inhibited the
viability of CT-26 cells (Fig. 1B).
The IC50 (50% growth inhibitory concentration) value of
calenduloside E 6′-methyl ester was approximately 10 μM. The
IC50 value of calenduloside E was between 20 and 25 μM.
However, oleanolic acid was demonstrated to have a low
cytotoxicity.
Calenduloside E 6′-methyl ester induced
apoptosis in CT-26 cells
To analyze whether the cytotoxic effect of
calenduloside E 6′-methyl ester was caused by apoptosis,
calenduloside E 6′-methyl ester-treated CT-26 cells were stained
with the annexin V-FITC conjugate, and monitored under confocal
microscopy (Fig. 2A and B). Cells
treated with calenduloside E 6′-methyl ester were readily stained
with annexin V-FITC; however, this was not observed for the
untreated control group. The cell cycle was also analyzed to
examine the effect of treatment on the sub-G1 apoptotic population
of CT-26 cells. Cells were treated with diverse concentrations
(2.5, 5, 10 and 25 μM) of calenduloside E 6′-methyl ester for 12 h
and their DNA contents were analyzed by flow cytometry after PI
staining. The number of cells in the sub-G1 population increased in
a dose-dependent manner (Fig. 2C and
D). After 12 h of incubation with concentrations of 2.5, 5, 10
and 25 μM, the number of cells in the sub-G1 populations increased
to 5.1, 6.4, 53.1 and 99.1%, respectively.
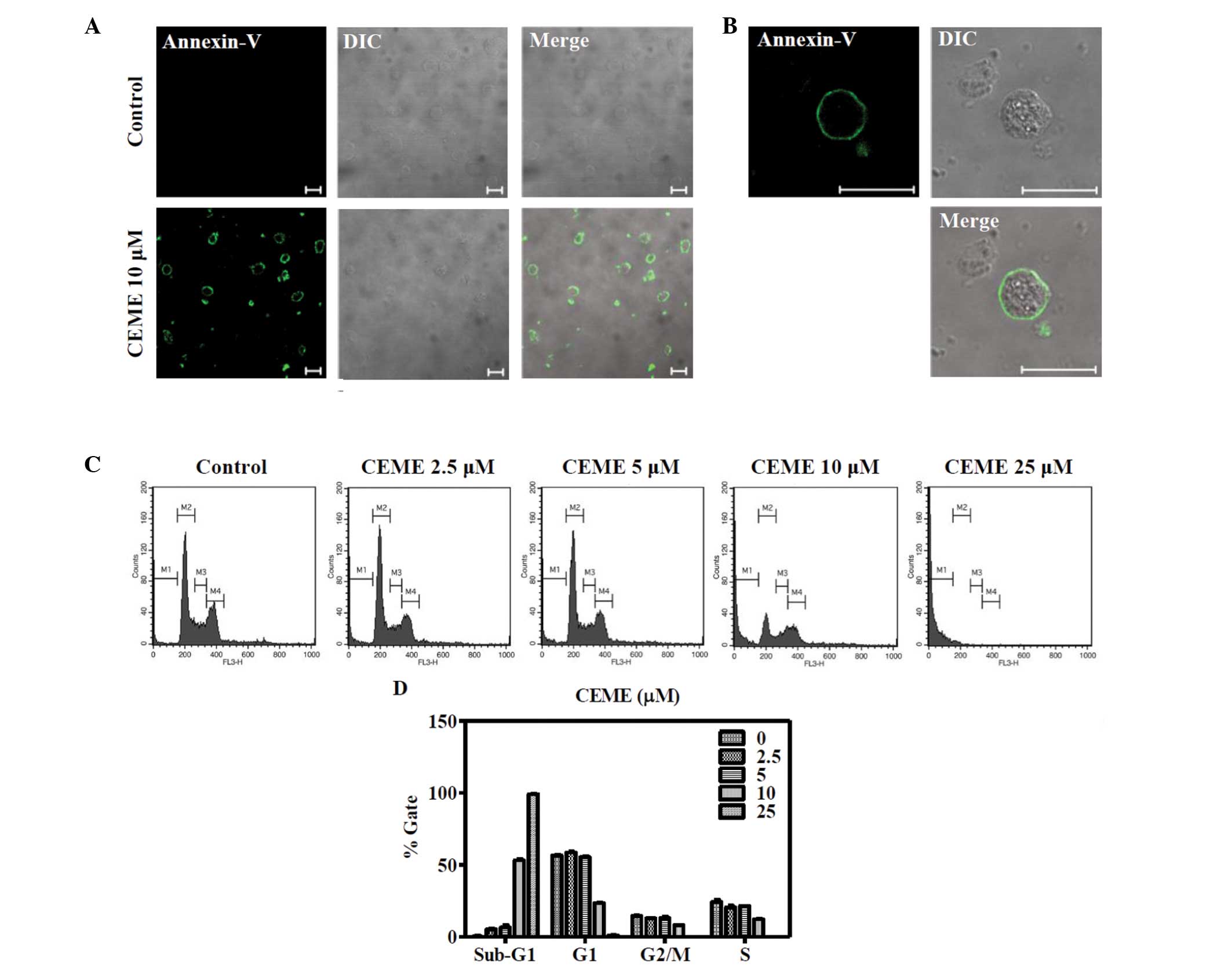 | Figure 2Annexin V and flow cytometric analysis
of calenduloside E 6′-methyl ester-treated CT-26 cells. (A)
Calenduloside E 6′-methyl ester increased the number of apoptotic
cells stained with annexin-V-FITC. Cells were treated with 10 μM
calenduloside E 6′-methyl ester for 6 h and stained with
annexin-V-FITC, then imaged under ×40 objective magnification using
a confocal microscope (bar, 20 μm). DIC, differential interference
