Introduction
Carcinosarcoma of the stomach is an extremely rare
mixed tumor comprising carcinoma and sarcoma components (1). The most common carcinoma component is
tubular or papillary adenocarcinoma, while neuroendocrine
carcinomatous differentiation is less common. The mesenchymal
sarcomatous component is variable and may include leiomyosarcoma,
rhabdomyosarcoma, osteosarcoma or chondrosarcoma (2).
Tumor in the upper gastrointestinal tract tends to
be localized in the esophagus where it represents approximately 3%
of all esophageal tumors. By contrast, only 52 cases of gastric
carcinosarcoma have been reported in the literature thus far, most
of which are described in the Japanese literature (3). Other terms for this tumor are
sarcomatoid carcinoma of the stomach and spindle cell carcinoma of
the stomach. The average survival of these patients is 2–6 months.
In the present study, we report a case of gastric carcinosarcoma
and review some of the literature. Consent was obtained from the
patient.
Case report
A 62 year-old woman was admitted to our division
reporting a history of epigastric pain with asthenia and weight
loss (8 kilos over 3 months). The physical examination revealed no
specific findings. The patient underwent the following laboratory
tests and endoscopic examinations: esophagogastroduodenoscopy
(EGD), ultrasound (US) and computed tomography (CT) scanning. The
EGD revealed an ulcerated mass in the gastric body-fundus.
On abdominal US, three hepatic mass lesions were
revealed, the largest one being 5×3 cm in size. Abdominal CT
revealed that the tumor had metastasized through the gastric wall
