Introduction
Breast cancer is the leading cause of cancer
mortality in women worldwide (1).
Despite advances in diagnosis and chemotherapy, numerous women with
breast cancer continue to succumb to this malignancy. The
underlying mechanisms that regulate the progression of breast
cancer are still poorly understood. A variety of factors and genes
are involved in breast cancer pathogenesis. The gene ING4 encodes a
protein belonging to the inhibitor of growth (ING) family, which
are considered to be candidate tumor suppressor genes, playing a
pivotal role in apoptosis, cell cycle regulation, DNA damage repair
(2–5) and control of cellular aging (6). Similar to other ING family members,
ING4 encodes a 249-amino acid protein containing a highly conserved
plant home domain (PHD) finger motif at the C-terminal region, a
conserved central region containing the nuclear localization signal
(NLS) and a variable N-terminal region (7). Various studies have suggested that
ING4 exerts its biological functions via its specific functional
domains, which in association with other regulatory molecular
partners, such as p53, p300, HBO1, NF-κB p65 and H3K4me (7–9),
activate cellular regulation networks. ING4 is also among the few
known regulatory proteins that are able to directly interact with
chromatin as well as being involved in transcription. In the
present study, to explore the role of ING4 in human breast cancer,
we used vector-mediated ING4 to upregulate its expression in the
human breast cancer cell line MCF-7. We investigated the effect of
ING4 overexpression on the proliferation, cell cycle and apoptosis
of MCF-7 cells.
Materials and methods
Cells, reagents and vectors
Human breast cancer cell line MCF-7 was obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). PcDNA3.1(+)/ING4 has been described in previous studies
(10). Cell culture medium
Dulbecco’s modified Eagle’s medium (DMEM) and fetal calf serum
(FCS) were bought from Gibco (Life Technologies, Grand Land, NY,
USA). TRIzol reagents and transfection reagent Lipofectamine™ 2000
were provided by Invitrogen Life Technologies (Carlsbad, CA, USA).
A microplate reader was purchased from Bio-Rad. Flow cytometry
analysis was undertaken with a BD FACS system. PrimeScript™ reverse
transcription reagent kit was purchased from Takara (Dalian,
China). SYBR-Green I kit was obtained from Roche Diagnostics
(Indianapolis, IN, USA). The primary antibody ING4 was obtained
from Santa Cruz (Santa Cruz, CA, USA), p21 and p53 were purchased
from Signalway Antibody Co. (Preland, TX, USA), bax was purchased
from Boster (Wuhan, China) and β-actin was purchased from Bioworld
(Dublin, OH, USA). Enhanced chemiluminescence detection reagents
were purchased from Applygen Technologies Incorporation (Beijing,
China).
Cell culture and transfection
MCF-7 cells were cultured in DMEM supplemented with
10% FCS, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin. All cells were subcultured in six-well plates (with
cells at 60–70% confluence) at 37°C in 5% CO2 and fed
every 2–3 days with complete medium. Transfection was performed
using Lipofectamine 2000 according to the manufacturer’s
instructions (the ratio of the plasmids to the transfection reagent
was 4 μg:10 μl). Three types of cells were utilized, including the
specific plasmid-transfected cells (pcDNA3.1(+)/ING4), and the
empty plasmid-transfected cells and the untransfected cells were
taken as controls. Stable cells were selected with G418 (400 μg/μl)
for three weeks. The stably transfected cells were subsequently
verified by real-time PCR and western blot analysis.
MTT assay
The three cell types were seeded in 96-well plates
at 2.5×103 cells/well in DMEM containing 10% FCS and
incubated for five days at 37°C with 5% CO2. During this
period, cells were selected from three wells from each group every
day at random for methylthiazolyltetrazolium (MTT, 100 μg/well)
assay at 37°C for 4 h. The formazan crystals in the cells were
solubilized with 150 μl/well DMSO for 15 min. Absorbance of the
plates was read using a microplate reader at 570 nm to determine
the inhibitory rates of cell growth.