contrast. (B) Image of the annexin-V-FITC-stained cells of (A) is
enlarged to better demonstrate the FITC signal (bar, 20 μm). (C)
Calenduloside E 6′-methyl ester increased sub-G1 cell populations
of CT-26 cells. The cells were treated with different
concentrations (2.5, 5, 10 and 25 μM) of calenduloside E 6′-methyl
ester for 12 h, and the DNA content was measured using a flow
cytometer after staining with PI. (D) Three independent experiments
of (C) were performed and data are shown as a bar diagram. CEME,
calenduloside E 6′-methyl ester; FITC, fluorescent dye fluorescein
isothiocyanate; PI, propidium iodide. |
Apoptosis was characterized by TUNEL and a DNA
fragmentation assay. Calenduloside E 6′-methyl ester-treated CT-26
cells were labeled with fluorescein-dUTP, and monitored under
confocal microscopy (Fig. 3A and
B). Fluorescein-labeled cells were observed after 12 h of
incubation with calenduloside E 6′-methyl ester, but not with the
control cells. The DNA fragmentation assay was also performed to
evaluate genomic DNA fragmentations in calenduloside E 6′-methyl
ester-treated CT-26 cells. Genomic DNA was purified from cells
treated with 10 μM calenduloside E 6′-methyl ester for the
indicated times (3, 6, 9 and 12 h) and subjected to agarose gel
electrophoresis to assess DNA fragmentation (Fig. 3C). DNA fragmentations were observed
in calenduloside E 6′-methyl ester-treated cells and increased in a
time-dependent manner. These results indicate that calenduloside E
6′-methyl ester induces apoptosis in CT-26 cells.
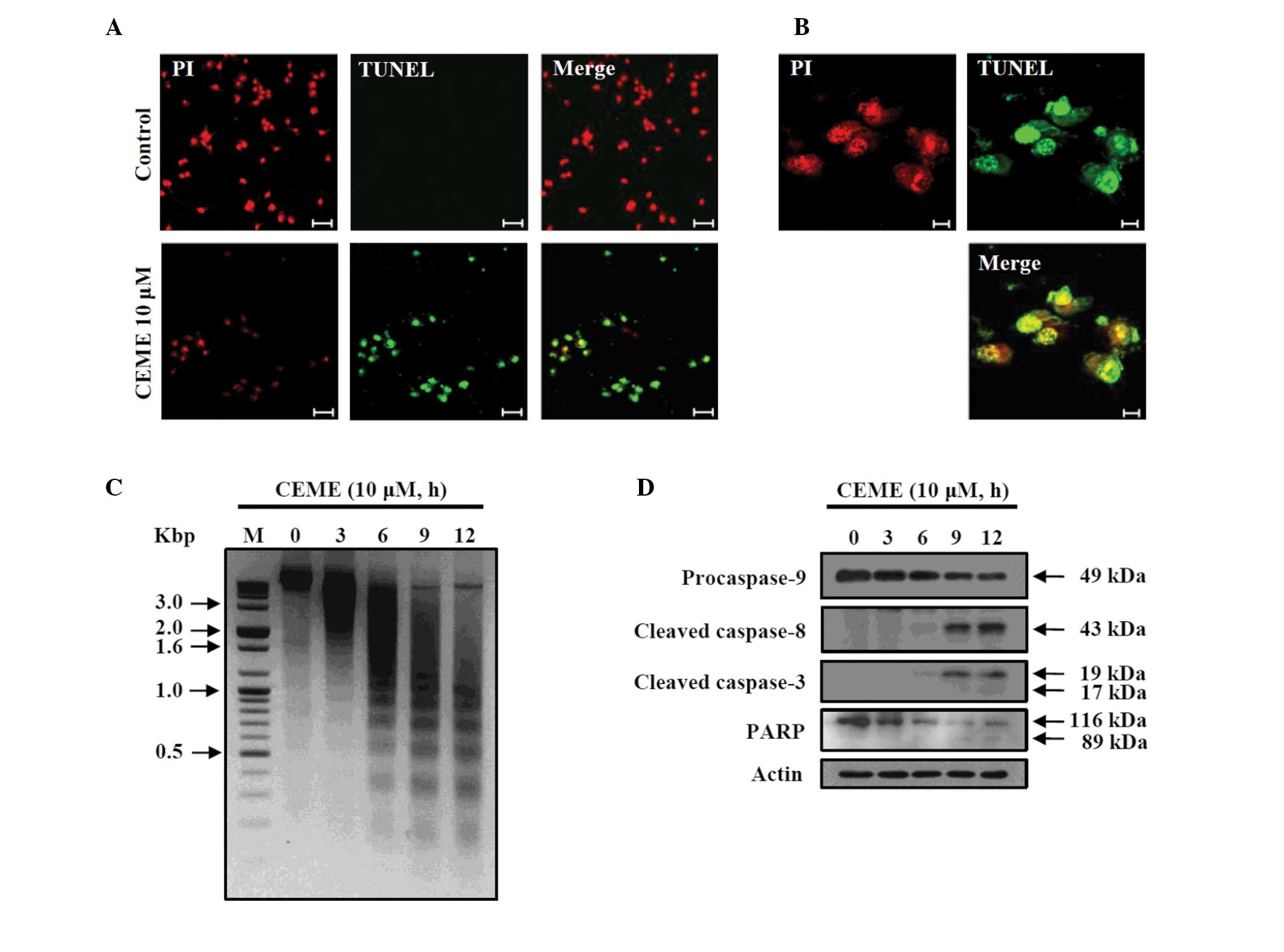 | Figure 3TUNEL, DNA fragmentation and caspase
cascade analysis of calenduloside E 6′-methyl ester-treated CT-26
cells. (A) The intensity of fluorescein-dUTP labeling of DNA strand
break was increased in calenduloside E 6′-methyl ester-treated
CT-26 cells. Cells were treated with 10 μM calenduloside E