up to the serose and confirmed the hepatic US report. The patient
subsequently underwent an exploratory laparotomy that revealed a
large mass (20×15 cm in size). The tumor had infiltrated the
pancreatic body-tail, gastric body fundus, splenic hilium and left
adrenal gland. A total gastrectomy with Roux-en-Y
esophagojejunostomy with body-tail pancreatic resection and left
surrenalectomy was performed.
Despite the poor prognosis, radical surgery was
performed since the tumor had invaded most of the organ and
perivisceral structures. Radiofrequency ablation (RFA) was used for
the treatment of the hepatic lesions.
Macroscopically, an ulcerative polypoid tumor (13×10
cm) arising from the gastric body fundus was observed. The mass had
infiltrated through all layers of the gastric wall and extended to
the pancreatic tail. The spleen was not infiltrated by the tumor.
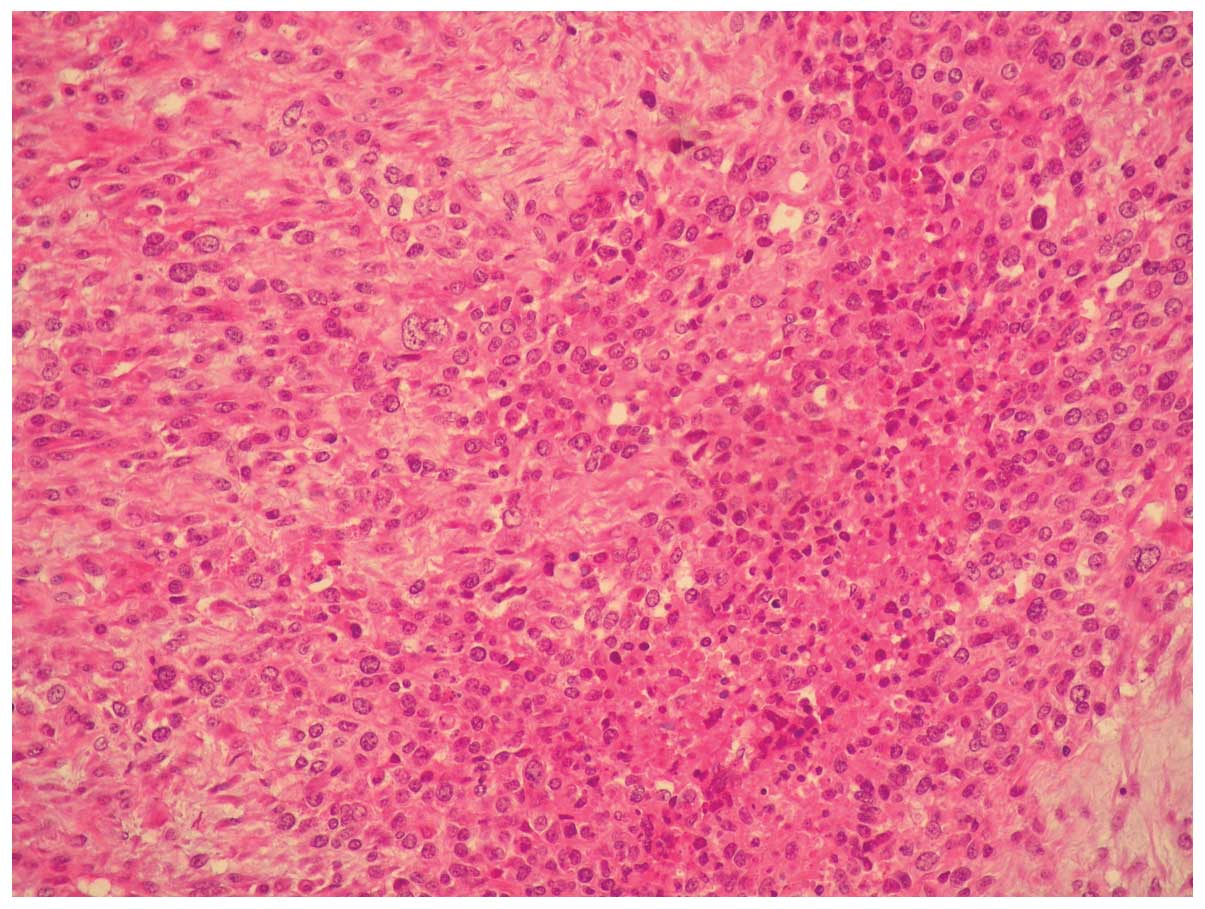
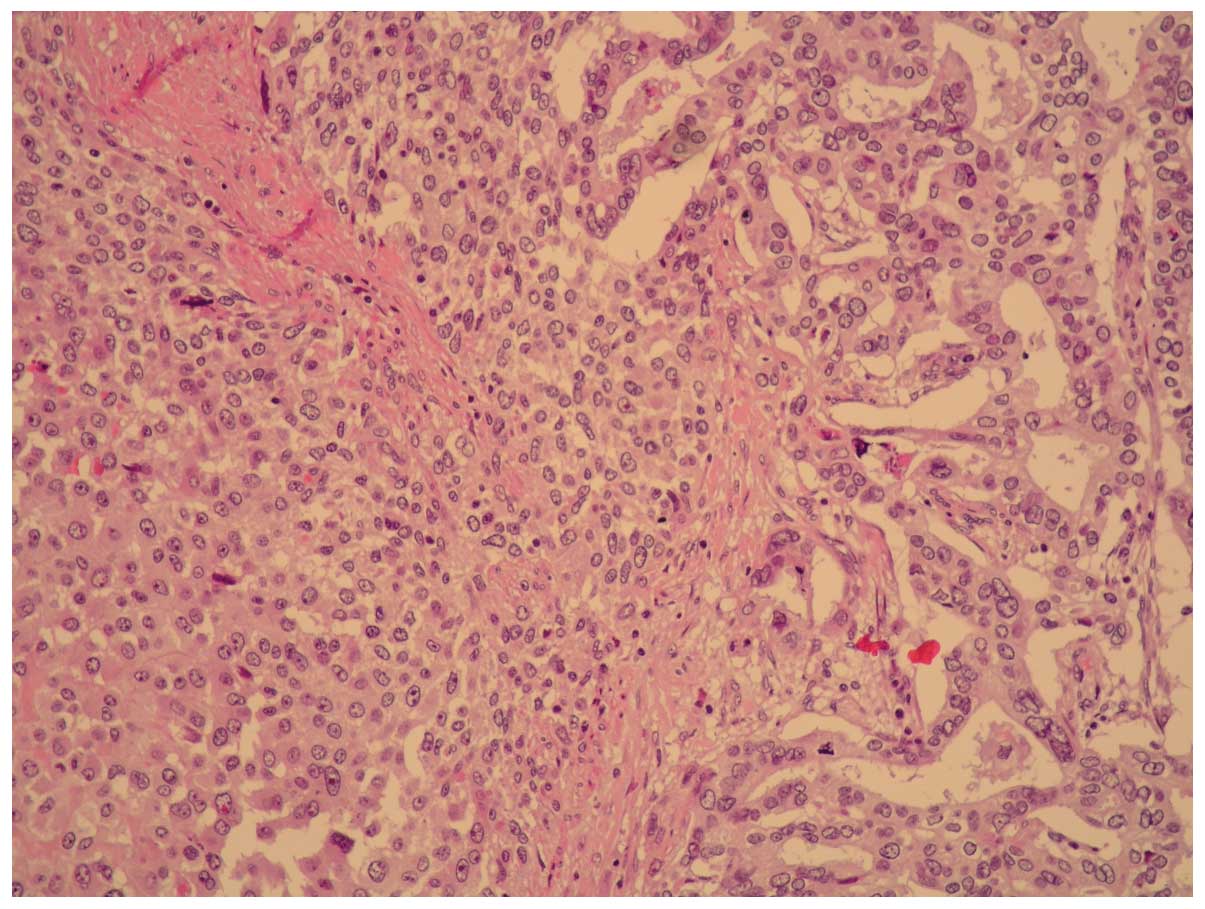
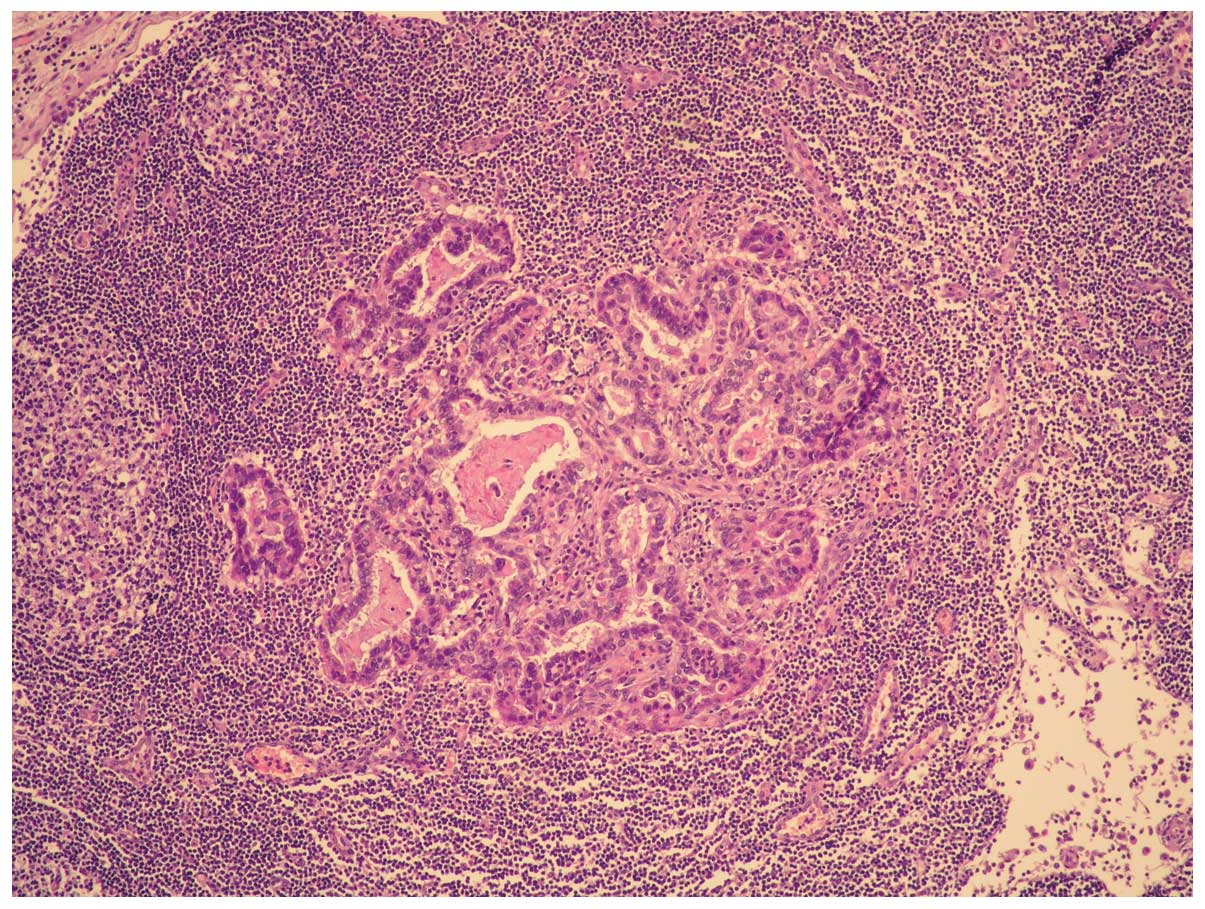
Microscopically, the tumor was composed of moderately
differentiated adenocarcinoma and poorly differentiated sarcoma
with a high mitotic index and necrotic areas (Figs. 1 and 2).
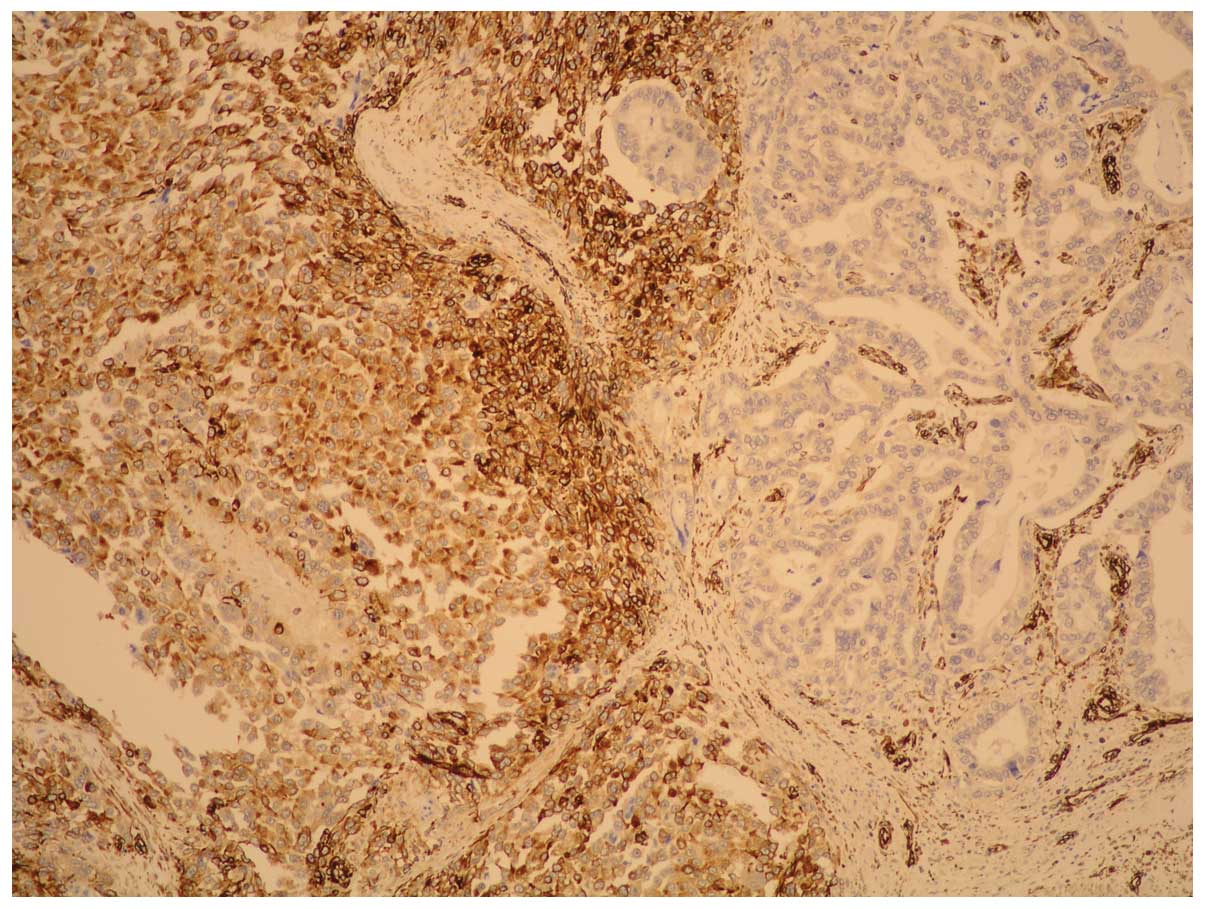
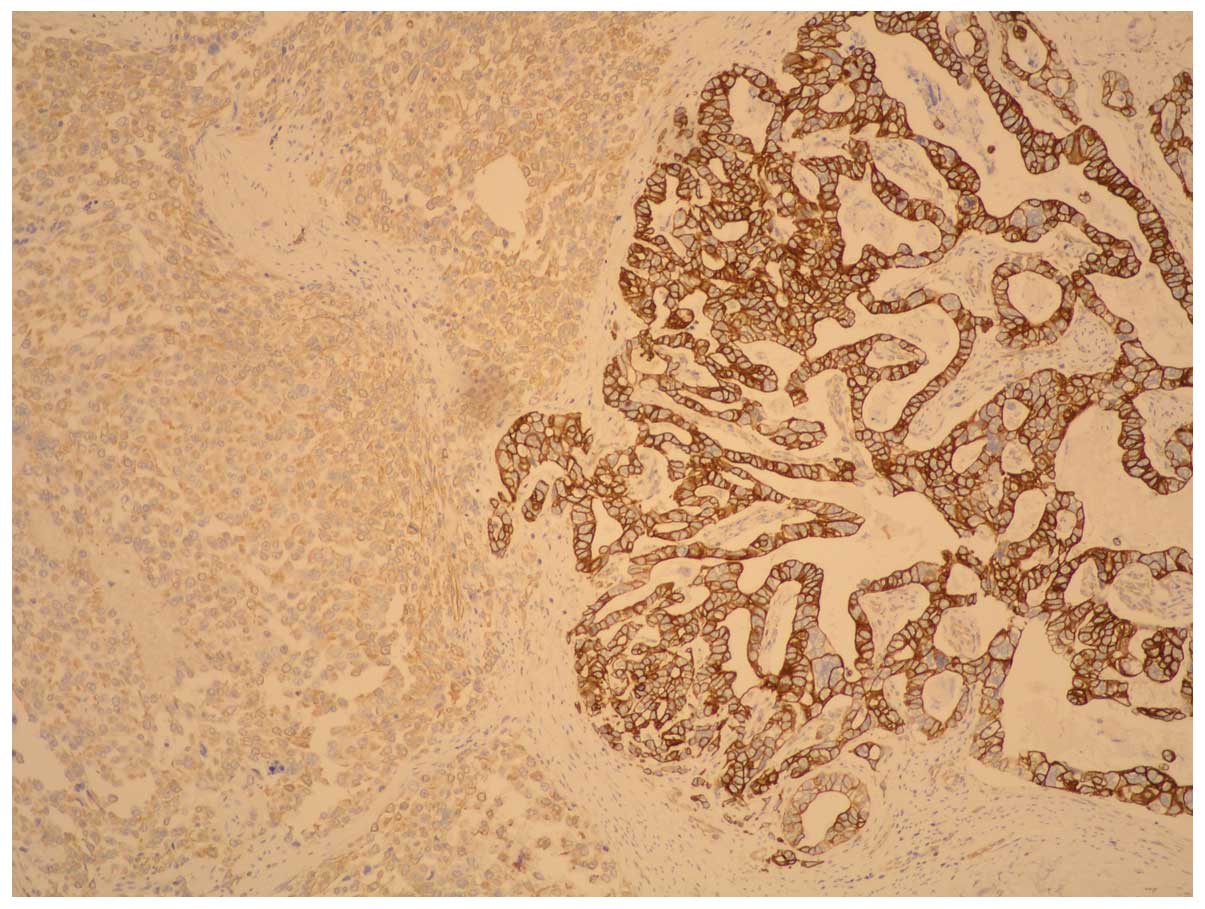
The carcinoma component exhibited a positive
reaction to pan-cytokeratin whereas fusiform cells showed positive
reactions to vimentin: HHF35; CD56; EMA (weak); desmin (singular
component) and negative reactions to cytokeratin, actine,
caldesmon, CD-34, S-100 and cromogranin; synaptophysin, CD-57 and
c-kit. These immunohistochemical findings led to a diagnosis of
gastric carcinosarcoma (Figs. 4 and
5).