Flow cytometric analysis of the cell
cycle
Cells were seeded into 6-well plates at a density of
2×105 cells and cultured for 24 h at 37°C. All harvested
cells were washed with ice-cold phosphate-buffered saline (PBS),
then fixed with cold 70% (vol/vol) ethanol overnight at 4°C. Cells
were resuspended in PBS buffer containing a final concentration of
20 μg/ml RNase A and 20 μg/ml propidium iodide for 20 min. The cell
cycle profiles were determined using flow cytometry (FACScan,
Beckton Dickinson, Franklin Lakes, NJ, USA) and analyzed using
CellQuest software. All of the samples were assayed in
triplicate.
Flow cytometric analysis of
apoptosis
An annexin V-fluorescein isothiocyanate kit (BD
Pharmingen, USA) was used to detect apoptosis. The cultured cells
were harvested and washed with ice-cold PBS buffer twice. Binding
buffer containing 5 μl FITC-labeled annexin V and 10 μl of 20 μg/ml
propidium iodide was added to an aliquot of the cell suspension.
Following incubation for 15 min in the dark at room temperature,
stained cells were tested by flow cytometry with ModiFit3.0
software. Cells were gated for analysis by a combination of forward
scatter channels (FSC) and side scatter channels (SSC). All samples
were assayed in triplicate.
Real-time polymerase chain reaction
To further explore the effect of ING4 on cell
proliferation, p21, p53 and bax mRNA were analyzed by real-time
PCR. Total RNA was extracted from the three types of cells using
TRIzol reagent (Invitrogen) according to the manufacturer’s
instructions. RNA was quantified by spectrophotometry and analyzed
by 1% agarose/formaldehyde denaturing gel electrophoresis to
confirm RNA integrity. An equal amount of RNA (500 ng) was used as
a template and reverse transcribed into cDNAs using the PrimeScript
RT kit, then amplified by using SYBR-Green mix kit. The melting
curve data were collected to verify PCR specificity. Each gene was
analyzed in triplicate to diminish operation error. The relative
gene expression levels were calculated according to housekeeping
gene β-actin. Relative gene expression was calculated using the
2-ΔΔCt method. Primers used in the experiments were as
follows: β-actin: F, 5′-AAAGACCTGTACGCCAACAC-3′ and R, 5′-GTCATA
CTCCTGCTTGCTGAT-3′; p21: F, 5′-GATTAGCAG CGGAACAAGGAGT-3′ and R,
5′-GGAGAAACGGGAA CCAGGACA-3′; p53: F, 5′-CAGATTGCAAGTTCACCT
GCCACTA-3′ and R, 5′-GATGAAGCCTGTGTAAGAACC GTCCT-3′; bax: F,
5′-TTTCTGACGGCAACTTCAACTG-3′ and R, 5′-GGAGTCTCACCCACCACCCT-3′;
ING4: F, 5′-GAGGCTGATCTCAAGGAGAA-3′ and R, 5′-TCC
ACAGGCATATCCAACAC-3′.
Western blot analysis
Collected cells were lysed in RIPA buffer [10 mmol/l
Tris-HCl (pH 7.4), 1% deoxycholate, 1% NP40, 150 mmol/l NaCl, 0.1%
SDS, 0.2 mmol/l phenylmethy sulfonyl fluoride, 1 μg/ml aprotinin
and 1 μg/ml leupeptin] for 30 min on ice, then the lysates were
centrifuged at 15,000 × g for 15 min to remove debris. Total
protein concentrations in the supernatants were quantified using
the Lowry method. Equal amounts (20 μg) of protein were denatured,
then separated by 15% SDS-polyacrylamide gel electrophoresis, and
transferred to PVDF membrane. Membranes were blocked in 3% bovine
serum albumin (10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20) for 2 h,
then incubated with primary antibodies against ING4 (1:1,000
dilution, Santa Cruz), p21 (1:500 dilution), p53 (1:500 dilution),
bax (1:200 dilution) and β-actin (1:5,000 dilution) in 3% bovine
serum albumin at 4°C overnight. Subsequently, the blots were
incubated with HRP-conjugated secondary antibodies following 3–5
washes with TBST. The protein bands were visualized by enhanced
chemiluminescence detection reagents and developed with Kodak film.
Normalization was made against β-actin expression.
Statistical analysis
Statistical analysis was carried out using Scheffe’s
F test and Student’s t-test. The differences were considered
statistically significant at P<0.05 and P<0.01.