6′-methyl ester for 12 h. The cells were fixed, permeabilized and
stained with enzyme buffer containing terminal deoxynucleotidyl
transferase and fluorescein-dUTP. After nuclei staining with PI,
cells were imaged at ×20 objective magnification using a confocal
laser scanning microscope (bar, 50 μm). (B) Image of the
TUNEL-stained cells of (A) is enlarged to better demonstrate the
fluorescein signal (bar, 10 μm). (C) Calenduloside E 6′-methyl
ester induced the DNA fragmentation of CT-26 cells. Cells were
treated with 10 μM calenduloside E 6′-methyl ester for the
indicated times. DNA fragmentation was analyzed by agarose gel
electrophoresis. M, 100 bp DNA ladder size markers. (D)
Calenduloside E 6′-methyl ester induced the activation of
caspase-8, -3, -9 and PARP. Protein extracts were prepared from
CT-26 cells treated with 10 μM calenduloside E 6′-methyl ester for
the indicated times. The cleavage of caspase-3, -8, -9 and PARP
were determined using western blot analysis. Actin was used as a
control. CEME, calenduloside E 6′-methyl ester; TUNEL, terminal
deoxynucleotidyl transferase mediated dUTP nick end labeling; PI,
propidium iodine; PARP, poly ADP-ribose polymerase. |
Calenduloside E 6′-methyl ester induced
the activation of the caspase cascade
The activation of the caspase cascade was determined
in CT-26 cells treated with 10 μM calenduloside E 6′-methyl ester
for the indicated times (3, 6, 9 and 12 h). The amount of cleaved
caspase-8 (~43 kDa) and caspase-3 (~19 and ~17 kDa) was increased
by treatment with calenduloside E 6′-methyl ester (Fig. 3D). Calenduloside E 6′-methyl ester
treatment caused a reduction in procaspase-9 (~49 kDa) and induced
cleavage of PARP. These results indicate that calenduloside E
6′-methyl ester induces apoptosis in CT-26 cells via the activation
of the caspase cascade.