The tumor was found to have infiltrated the
perivisceral fat and peripancreatic areas. There was neoplastic
vein thrombosis of the splenic hilium and, for 2 of 13 regional
nodes, metastases were present (Fig.
3). These metastases belonged only to the carcinoma component
(pTNM classification was: T4 N1 G3 R0). The post-operative course
was unremarkable. Due to poor general health conditions, the
patient did not undergo administration of chemotherapy. The patient
succumbed to the disease approximately 4 months later.
Discussion
Carcinosarcoma is defined by the WHO as ‘a malignant
tumor composed of intimately mixed epithelial and mesenchymal
elements of a type ordinarily found in malignancies of adults’.
This definition is based on traditional histological findings
(4). The localizations of this
tumor are widespread.
The most common site of origin for these tumors is
the uterus. Several other organs such as the salivary gland,
thyroid gland, breast, gallbladder, esophagus, stomach, small and
large intestine, pancreas, urinary system and the prostate gland
may also be affected by this type of tumor.
Localization in the stomach has been less frequently
reported (5); male (M) gender is
more affected than female (F) (M:F=2.8:1). These data are
considerably different from that of other locations where
localization to other sites occurs more frequently in women (i.e.,
gallbladder M:F=1:3.25).
The median age of patients affected by gastric
carcinosarcoma was 62 years (range, 29–80), slightly lower than
other localizations in which the most frequent onset age is the
geriatric age (6). The youngest
patient was 29 years old and this case was originally reported by
Saito in 1916 [described by Ashida et al (7)], while the oldest patient was 80 years
old, as reported by Ooi in 1982 (8). The median age of male and female
patients was 60.9 and 61.3 years, respectively.
The dimensions of this type of tumor calculated on
data from 33 reviewed cases and our case report range in size from
4 cm [Cho et al (9)] to 15
cm [Saito, described in (7)]. The
median dimension of the tumors was 9 cm. According to the
macroscopic pattern of growth and particularly in relation to the
gastric wall, carcinosarcoma has been classified into three types
(10): i) a predominantly
intramural infiltration; ii) a predominantly extramural mass; iii)
a predominantly intramural mass with exophytic or crater-shaped
growth.
Microscopically, carcinosarcoma is classified into
two types: true carcinosarcoma and false carcinosarcoma or
so-called sarcomatoid carcinoma. Most of the reviewed cases were
polypoid (20 cases) or ulcerated (19 cases) in appearance. This
type of tumor, as with all visceral carcinomas, tends to develop
rapidly, appearing similar to an endophytic polypoid mass. It can
arise from all areas of the stomach. Cancer does not occur more
frequently in any one area. The exact histogenesis remains
controversial and remains unknown. However, some authors have
proposed two hypotheses (11). The
first is the biclonal origin hypothesis that supports the collision
tumor theory, according to which the carcinosarcoma originates from
two different tumor cell clones. The second is the monoclonal
origin hypothesis, whereby the carcinosarcoma may originate from a
stem cell that is capable of undergoing both epithelial and
mesenchymal differentiation.
In most cases, no specific symptoms of
carcinosarcoma were reported and it was the epiphenomenon of
locally advanced gastric cancer: asthenia, epigastric pain,
dysphagia and vomiting. However, the occurrence of hematemesis and
melena were infrequent (12). A
mass in the epigastric region is frequently revealed on physical
examination. Endoscopic examination is the gold standard in
diagnosis as is contrast-enhanced CT in the staging of the disease.
However, clinical symptoms of carcinosarcomas do not differ from
gastric adenocarcinomas, and a discriminating diagnosis is
endoscopically or radiologically impossible. Furthermore, only an
epithelial or sarcomatous component of the tumor may be observed in
small endoscopic biopsies (13).
In 89% of patients a surgical procedure was
performed. In most cases curative surgery was performed. In some
rare cases palliative surgery was carried out to restore intestinal
continuity or cytoreductive surgery to remove a mass necrosis
(14).
The most frequent surgical procedure performed was
total gastrectomy, which was often carried out on principle and not
of necessity (the tumor had invaded the majority of the organ
and/or perivisceral structures, or prior gastrectomy for peptic
ulcer disease).
Splenectomy and partial pancreatectomy were not
performed on principle but only as a necessity to intervene in
patients with tumors that had invaded the surrounding structures.
When feasible, resection of liver metastasis was performed at the
same time as gastrectomy (15).
In the past, the diagnosis of carcinosarcoma was
obtained by conventional histology. The first association between
traditional histology and immunohistochemistry in the diagnosis of
carcinosarcoma was in a case report in 1988 (16). A third neuroendocrin component has
been identified in certain cases in addition to the carcinoma and
sarcoma components
CEA, EMA, pancreatin, chromogranin A, CD56 and
synaptophysin staining are highly specific markers used to identify
carcinomatous components, whereas desmin, vimentin and α-smooth
muscle/sarcomeric actin show affinity for the sarcomatous elements
(17).