Results
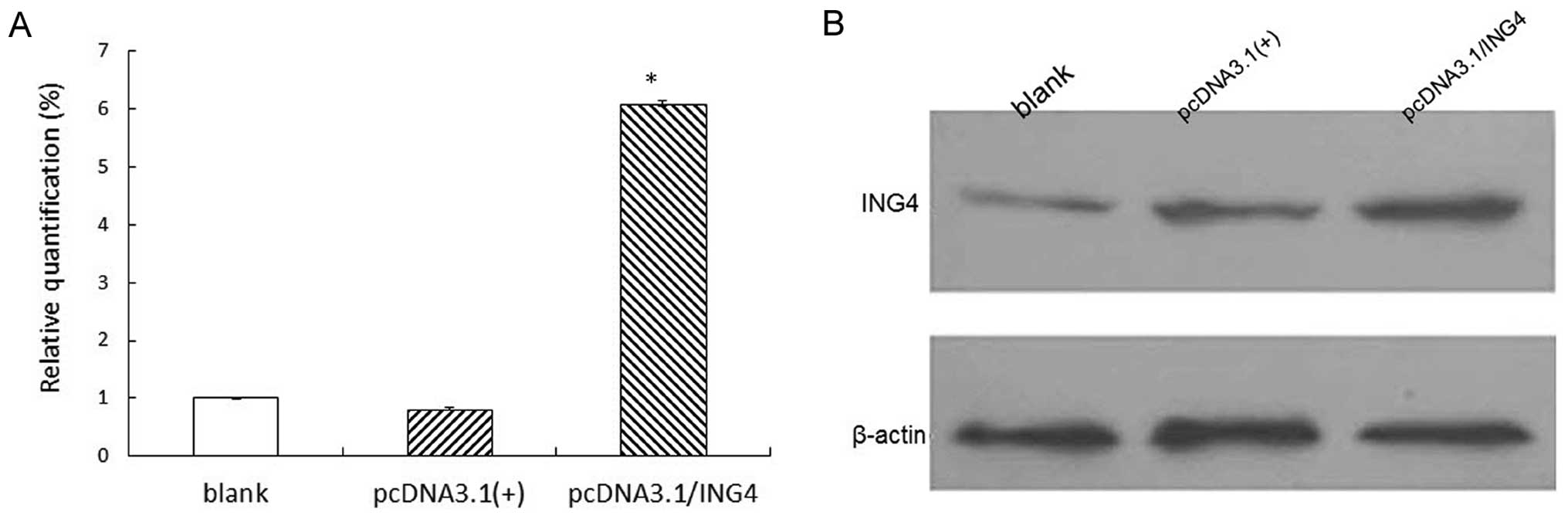
ING4 overexpression in MCF-7 cells by
transfection
Real-time PCR and western blot analysis were used to
evaluate ING4 expression. It was observed that the expression of
ING4 in the stable cell group with exogenous ING4 was increased
significantly in comparison to the negative group and blank group
(P<0.05), while there were no significant differences between
the negative group and the blank group (P>0.05; Fig. 1).
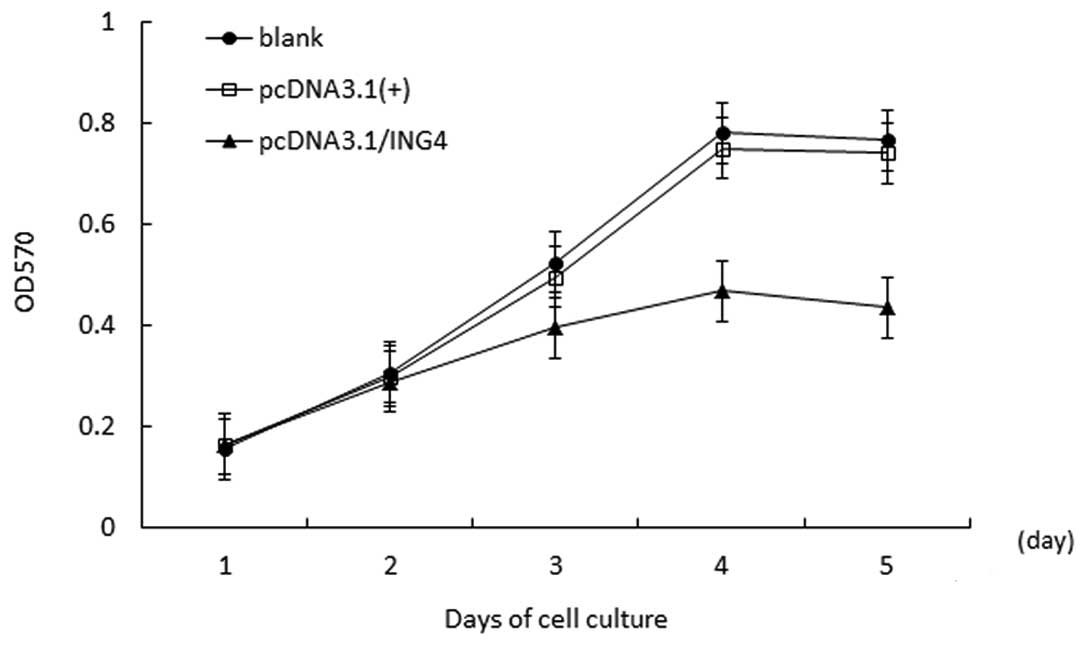
ING4 inhibites MCF-7 cell growth
The cell growth curve demonstrated that compared
