Introduction
Esophageal cancer is the third leading cause of
gastrointestinal malignancy and the sixth most frequent cause of
cancer-related mortality worldwide (1). In China, esophageal cancer is the
fourth leading cause of cancer-related mortality, with esophageal
squamous cell carcinoma (ESCC) being the major histological subtype
(2–4). Statistics indicate the insidious
nature of this malignancy and support the importance of developing
improved treatments and preventive strategies. Chemoprevention
plays an integral role in reducing the incidence of cancer and it
is a potentially viable approach to reduce the risk of esophageal
cancer in high-risk individuals (5). Herbal and natural products are
valuable resources for anticancer drugs (6). Plant-derived active principles, as
well as their semi-synthetic and synthetic analogs, have served as
one of the major sources for new anticancer drugs (7,8).
Several plant-derived anticancer agents, including flavopiridol,
acronycine, bruceantin and thalicarpine, are currently being used
in US clinical trials (8).
Consequently, natural products have been the mainstay of cancer
chemotherapy for a number of years (8).
Screening hundreds of traditional Chinese medicines
revealed that the extracts of Cortex periplocae (CP) have
cancer-preventive properties. CP is the dry root of the traditional
Chinese herb Periplocae sepium Bunge, which is referred to
as Xiangjiapi in Chinese. It is a traditional type of medicine
commonly used to treat inflammation, enhance bone density and
muscle mass, and to stimulate the nervous system (9). Itokawa et al found that
periplocoside A, which is extracted from CP, markedly inhibited the
growth of ascite cancer S180 cells (10). Lupeal acetate
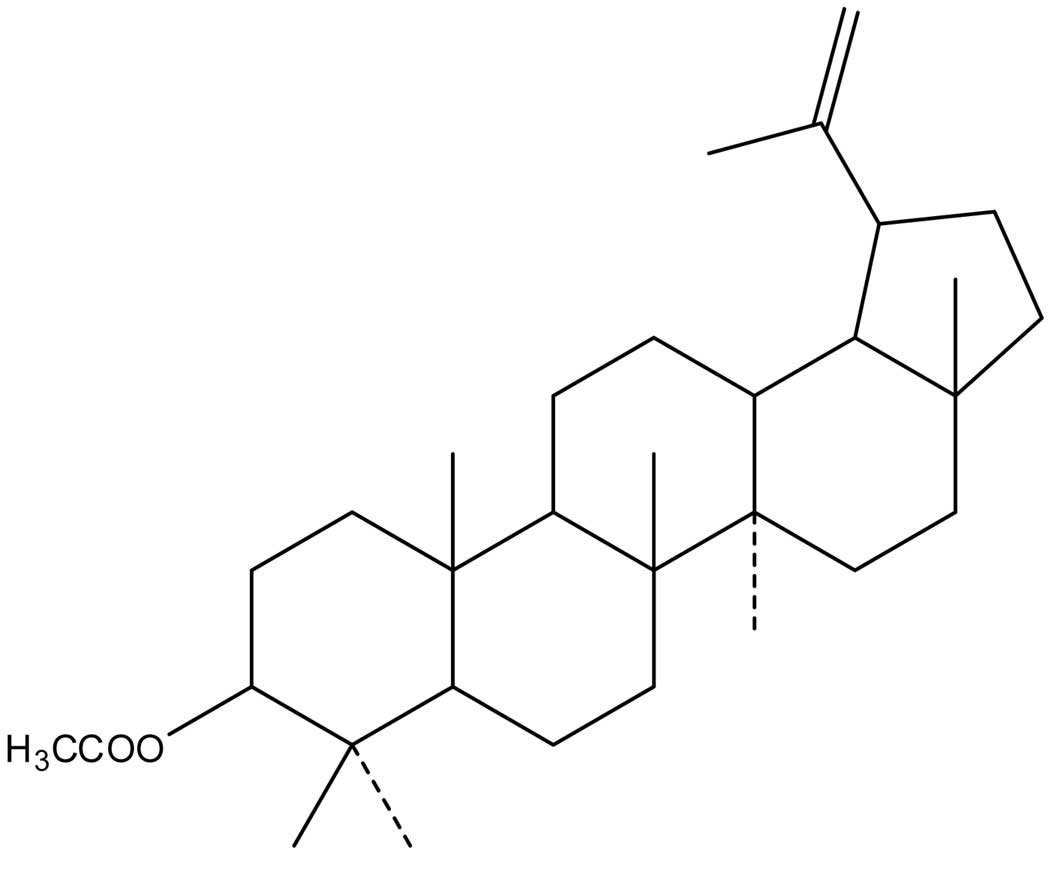
(C32H52O2; MW, 468) (Fig. 1), a triterpene compound extracted
from CP, is known to significantly inhibit the growth of esophageal
cancer, leukemia and breast cancer cells (11,12).