Calenduloside E 6′-methyl ester inhibited
tumor growth in the CT-26 allograft colon carcinoma animal
model
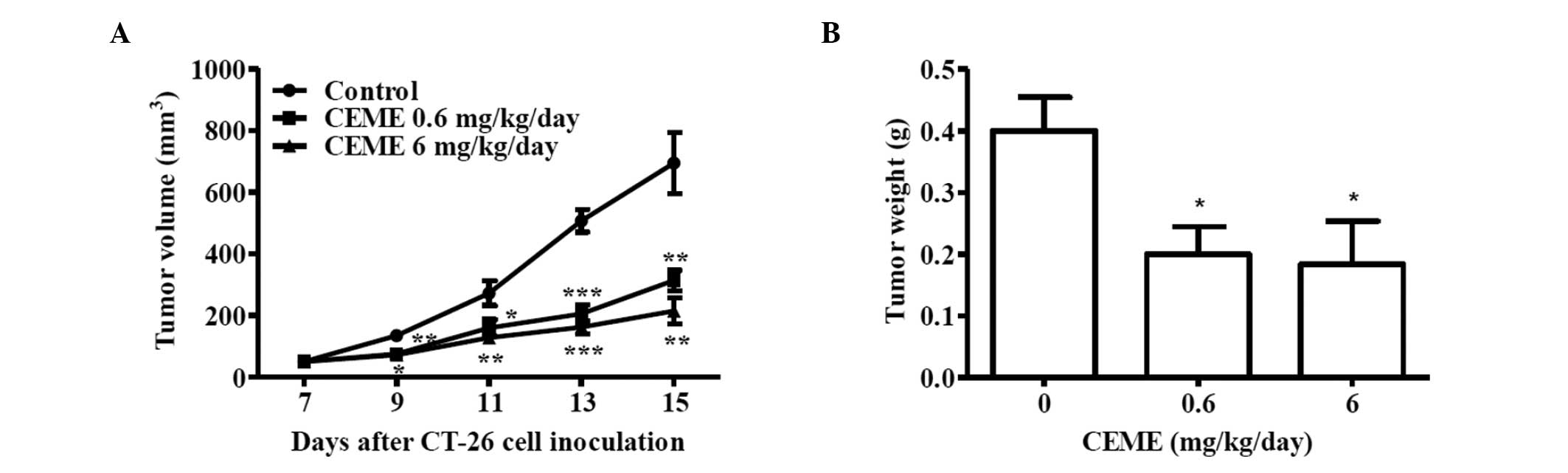
The anti-tumor activity of calenduloside E 6′-methyl
ester was examined in a CT-26 colon carcinoma animal model using
BALB/c mice. After 7 days subcutaneous implantation of CT-26
allograft, the average volumes of tumors were approximately 50
mm3. BALB/c mice were divided into groups and treated
daily with a peritumor injection of calenduloside E 6′-methyl ester
for 8 days. No acute side effects, including body weight loss, hair
loss, lethargy and mortality, were detected (data not shown). In
the control group, tumors grew rapidly and reached an average
volume of 694.4±99.3 mm3 (mean ± SE) on day 15 after
inoculation with CT-26 cells (Fig.
4A). The size of the primary tumor (313.74±33, 214.3±42.5
mm3) in 0.6 and 6 mg/kg/day calenduloside E 6′-methyl
ester-treated animals was reduced to 45.2 and 30.9% of the control
group size at 15 days, respectively. Similarly, the tumor weight
for the 0.6 and 6 mg/kg/day calenduloside E 6′-methyl ester-treated
group was reduced to 50 and 46% of the control group weight,
respectively (Fig. 4B). Taken
together, these results indicate that calenduloside E 6′-methyl
ester inhibited tumor growth in the CT-26 allograft colon carcinoma
animal model.