A differential diagnosis between GIST and
mesotelioma is crucial. GIST often occurs as a large
intra-abdominal tumor and sometimes consists of spindle-shaped or
epithelioid cells. However, carcinosarcoma shows no
immunoreactivity for CD117 or CD34 and it does not demonstrate
papillary or glandular structures on H&E sections, such as
those usually observed in malignant mesotheliomas. It also tests
negative for mesothelial cell markers, such as calretinin.
In all reviewed cases the mean survival period was
extremely poor, approximately 6.5 months excluding the four major
(up to 2 years) and minor survivals (less than 30 days). There was
no difference in the survival of patients with gastric cancer where
the neuroendocrin component was present. Overall tumor recurrence
in the first postoperative year was greater than 50%.
From the review of the literature it was not
possible to identify any prognostic factor because only a few cases
had a survival of longer than 12 months: i) in the case report
described by Ashida et al (7), the patient survived 7 years; ii) in
that by Tominaga [described in (7)], the patient survived 5 years; iii) in
that by Kyogoku et al (18),
the patient survived 3 years; iv) in that by Kumagai et al
(19), the patient survived 2
years; v) in the one by Teramachi et al (1) the patient survived 20 months; and vi)
in the case report described by Kitamura (20), the patient survived for 1 year
(Table I).
 | Table IReported cases of gastric
carcinosarcoma. |
Table I
Reported cases of gastric
carcinosarcoma.
| Author (Ref.) | Age | Gender | Medical history | Endoscopy | CT | Laboratory | Treatment | Histology | IHC (positivity) | Follow-up |
|---|
| Randjelovic et
al (9) 2007 | 62 | M | Epigastric pain,
nausea, weight loss and intermittent bleeding from the upper
gastrointestinal tract. In the epigastric region, an elastic,
resistant, fixed mass. | Exophytic, lobulated
mass that infiltrates the entire posterior wall of the stomach,
obturating the lumen throughout. | Irregular,
inhomogeneous, prominent formation (120×80×50 mm) in the
stomach. | Hb: 10 g/dl; Hct:
26%; MCV 78 fL; CA 72.4: 110 U/ml. | Total gastrectomy
with Roux-en-Y esophagojejunostomy and resection of the affected
lymph nodes. | Moderately to
well-differentiated adenocarcinoma with traces of neuroendocrinous
elements. | Cytokeratin 18, EMA,
CEA, vimentin. | Eight months
following surgery, liver metastases were observed on CT scanning.
His general condition did not allow the administration of
chemotherapy. He died approximately 4 months later. |
| Ikeda et al
(7) 2007 | 70 | F | Epigastric discomfort
that had lasted >1 year prior to admission, elastic mass in the
epigastric region, fever, extremely marked emaciation. | Submucosal tumor with
an open ulcer in the anterior wall of the cardia. | Upper abdominal mass
(21×14×8 cm) adjacent to the lesser curvature of the stomach. | Hb: 7.3 g/dl; CA
19-9: 71 U/ml; CA125: 47 U/ml | Palliative
surgery. | | CAM, vimentin,
muscular markers, HHF35. | The patient’s
condition rapidly deteriorated and he died 16 days
postoperatively. |
| Teramachi et
al (1) 2003 | 62 | M | Epigastric pain and
anorexia. | Large ulcerative
lesion in the stomach. | | No
abnormalities. | Total
gastrectomy. | The carcinoma
component was predominantly (95%) composed of undifferentiated
carcinoma cells. The sarcoma component consisted of atypical
spindle cells showing rhabdomyo-, chondro-or osteosarcomatous
differentiation. | CAM5.2, EMA, αSMA,
desmin. | Disease-free for 20
months following surgery. |
| Nakayama et al
(5) 1997 | 69 | M | Partial gastrectomy
(Billroth II) for a duodenal ulcer 30 years earlier, epigastric
pain, emaciation, anemia, elastic soft mass in the left upper
quadrant of the abdomen. | A large polypoid
tumor located on the greater curvature of the remnant stomach. | Huge tumor in the
dilated stomach. There was no metastasis in the liver, the
intra-abdominal lymph node or other organs. | Hb: 6.4 g/dl; Hct:
21.6%; normal CEA level. | Palliative
therapy. | Diffuse sarcomatous
and carcinomatous elements with large areas of necrosis. | Vimentin, desmin,
HHF35, αSMA, EMA, cytokeratins (35pH11 and 34pE12). | |
| Kayaselcuk et
al (12) 2002 | 53 | M | Weight loss,
asthenia, and gastric hemorrhage. | Tumoral mass in the
antrum. | Liver showed many
areas consistent in appearance with metastasis. | | Subtotal gastrectomy
and liver wedge resection. | | Pancytokeratin, EMA,
CEA, vimentin, desmin, αSMA. | The patient underwent
adjuvant chemotherapy. Metastatic focus was determined in the first
8 months. |
| Yamazaki (17) 2003 | 56 | M | Anorexia, rapid