Numerous studies suggest that the activation of the
Wnt/β-catenin signaling pathway is important in human tumorigenesis
(13–16). Therefore, various components of this
signaling pathway may serve as rational targets for the development
of anticancer drugs. Given the complexity of the Wnt/β-catenin
signaling pathway, it is conceivable that potential cancer drugs
may be developed through targeting various nodal points (17). In the Wnt/β-catenin signaling
pathway, glycogen synthase kinase-3β (GSK-3β) mediates the
degradation of β-catenin molecules through phosphorylating specific
amino acid residues. These residues mark the protein that triggers
β-catenin degradation by the 26S proteasome complex (18,19).
When the Wnt proteins bind to the Frizzled/low-density lipoprotein
receptor-related protein (Fz/LRP) receptor complex, cytoplasmic
disheveled (Dvl), a protein downstream of the receptor complex, is
phosphorylated and inhibits GSK-3β. This process occurs by causing
GSK-3β retention at the scaffolding protein axin, which results in
the accumulation of non-phosphorylated β-catenin in the cytoplasm.
Non-phosphorylated β-catenin avoids degradation and translocates
into the nucleus in which β-catenin forms a complex with the
transcription factor TCF and induces the transcription of
downstream target genes, including c-myc and survivin (20–22).
Therefore, GSK-3β is crucial in the regulation of Wnt/β-catenin
target gene expression through controlling the level of cytoplasmic
β-catenin (23). Overall, an
aberrant activation of β-catenin-dependent signaling is a major
contributor in the pathogenesis of ESCC. Therefore, targeting this
pathway may have vital implications in controlling the progression
of ESCC. The present study was conducted to determine whether
pretreatment with lupeal acetate of CP (CPLA) inhibits tumor
development in N-nitrosomethylbenzylamine (NMBA)-induced rat
esophagus by modulating β-catenin, GSK-3β and c-myc expression.
Materials and methods
Animals
A total of 135 male F344 rats (5–6 weeks of age)
were purchased from Shanghai Slac Laboratory Animal Co., Ltd.
(Shanghai, China). The animals were housed 3 per cage and were kept
under standard conditions (20±2°C, 50±10% relative humidity and
12-h light/dark cycles) in our animal center where food and water
were readily available. Hygienic conditions were maintained by cage
changes twice a week and routine cleaning of animal rooms. This
study was conducted in accordance with the internationally accepted
principles for laboratory animal use and the experimental protocols
duly approved by the Institutional Ethics Committee of Hebei
Medical University.
Chemicals and reagent kits
NMBA was purchased from Nard Co., Ltd. (Osaka,
Japan) and CPLA (>85% purity), identified by Professor Ren, was
from New Drug Research and Development Co., Ltd., (North China
Pharmaceutical Co., Ltd., Shijiazhuang, China). NMBA was dissolved
in saline solution, while CPLA was dissolved in soya oil
(Jinghaitang Co., China).
Experimental procedure
Following a 2 week acclimation period to the animal
facility, 135 male F344 rats were randomly divided into 5
experimental groups according to their assigned regimens. The study
consisted of the following groups: 1, 15 rats which subcutaneously
(s.c.) received saline (1 ml/kg); 2, 15 rats which were
intramuscularly (i.m.) injected with soya oil (1 ml/kg); 3, 15 rats
that were i.m. treated with CPLA (20 mg/kg); 4, 45 rats that
received NMBA (0.5 mg/kg) s.c. and CPLA (20 mg/kg) i.m.; 5, 45 rats
treated s.c. with NMBA (0.5 mg/kg). Groups 1, 2 and 3 were negative
controls and group 5 was a positive control. The drugs were
administered 3 times a week for 5 weeks. At weeks 9, 15 and 25, 5
rats from groups 1, 2 and 3, and 15 rats from groups 4 and 5 were
euthanized using pentobarbital sodium and were subjected to gross
necropsy. The esophagus of each rat was excised, opened
longitudinally and cut into thirds. Esophagi were then fixed in 10%
phosphate-buffered formalin solution and routinely embedded in
paraffin for H&E staining to observe any pathological changes
in the tissue. During this time, the β-catenin and GSK-3β protein
expression was detected using western blot analysis and the
expression of c-myc mRNA was detected using RT-PCR. The food intake
and body weight of each rat were recorded weekly.
Pathological diagnosis
The entire esophagus was removed and longitudinally
opened to examine for evidence of gross abnormalities. Samples of
esophageal tissue were fixed in 10% buffered formalin for 24 h and
transferred into 80% ethanol. The formalin-fixed esophagus was then
embedded in paraffin and serial sections (4 μm) were mounted onto
glass slides for histopathological analyses. Pathological diagnosis
of esophageal cancer was determined by a skilled pathologist, who
did not know the background of the administered drugs. According to
Xiang et al (24), the
histopathological features of NMBA-induced tumors in the rat
esophagus were classified into papilloma, involving endophytic
growth of the epithelium; papilloma with atypia, involving
pre-cancerous changes; and carcinoma, involving malignant changes
of basal cells, malignant changes of papilloma, carcinoma in
situ and early infiltrative carcinoma.