Discussion
Calenduloside E 6′-methyl ester, which is an
oleanane-type triterpenoid, has been isolated from Brazilian
ginseng, Pfaffia paniculata (13); however, its effects on colon cancer
cells have not yet been reported. In this study, the cytotoxic
effect of calenduloside E 6′-methyl ester isolated from
Acanthopanax sessiliflorus fruits, was investigated in mouse
colon carcinoma CT-26 cells. Calenduloside E 6′-methyl ester
significantly decreased the cell viability of CT-26 cells when
compared with its aglycone and parental structures, oleanolic acid
and calenduloside E. When cells were treated with 10 μM
calenduloside E 6′-methyl ester for 24 h, the viability of CT-26
cells decreased to approximately 50% relative to the untreated
control cells. Oleanolic acid exhibits cytotoxic effects in a
variety of cell lines, including A549 (non-small cell lung),
SK-OV-3 (ovary), SK-MEL-3 (melanoma), XF498 (central nerve system),
HCT15 (colon) and HONE-1 (nasopharyngeal carcinoma) (18–19).
However, the oleanolic acid slightly decreased the viability of
CT-26 cells at concentrations of 20 and 25 μM. The cytotoxic effect
was not observed in 10 μM oleanolic acid-treated CT-26 cells.
Calenduloside E also inhibited the viability of CT-26 cells.
However, the ID50 of calenduloside E was higher than
that of calenduloside E 6′-methyl ester. Therefore, these results
suggest that the presence of glucoronopyranoside or
glucoronopyranoside-6′-methyl ester in C-3 position contributes to
the cytotoxic activity of oleanolic acid derivatives. Notably, our
findings on a high cytotoxic activity of calenduloside E 6′-methyl
ester compared to calenduloside E are in accordance with a previous
study which demonstrated that CDDO-Me exhibits more activity than
CDDO against cancer cells due to the presence of the methyl ester
group (20).
To confirm that the cytotoxic effect of
calenduloside E 6′-methyl ester was induced by apoptosis,
calenduloside E 6′-methyl ester-treated CT-26 cells were
characterized by detections of cell surface annexin-V and sub-G1
apoptotic cell populations, TUNEL and DNA fragmentation
experiments. Calenduloside E 6′-methyl ester increased the cells
immunostained with annexin-V-FITC and sub-G1 apoptotic cell
populations (Fig. 2). It also
increased terminal deoxynucleotidyl transferase dUTP nick
end-labeled CT-26 cells (Fig. 3A and
B) and induced DNA fragmentation (Fig. 3C). Taken together, these indicate
that calenduloside E 6′-methyl ester induced apoptosis in CT-26
cells and treatment with Calenduloside E 6′-methyl ester resulted
in the reduction of procaspase-9 and the increase of cleaved
caspase-8 (Fig. 3D). Thus,
calenduloside E 6′-methyl ester-induced apoptosis is mediated by
the activation of the caspase cascade which is involved in
apoptotic mechanisms (21,22). Calenduloside E 6′-methyl ester
activates two major apoptotic signaling pathways, intrinsic and
extrinsic, as is the case for other reported triterpenoids to
induce apoptosis through mitochondria-mediated intrinsic and death
receptor-induced extrinsic pathways (23).
Furthermore, we investigated the anti-tumor activity
of calenduloside E 6′-methyl ester in a CT-26 colon carcinoma
animal model using BALB/c mice. Calenduloside E 6′-methyl ester
markedly reduced the tumor size and weight without acute side
effects, including body weight loss, hair loss, lethargy and
mortality. This is, to the best of our knowledge, the first study
demonstrating that calenduloside E 6′-methyl ester inhibits tumor
growth in a CT-26 colon carcinoma animal model.
In conclusion, our results demonstrated for the
first time that calenduloside E 6′-methyl ester induces apoptosis
and inhibits tumor growth in mouse colon carcinoma CT-26 in
vitro and in vivo. Moreover, it induced apoptosis in
CT-26 cells, which is mediated by extrinsic and intrinsic apoptotic
signaling pathways, and inhibited tumor growth in a CT-26 colon
carcinoma animal model. These findings suggest that calenduloside E
6′-methyl ester of Acanthopanax sessiliflorus fruits is a
good source of chemotherapeutic agents involved in the inhibition
of tumor growth.
Acknowledgements
This study was supported by grants from the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (20110003112), and from Kyung Hee University in 2010
(KHU-20110257).
References
|
1
|
Kristó TS, Terdy PP, Simándi B, Szöke E,
Lemberkovics E and Kéry A: Efficiency of supercritical fluid
extraction for the production of non-volatile terpenoids from
Taraxaci radix. Acta Pharm Hung. 71:318–324. 2001.PubMed/NCBI
|
|
2
|
Brieskorn CH and Süss HP: Triterpenoids
from the peels of pear and apple. Arch Pharm. 307:949–960.