weight loss. | Infiltrating
ulcerated gastric tumor in the posterior wall of the gastric
body. | Swellings of para-
abdominal aortic lymph nodes were revealed. The left subclavicular
lymph node was also swollen. | Hb: 10.9 g/dl; CEA:
87.9 ng/ml; AFP 16 ng/ml; CA19-9: 1093 U/ml | Total
gastrectomy | Three distinct,
components: well to moderately differentiated tubular carcinoma
(70%) neuroendocrine carcinoma (15%) and sarcoma (15%). | CAM5.2, αSMA,
desmin. | The patient died of
esophageal obstruction due to local recurrence of the tumor and
liver metastasis approximately 2 months following surgery. |
| Kuroda et al
(12) 2006 | 59 | M | Epigastric pain and
anorexia. | | | CEA elevated. | Total
gastrectomy | The gastric tumor
consisted of both epithelial and spindle cells. | Chromogranin A,
synaptophysin, αSMA, h-caldesmon, S-100, CAM5.2. | |
| Matsukuma et
al (18) 1997 | 74 | M | Weight loss,
asthenia. | | | | Total
gastrectomy | Adenocarcinomatous
and sarcomatous component. | EMA, CEA, S-100
protein, desmin, vimentin. | Liver metastasis 4
months after surgery. |
| Pase et al
(22) 2005 | 53 | M | Epigastric pain and
anorexia. | A gastric polypoid
tumor. | Round nodular lesion
(2 cm) on the lesser curvature. | No
abnormalities. | Subtotal
gastrectomy | Well-differentiated
adenocarcinoma and carcinoid. | Chromogranin,
Synaptophysin, NSE, CD56. | 6 months
survival. |
| Melato et al
(23) 1993 | 55 | M | Epigastric pain,
weight loss. | | | | Total
gastrectomy | Adenocarcinoma and
fibro-mio-chondro-osteosarcoma highly indifferentiated with mixoid
areas. | NSE, chromogranin,
CEA, desmin calcitonin, synaptophysin. | |
| Tsuneyama et
al (24) 1999 | 63 | M | Epigastric pain and
anorexia. | Large polypoid lesion
on pylorus. | Partial
gastrectomy. | | | Adenocarcinoma with
rhabdomyosarcomatous and neuroendocrine tissue. | EMA, CEA, desmin,
vimentin, chromogranin. | |
| Cruz et al
(25) 1991 | 67 | M | Asthenia, anorexia,
fever. | Large polypoid mass
of the lesser curvature of the stomach. | | Anemia | Total
gastrectomy | | Vimentin, CEA, EMA,
chromogranin | 4 months
survival. |
| Cirocchi et
al (Present study) | 62 | F | Epigastric pain
with marked asthenia and weight loss (8 kg over 3 months). | Ulcerated mass in
the body-fundus gastric. | Abdominal CT
revealed the tumor has spread through the gastric wall until the
serose and three hepatic mass lesions. | Anemia | Total gastrectomy
with Roux-en-Y esophagojejunostomy with body-tail, pancreatic
resection and left surrenalectomy. | Moderately
differentiated adenocarcinoma and poorly differentiated
sarcoma. | HHF35, CD56, EMA,
desmin. | 4 months
survival. |
The most common site of recurrence is the liver
where metastases occur immediately after surgery (17,21).
In the present case report, liver metastases originate from the
adenocarcinoma component.
In conclusion, we reported a case of carcinosarcoma
and the procedure for achieving a definitive diagnosis. The
simultaneous presence of epithelial and mesenchymal elements in a
gastric tumor is a rare event, found almost exclusively in areas
with high incidence of gastric cancer and with only few cases
reported in literature. Carcinosarcoma of the stomach is a rare
malignant tumor of often unclear etiology and pathogenesis. At
present, the gold standard for definitive diagnosis is based on
immunohistochemical staining of endoscopic biopsy or surgical
findings. Radical gastrectomy is the treatment of choice when
feasible even if the tumor has rapid growth and malignant
potential. However, the recurrence of this type of tumor may be
expected within the first postoperative year. Therefore, more
effective diagnostic techniques should be identified to improve
patient survival.
Acknowledgements
The authors thank Professor Angelo Sidoni and Dr
Marta Sbaraglia of the Pathological Anatomy Unit, University of
Perugia, for the histopathological analysis.
References
|
1
|
Teramachi K, Kanomata N, Hasebe T, Ishii
G, Sugito M and Ochiai A: Carcinosarcoma (pure endocrine cell
carcinoma with sarcoma components) of the stomach. Pathol Int.
53:552–556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khan AR: Sarcomatoid carcinoma of the
stomach with heterologous elements. Ann Saudi Med. 19:135–136.
1999.PubMed/NCBI
|
|
3
|
Solerio D, Ruffini E, Camandona M, Raggio
E, Castellano I and Dei Poli M: Carcinosarcoma of the
esophagogastric junction. Tumori. 94:416–418. 2008.PubMed/NCBI
|
|
4
|
Maiorana A, Fante R, Maria Cesinaro A and
Adriana Fano R: Synchronous occurrence of epithelial and stromal
tumors in the stomach: a report of 6 cases. Arch Pathol Lab Med.