Western blot analysis of β-catenin and
GSK-3β expression
Western blot analysis was performed to determine the
β-catenin and GSK-3β protein expression in the esophageal
epithelium. Total protein was isolated from frozen esophageal
epithelium by homogenization in ice-cold buffer containing 20
mmol/l HEPES (pH 7.5), 1.5 mmol/l MgCl2, 0.1 mmol/l
dithiothreitol, 0.4 mol/l NaCl, 20% glycerol, 0.5 mmol/l
phenylmethylsulfonyl fluoride and 0.5 mmol/l leupeptin at 4°C. The
insoluble cellular material was removed using microcentrifugation
at 16,000 rpm for 5 min and the total protein expression was
determined spectrophotometrically. The protein samples were
separated using SDS/polyacrylamide gel electrophoresis and
transferred to the nitrocellulose membrane for western blot
analysis (25).
Semi-quantitative RT-PCR for c-myc
expression
Total RNA was extracted from esophageal tissue using
TRIzol isolation reagent (Gibco-BRL, Carlsbad, CA, USA), according
to the manufacturer’s instructions. RNA concentration was measured
spectrophotometrically at 260 nm, and the integrity was determined
by separating the RNA on 1% agarose gel and estimating the ratio of
18S/28S rRNA. cDNA was synthesized by the reverse transcription of
2 μg of total RNA at 37°C for 45 min. First-strand cDNA was then
performed using an RT-PCR kit (Sino-American Co., Zhejiang, China)
in a 30-μl reaction volume, following the manufacturer’s
instructions.
Following initial denaturation for 5 min at 95°C,
amplification was conducted for 30 cycles as follows: denaturation
at 95°C for 30 sec, annealing at 55°C for 30 sec and extension at
72°C for 30 sec and again at 72°C for 5 min. PCR products were
analyzed using electrophoresis on a 1.5% agarose gel and images
were captured to determine the density of the bands. The relative
values of the c-myc and β-actin bands were calculated in each
sample. The sequences of the primers (synthesized at Sangon,
Shanghai, China) used in the RT-PCR are shown in Table I.
 | Table Ic-myc and β-actin primer
sequences. |
Table I
c-myc and β-actin primer
sequences.
| Gene | Primer sequence | Product size
(bp) |