1974.PubMed/NCBI
|
|
3
|
Urech K, Scher JM, Hostanska K and Becker
H: Apoptosis inducing activity of viscin, a lipophilic extract from
Viscum album L. J Pharm Pharmacol. 57:101–109. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petronelli A, Pannitteri G and Testa U:
Triterpenoids as new promising anticancer drugs. Anticancer Drugs.
20:880–892. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Konoshima T, Takasaki M, Tokuda H, Masuda
K, Arai Y, Shiojima K and Ageta H: Anti-tumor-promoting activities
of triterpenoids from ferns. I Biol Pharm Bull. 19:962–965. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishino H, Nishino A, Takayasu J, Hasegawa
T, Iwashima A, Hirabayashi K, Iwata S and Shibata S: Inhibition of
the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate
by some oleanane-type triterpenoid compounds. Cancer Res.
48:5210–5215. 1998.PubMed/NCBI
|
|
7
|
Ryu SY, Oak MH, Yoon SK, Cho DI, Yoo GS,
Kim TS and Kim KM: Anti-allergic and anti-inflammatory triterpenes
from the herb of Prunella vulgaris. Planta Med. 66:358–360.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Honda T, Rounds BV, Gribble GW, Suh N,
Wang Y and Sporn MB: Design and synthesis of
2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly
active inhibitor of nitric oxide production in mouse macrophages.
Bioorg Med Chem Lett. 8:2711–2714. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honda T, Rounds BV, Bore L, Favaloro FG
Jr, Gribble GW, Suh N, Wang Y and Sporn MB: Novel synthetic
oleanane triterpenoids: a series of highly active inhibitors of
nitric oxide production in mouse macrophages. Bioorg Med Chem Lett.
9:3429–3434. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Assefa H, Nimrod A, Walker L and Sindelar
R: Synthesis and evaluation of potential complement inhibitory
semisynthetic analogs of oleanolic acid. Bioorg Med Chem Lett.
9:1889–1894. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Konopleva M, Tsao T, Ruvolo P, Stiouf I,
Estrov Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE,
Munsell M, Suh N, Gribble G, Honda T, May WS, Sporn MB and Andreeff
M: Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and
differentiation in acute myelogenous leukemia. Blood. 99:326–335.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamaguchi Y, Yamada K, Yoshikawa N,
Nakamura K, Haginaka J and Kunitomo M: Corosolic acid prevents
oxidative stress, inflammation and hypertension in SHR/NDmcr-cp
rats, a model of metabolic syndrome. Life Sci. 79:2474–2479. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Jadhav AN and Khan IA: Triterpenoids
from Brazilian ginseng, Pfaffia paniculata. Planta Med.
76:635–639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hahn DR, Kim CJ and Kim JH: A study on
chemical constituents of Acanthopanax koreanum Nakai and its
pharmaco-biological activities. Yakhak Hoeji. 29:357–361. 1985.
|
|
15
|
Yook CS, Rho YS, Sed SH, Leem JY and Han
SH: Chemical components of Acanthopanax divaricatus and
anticancer effect in leaves. Yakhak Hoeji. 40:251–261. 1996.
|
|
16
|
Fujikawa T, Yamaguchi A, Morita I, Takeda
H and Nihshibe S: Protective effects of Acantopanax
senticosus Harms from Hokkai do and its components on gastric
ulcer in restrained cold water stressed rats. Biol Pharm Bull.
19:1227–1230. 1996.
|
|
17
|
Alessandria G, Filippeschi S, Sinibaldi P,
Mornet F, Passera P, Spreafico F, Cappa PM and Gullino PM:
Influence of gangliosides on primary and metastatic neoplastic
growth in human and murine cells. Cancer Res. 47:4243–4247.
1987.PubMed/NCBI
|
|
18
|
Chiang YM, Chang JY, Kuo CC, Chang CY and
Kuo YH: Cytotoxic triterpenes from the aerial roots of Ficus
microcarpa. Phytochemistry. 66:495–501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YK, Yoon SK and Ryu SY: Cytotoxic
triterpenes from stem bark of Physocarpus intermedius.
Planta Med. 66:485–486. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deeb D, Gao X, Dulchavsky SA and Gautam
SC: CDDO-me induces apoptosis and inhibits Akt mTOR and NF-kappa B
signaling proteins in prostate cancer cells. Anticancer Res.
27:3035–3044. 2007.PubMed/NCBI
|
|
21
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar
|
|
22
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: a link between cancer genetics and chemotheraphy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|