with the negative group and the blank group, proliferation in the
stably transfected pcDNA3.1(+)/ING4 group was inhibited
significantly in a time-dependent manner, and the highest
inhibitory rate was 2.58±2.93% (P<0.05) on day 4. However, there
was no obvious difference in cell proliferation in the control
groups (P>0.05; Fig. 2).
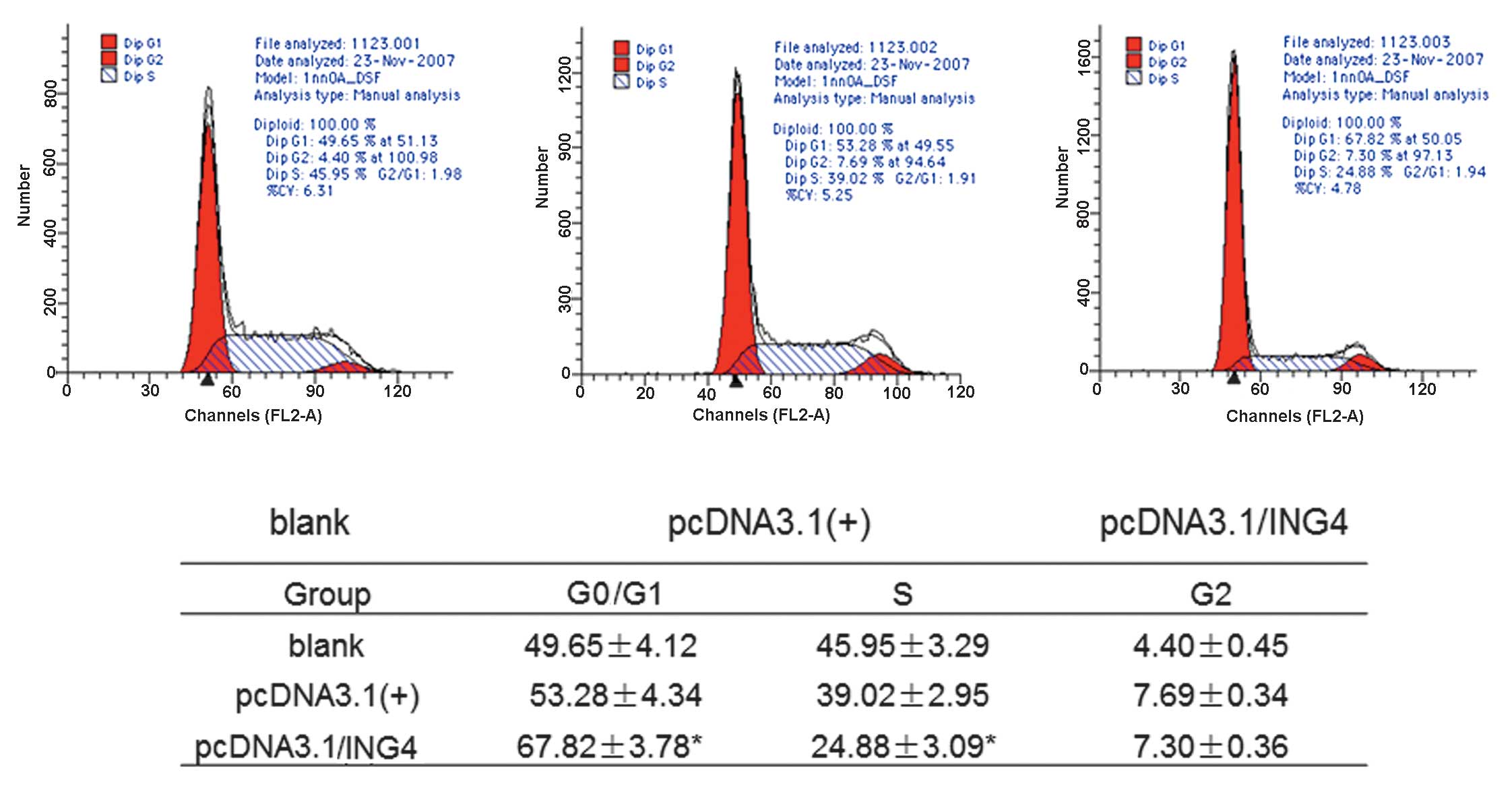
Overexpression of ING4 induces MCF-7 cell
cycle arrest
To investigate the underlying mechanism by which
ING4 suppresses cell growth, cell cycle distribution analysis by