|---|
| C-myc |
| Sense |
5′CTCCGTCCTATGTTGCG3′ | 275 |
| Anti-sense |
5′GCTGGTGCTGTCTTTGC3′ | |
| β-actin |
| Sense |
5′CCTCTATGCCAACAGTGC3′ | 211 |
| Anti-sense |
5′GTACTCCTGCTTGCTGATCC3′ | |
Statistical analysis
Data were shown as the mean ± standard deviation
(SD). Statistical significance between the groups was determined
using the one-way ANOVA and t-test. The χ2 analyses were
used to compare the incidences of tumor presentation between the
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
General observations
The mean body weights and food consumption levels in
all rats were not significantly different throughout the bioassay
(data not shown). No observable gross or histopathological changes
occurred in the lungs, liver, kidneys, small intestine or colon of
CPLA-treated rats.
Effect of CPLA on preneoplastic lesions
in NMBA-treated rat esophagi
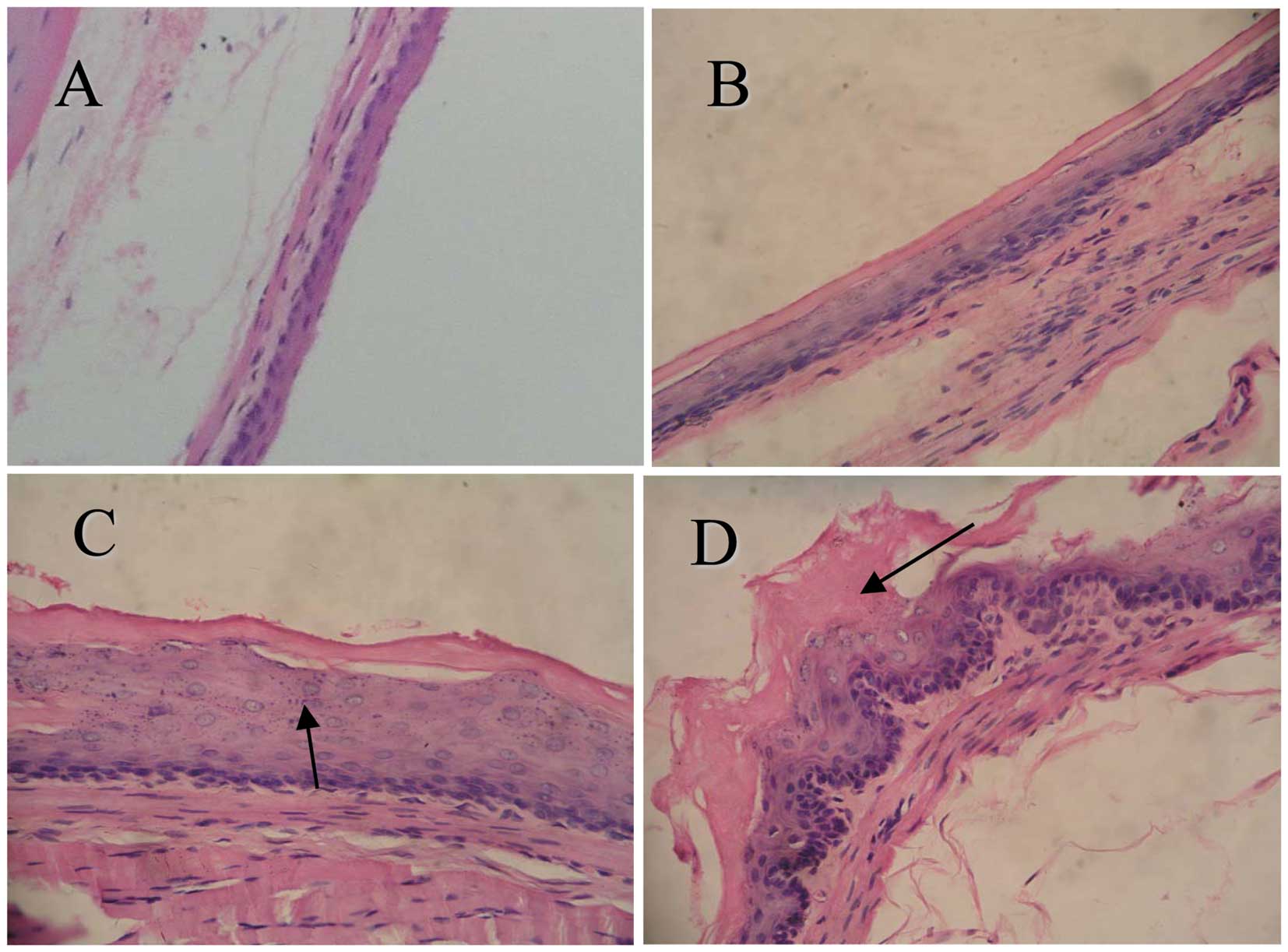
To determine the effects of CPLA on microscopic
NMBA-induced preneoplastic lesions, we used a histological grading
scheme (Fig. 2). At weeks 9 and 15
of the bioassay, <10 and 20% of the esophagi had tumors
(papillomas), respectively. However, the tumor responses (incidence
and multiplicity) at these time points were too weak to determine
whether CPLA treatment produced any inhibitory effects. At the end
of the bioassay (week 25), no rats in group 1 (treated with
saline), group 2 (treated with soya oil) or group 3 (treated with
CPLA) developed tumors. In the rats treated with NMBA, CPLA
significantly (P<0.05) reduced the incidence of esophageal
tumors from 93.3% in group 5 (treated with NMBA only), to 33.3% in
group 4 (treated with NMBA and CPLA) (Table II, Fig.
2). Since the esophagi treated with CPLA alone were
histologically similar to the vehicle controls, data from group 3
(treated with CPLA alone) are not shown.
 | Table IIEffects of CPLA on preneoplastic
esophageal lesion formation in F344 rats administered with NMBA and
euthanized at week 9, 15 or 25. |
Table II
Effects of CPLA on preneoplastic
esophageal lesion formation in F344 rats administered with NMBA and
euthanized at week 9, 15 or 25.