124:682–686. 2000.PubMed/NCBI
|
|
5
|
Nakayama Y, Murayama H, Iwasaki H, Iwanaga
S, Kikuchi M, Ikeda S, Okada M, Iizuka Y and Iwashita A: Gastric
carcinosarcoma (sarcomatoid carcinoma) with rhabdomyoblastic and
osteoblastic differentiation. Pathol Int. 47:557–563. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guerra Bautista JA, Ibáñez Delgado F,
Hernández de la Torre Bustillo JM and Alcántara Gijón F: Gastric
carcinosarcoma. Rev Esp Enferm Dig. 98:146–147. 2006.
|
|
7
|
Ashida K, Wamata T, Sugesawa A, Miyano Y,
Iwai N and Tani H: A case of so-called carcinosarcoma of the
stomach. J Jpn Surg Assoc. 59:702–706. 1998. View Article : Google Scholar
|
|
8
|
Ooi A, Okada Y, Nakanishi I and Nakajima
Y: A case of so-called carcinosarcoma of the stomach (in Japanese).
Jpn J Cancer Clin. 28:1300–1304. 1982.
|
|
9
|
Cho KJ, Myong NH, Choi DW and Jang JJ:
Carcinosarcoma of the stomach. A case report with light
microscopic, immunohistochemical, and electron microscopic study.
APMIS. 98:991–995. 1990.PubMed/NCBI
|
|
10
|
Jang SM, Jang SH, Min KW, Na W, Jun YJ and
Paik SS: A case of gastric carcinosarcoma with neuroendocrine and
smooth muscle differentiation. Korean J Pathol. 44:87–91. 2010.
View Article : Google Scholar
|
|
11
|
Randjelovic T and Filipovic B, Babic D,
Cemerikic V and Filipovic B: Carcinosarcoma of the stomach: a case
report and review of the literature. World J Gastroenterol.
13:5533–5536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuroda N, Oonishi K, Iwamura S, Ohara M,
Hirouchi T, Mizumo K, Miyazaki E and Enzan H: Gastric
carcinosarcoma with neuroendocrine differentiation as the carcinoma
component and leiomyosarcomatous and myofibroblastic
differentiation as the sarcomatous component. APMIS. 114:234–238.
2006. View Article : Google Scholar
|
|
13
|
Kikuyama R, Tanaka K, Tano S, et al: A
case of gastric carcinosarcoma. Endoscopy. 41:e220–e221. 2009.
View Article : Google Scholar
|
|
14
|
Ikeda Y, Kosugi S, Nishikura K, et al:
Gastric carcinosarcoma presenting as a huge epigastric mass.
Gastric Cancer. 10:63–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kayaselcuk F, Tuncer I, Toyganozu Y, et
al: Carcinosarcoma of the stomach. Pathol Oncol Res. 8:275–277.
2002. View Article : Google Scholar
|
|
16
|
Siegal A, Freund U and Gal R:
Carcinosarcoma of the stomach. Histopathology. 13:350–353. 1988.
View Article : Google Scholar
|
|
17
|
Yamazaki K: A gastric carcinosarcoma with
neuroendocrine cell differentiation and undifferentiated
spindle-shaped sarcoma component possibly progressing from the
conventional tubular adenocarcinoma; an immunohisto-chemical and
ultrastructural study. Virchows Arch. 442:77–81. 2003.
|
|
18
|
Kyogoku M, Okukubo T and Aoki S: An
autopsy case of carcinosarcoma which originated in the stomach.
Gann. 51:278–279. 1960.
|
|
19
|
Kumagai K, Kawai K, Kusano H, Matsuo K,
Irie J, Tsuchiyama H and Aridome Y: A case of so-called
carcinosarcoma of the stomach. Gan No Rinsho. 30:1931–1936.
1984.PubMed/NCBI
|
|
20
|
Kitamura S: Study on carcinosarcoma of
stomach. Gann. 41:15–27. 1950.PubMed/NCBI
|
|
21
|
Matsukuma S, Wada R, Hase K, Sakai Y,
Ogata S and Kuwabara N: Gastric stump carcinosarcoma with
rhabdomyosarcomatous differentiation. Pathol Int. 47:73–77. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pase F, Galassi A, Tormen D, Missaglia C,
Petrelli G and D’Amore ES: Composite tumour of the stomach: a case
report and review of the literature. Chir Ital. 57:99–102.
2005.PubMed/NCBI
|
|
23
|
Melato M, Bucconi S, Grillo BP, Angelucci
D, Di Stefano P and Natoli C: Carcinosarcoma and separate
neuroendocrine malignant tumor of a malignancy promoter, the
gastric stump. Anticancer Res. 13:2485–2488. 1993.PubMed/NCBI
|
|
24
|
Tsuneyama K, Sasaki M, Sabit A, Yokoi K,
Arano Y, Imai T, et al: A case report of gastric carcinosarcoma
with rhabdomyosarcomatous and neuroendocrinal differentiation.
Pathol Res Pract. 195:93–97; discussion 8. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cruz JJ, Paz JI, Cordero M, Martin J and
del Mar Abad M: Carcinosarcoma of the stomach with endocrine
differentiation. A case report. Tumori. 77:355–357. 1991.PubMed/NCBI
|