| | Pathological changes
in esophageal tissue |
|---|
| |
|
|---|
| | Normal | Grade I
(dysplasia) | Grade II
(preneoplastic lesion) |
|---|
| |
|
|
|
|---|
| Rat treatment | Total n | n | n | Incidence | n | Incidence (%) |
|---|
| Week 9 of
study |
| (−) | 5 | 5 | 0 | 0 | 0 | 0 |
| Soya oil | 5 | 5 | 0 | 0 | 0 | 0 |
| NMBA | 15 | 3 | 9 | 9/15 | 3 | 3/15 (20.0) |
| NMBA + CPLA | 15 | 9b | 6 | 6/15 | 0 | 0a |
| Week 15 of
study |
| (−) | 5 | 5 | 0 | 0 | 0 | 0 |
| Soya oil | 5 | 5 | 0 | 0 | 0 | 0 |
| NMBA | 15 | 0 | 8 | 8/15 | 7 | 7/15 (46.7) |
| NMBA + CPLA | 15 | 8b | 7 | 7/15 | 0 | 0a |
| Week 25 of
study |
| (−) | 5 | 5 | 0 | 0 | 0 | 0 |
| Soya oil | 5 | 5 | 0 | 0 | 0 | 0 |
| NMBA | 15 | 0 | 1 | 1/15 | 14 | 14/15 (93.3) |
| NMBA + CPLA | 15 | 0 | 10 | 10/15 | 5 | 5/15 (33.3)a |
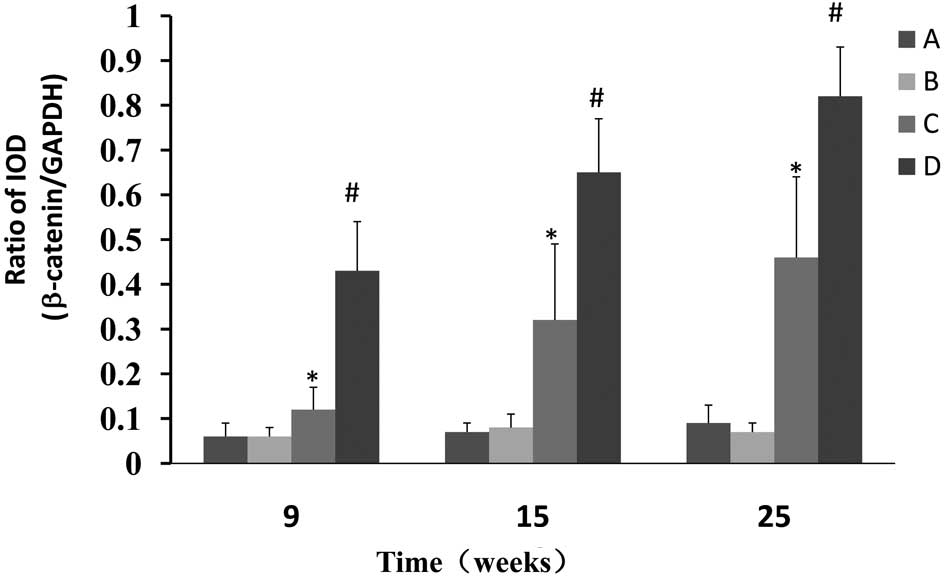
CPLA inhibits the protein expression of
β-catenin and upregulates the expression of GSK-3β
Western blot analysis was used to determine whether
CPLA inhibits β-catenin protein expression in preneoplastic lesions
at various times and, if so, whether this inhibition is associated
with the modulation of GSK-3β. The NMBA-induced overexpression of
β-catenin was significantly (P<0.05) decreased from 7.2- to
2.0-fold, 9.3- to 4.6 fold and 9.1- to 5.1 fold, in rats treated
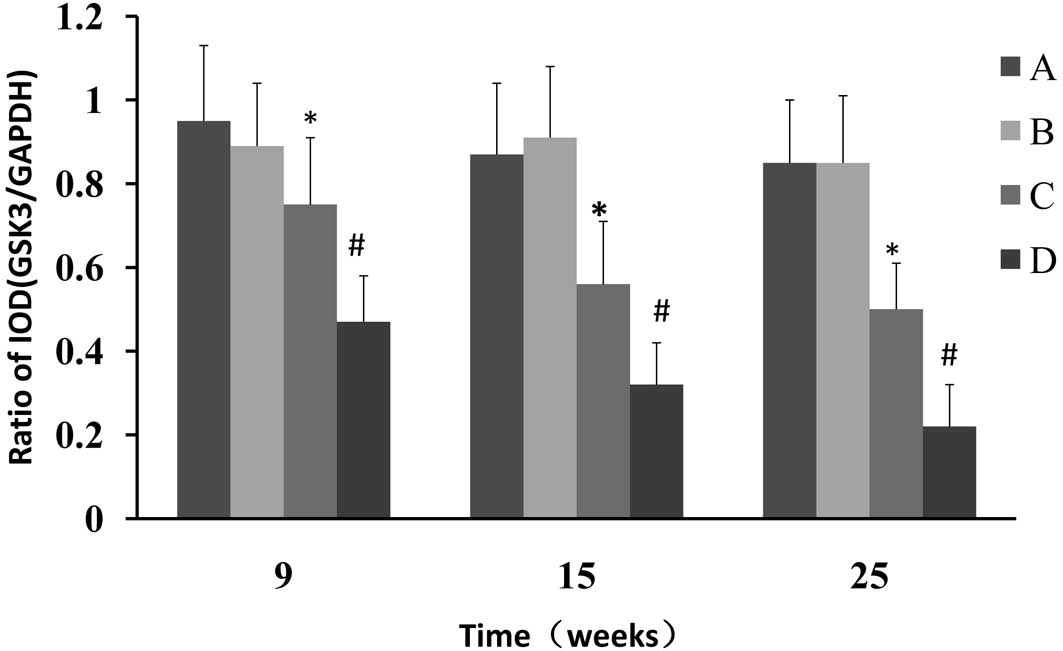
with NMBA and CPLA at 9, 15 and 25 weeks, respectively (Fig. 3). It was also found that CPLA
significantly (P<0.05) upregulates the protein expression of
GSK-3β in NMBA-induced rats at weeks 9, 15 and 25 (Fig. 4).
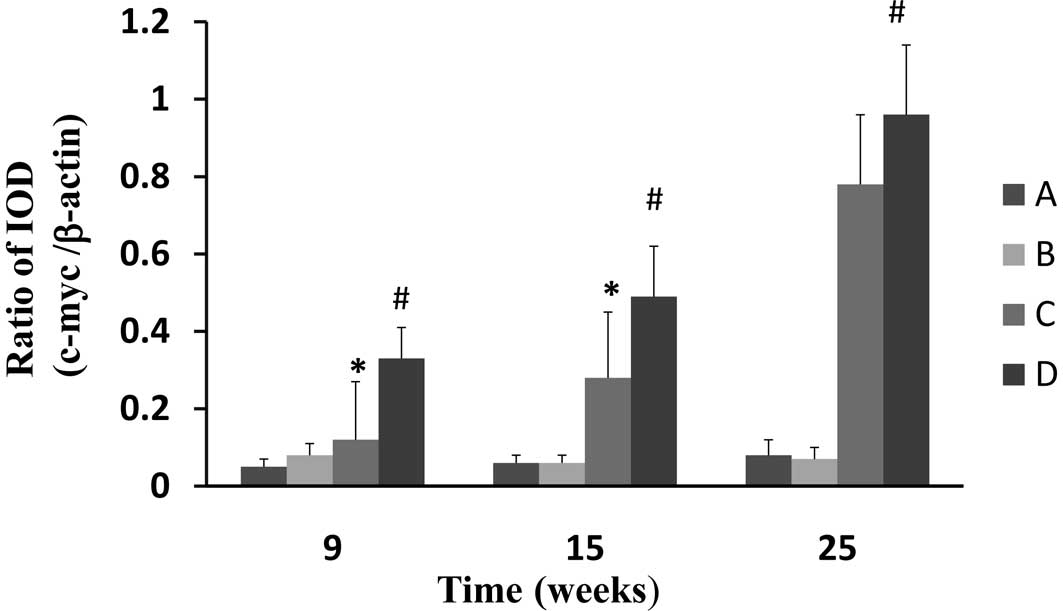
CPLA downregulates mRNA expression of
c-myc
Gene expression of c-myc was assessed using RT-PCR
with β-actin as the internal standard. The gene expression of c-myc
in the esophageal epithelium of the NMBA-treated rats was
significantly (P<0.05) increased at weeks 9, 15 and 25, in
comparison to the normal control (Fig.
5). CPLA treatment significantly (P<0.05) suppressed the
mRNA expression of c-myc at weeks 9 and 15, but not at 25.
Discussion
In the present study, the potential inhibitory
effects of CPLA, an extract from CP, on NMBA-induced esophageal
tumorigenesis in F344 rats, were investigated. We demonstrated that
pretreatment with 20 mg/kg CPLA reduced the tumor incidence rate in
NMBA-treated rats compared to NMBA controls. The inhibition of
tumor development correlated with reductions in esophageal cell
proliferation. Further investigations demonstrated that CPLA acted
as a tumor inhibitor by suppressing β-catenin protein expression
and c-myc gene expression, two key proteins of the Wnt signal
pathway that have previously been identified to be upregulated in
esophageal carcinomas (26).
Additionally, CPLA upregulated the expression of GSK-3β, a
multifaceted kinase in the Wnt signaling pathway (27).
Histopathological data revealed that treating rats
with CPLA alone led to few, if any, changes in the esophagus in
comparison to the normal control group of rats. By contrast,
treatment with NMBA led to basal cell proliferation, cytotoxicity
and inflammatory changes in the esophagus. The histological
appearance of the esophagus in rats treated with CPLA and NMBA
showed a greater degree of normality in comparison to the NMBA
control group of rats. Additionally, CPLA reduced the incidence of
esophageal tumors from 93.3% in NMBA controls to 33.3% in rats
treated with NMBA and CPLA. Therefore, our study provided evidence
that CPLA significantly suppresses carcinoma development.
Wnt proteins are a large family of secreted
glycoproteins that activate signal transduction pathways to control
a variety of cell processes, which include determination of cell
fate, proliferation, migration and polarity. In normal mature
cells, the Wnt pathway regulates normal cellular activities. The
majority of β-catenin within cells binds to E-cadherin on the cell
membrane to form a complex-epidermal catenin and cadherin unit
(ECCU). Free β-catenin levels are normally kept low through a
phosphorylation event that is mediated by GSK-3, α and β isoforms,
which target β-catenin for ubiquitylation and proteasomal
degradation. When the Wnt pathway is activated by the abnormal
expression of oncogenes, antioncogenes and cellular adhesion
molecules, GSK-3β is suppressed (27), and β-catenin is accumulated in the
cytoplasm without degradation. β-catenin is then translocated into
the nucleus, where it binds to Tcf/Lef and initiates transcription
of its target genes, which include c-myc; this results in cellular
canceration (20,28). The β-catenin oncogenic protein is
widely expressed in a number of human malignancies (29), including ESCC (30), head and neck squamous cell carcinoma
(31–33) and colorectal cancer (34). Additionally, it has been reported
that elevated β-catenin levels promote early neoplastic change
through oncogenic signaling within cells (35,36).
Therefore, targeting the oncogenic protein β-catenin may enhance
chemotherapy outcome against solid human cancers (37,38).
The inactivation of GSK-3β has been reported in numerous cancers
with epithelial origin, which include skin, breast, oral, salivary
gland, laryngeal and esophageal cancers (39). In accordance with these findings,
our study demonstrated that GSK-3β was suppressed in the esophagi
of rats in the NMBA control group, while these esophagi also
contained the highest levels of β-catenin. Furthermore, we
demonstrated that CPLA induces the inhibition of proliferation in
preneoplastic epithelium by modulating the β-catenin signaling
pathway. Our results also demonstrated that CPLA significantly
downregulates β-catenin protein expression and upregulates GSK-3β
protein expression, suggesting that CPLA is involved in the
inhibition of the Wnt/β-catenin pathway in rat esophageal
tumorigenesis.
One of the downstream targets of the β-catenin
transcriptional activity involved in cell cycle regulation is
c-myc. c-myc, which appeared to have the strongest evidence as an
independent predictor of ESCC patient outcome (40), regulates processes involved in
numerous aspects of cell fate. Additionally, c-myc is deregulated
in several human neoplasias as a result of genetic and epigenetic
alterations. The near ‘omnipotency’ together with a number of
regulation levels, makes c-myc an attractive target for tumor
intervention therapy (41). In the
present study, c-myc was overexpressed in the esophagi of NMBA
control rats. CPLA significantly downregulated c-myc expression,
suggesting its involvement in inhibiting cell proliferation in
rats.
We demonstrated for the first time that CPLA, a
novel antitumor active extract from a traditional Chinese medicinal
plant, inhibited NMBA-induced rat carcinogenesis via activation of
GSK-3β expression and suppression of β-catenin and c-myc
expression.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Grant No. 30772752). We thank the New
Drug Research and Development Co., Ltd., North China Pharmaceutical
Corporation, China, for their support.
References
|
1
|
Blot WJ and McLaughlin JK: The changing
epidemiology of esophageal cancer. Semin Oncol. 26:2–8.
1999.PubMed/NCBI
|
|
2
|
American Cancer Society. Cancer facts and
figures, 2005. Atlanta, GA: American Cancer Society; 2005
|
|
3
|
Zou XN, Chen WQ, Zhang SW, Li LD, Lu FZ
and Chen YH: An analysis of esophageal cancer incidence and
mortality from 30 cancer registries in China, 1998–2002. Chin
Cancer. 161:142–146. 2007.
|
|
4
|
Stoner GD and Gupta A: Etiology and
chemoprevention of esophageal squamous cell carcinoma.
Carcinogenesis. 22:1737–1746. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kakizoe T: Chemoprevention of cancer -
focusing on clinical trials. Jpn J Clin Oncol. 33:421–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cragg GM, Grothaus PG and Newman DJ:
Impact of natural products on developing new anti-cancer agents.
Chem Rev. 109:3012–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koehn FE and Carter GT: The evolving role
of natural products in drug discovery. Nat Rev Drug Discov.
4:206–220. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mann J: Natural products in cancer
chemotherapy: past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
National Committee of Pharmacopoeia.
Pharmacopoeia of the People’s Republic of China. Chemical Industry
Publishing Company; Beijing, China: pp. 1812005, (In Chinese).
|
|
10
|
Itokawa H, Xu JP and Takeya K: Studies on
chemical constituents of antitumor fraction from Periploca
sepium. V Structures of new pregnane glycosides, periplocosides
J, K, F and O. Chem Pharm Bull. 36:4441–4446. 1998.PubMed/NCBI
|
|
11
|
Zhang J, Shan BE and Liu GS: Apoptosis
induced by ethyl acetate extract from Cortex periplocae in
human breast cancer cell line MCF-7. Tumor. 26:418–421. 2006.(in
Chinese).
|
|
12
|
Zhao LM, Shan BE, Ai J, Ren FZ and Lian
YS: Effects of periplocin from Cortex periplocae on
proliferation of human esophageal carcinoma cells TE-13 and related
mechanisms. Tumor. 28:203–206. 2008.(in Chinese).
|
|
13
|
Polakis P: The oncogenic activation of
beta-catenin. Curr Opin Genet Dev. 9:15–21. 1999. View Article : Google Scholar
|
|
14
|
Waltzer L and Bienz M: The control of
beta-catenin and TCF during embryonic development and cancer.
Cancer Metastasis Rev. 18:231–246. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Behrens J: Control of beta-catenin
signaling in tumor development. Ann NY Acad Sci. 910:21–33. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv J, Cao XF, Ji L, Zhu B, Tao L and Wang
DD: Association of Wnt1/beta-catenin with clinical pathological
characteristics and prognosis of esophageal squamous cell
carcinoma. Genet Test Mol Biomarkers. 14:363–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luu HH, Zhang R, Haydon RC, Rayburn E,
Kang Q, Si W, Park K, Wang H, Peng Y, Jiang W and He TC:
Wnt/beta-catenin signaling pathway as a novel cancer drug target.
Curr Cancer Drug Targets. 4:653–671. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitagawa M, Hatakeyama S, Shirane M,
Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A and
Nakayama K: An F-box protein, FWD1, mediates ubiquitin-dependent
proteolysis of beta-catenin. EMBO J. 18:2401–2410. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moon RT, Bowerman B, Boutros M and
Perrimon N: The promise and perils of Wnt signaling through
beta-catenin. Science. 296:1644–1646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akiyama T: Wnt/beta-catenin signaling.
Cytokine Growth Factor Rev. 11:273–282. 2000. View Article : Google Scholar
|
|
23
|
Takahashi-Yanaga F and Sasaguri T: Drug
development targeting the glycogen synthase kinase-3beta
(GSK-3beta)-mediated signal transduction pathway: inhibitors of the
Wnt/beta-catenin signaling pathway as novel anticancer drugs. J
Pharmacol Sci. 109:179–183. 2009. View Article : Google Scholar
|
|
24
|
Xiang YY, Wang DY, Tanaka M, Igarashi H,
Kamo T, Shen Q, Sugimura H and Kino I: Efficient and specific
induction of esophageal tumors in rats by precursors of
N-nitrososarcosine ethyl ester. Pathol Int. 45:415–421. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Lu A, Liu X, Sang M, Shan B, Meng
F, Cao Q and Ji X: The flavonoid Baohuoside-I inhibits cell growth
and downregulates survivin and cyclin D1 expression in esophageal
carcinoma via β-catenin-dependent signaling. Oncol Rep.
26:1149–1156. 2011.PubMed/NCBI
|
|
26
|
Osterheld MC, Bian YS, Bosman FT,
Benhatter J and Fontolliet C: Beta-catenin expression and its
association with prognostic factors in adenocarcinoma developed in
Barrett esophagus. Am J Clin Pathol. 117:451–456. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu D and Pan W: GSK3: a multifaceted
kinase in Wnt signaling. Trends Biochem Sci. 35:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-myc as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
30
|
Situ DR, Hu Y, Zhu ZH, Wang J, Long H and
Rong TH: Prognostic relevance of β-catenin expression in T2-3N0M0
esophageal squamous cell carcinoma. World J Gastroenterol.
16:5195–5202. 2010.
|
|
31
|
Yang F, Zeng Q, Yu G, Li S and Wang CY:
Wnt/beta-catenin signaling inhibits death receptor-mediated
apoptosis and promotes invasive growth of HNSCC. Cell Signal.
18:679–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goto M, Mitra RS, Liu M, Lee J, Henson BS,
Carey T, Bradford C, Prince M, Wang CY, Fearon ER and D’Silva NJ:
Rap1 stabilizes beta-catenin and enhances beta-catenin-dependent
transcription and invasion in squamous cell carcinoma of the head
and neck. Clin Cancer Res. 16:65–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsai YP, Yang MH, Huang CH, Chang SY, Chen
PM, Liu CJ, Teng SC and Wu KJ: Interaction between HSP60 and
beta-catenin promotes metastasis. Carcinogenesis. 30:1049–1057.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dilek FH, Topak N, Tokyol C, Akbulut G and
Dilek ON: β-Catenin and its relation to VEGF and cyclin D1
expression in pT3 rectosigmoid cancers. Turk J Gastroenterol.
21:365–371. 2010.
|
|
36
|
DasGupta R, Kaykas A, Moon RT and Perrimon
N: Functional genomic analysis of the Wnt-wingless signaling
pathway. Science. 308:826–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saifo MS, Rempinski DR Jr, Rustum YM and
Azrak RG: Targeting the oncogenic protein beta-catenin to enhance
chemotherapy outcome against solid human cancers. Mol Cancer.
9:310–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paul S and Dey A: Wnt signaling and cancer
development: therapeutic implication. Neoplasma. 55:165–176.
2008.PubMed/NCBI
|
|
39
|
Ma C, Wang J, Gao Y, Gao TW, Chen G, Bower
KA, Odetallah M, Ding M, Ke Z and Luo J: The role of glycogen
synthase kinase 3beta in the transformation of epidermal cells.
Cancer Res. 67:7756–7764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang W, Xue L and Wang P: Prognostic value
of β-catenin, c-myc, and cyclin D1 expressions in patients with
esophageal squamous cell carcinoma. Med Oncol. 28:163–169.
2011.
|
|
41
|
Albihn A, Johnsen JI and Henriksson MA:
MYC in oncogenesis and as a target for cancer therapies. Adv Cancer
Res. 107:163–224. 2010. View Article : Google Scholar : PubMed/NCBI
|