flow cytometry was performed for stably transfected cells and
control cells. Compared with control group cells, the stably
transfected ING4 cells were blocked in the G0/G1 phase by
67.82±3.78% (P<0.05) and reduced in the S phase by 24.88±3.09%
(P<0.05; Fig. 3). These data
suggest that ING4 inhibited the cell proliferation by inducing
G0/G1 arrest and S phase inhibition in the stably transfected
cells.
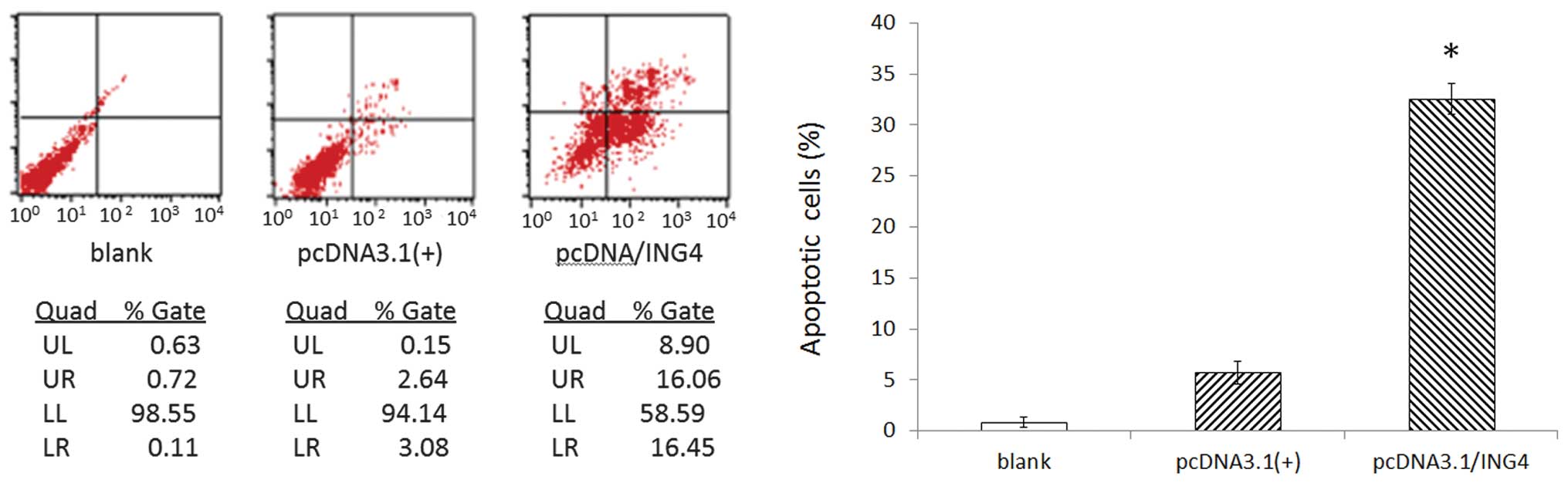
Overexpression of ING4 induces MCF-7 cell
apoptosis
Cell apoptosis detected by flow cytometry revealed
that compared with control group cells, the apoptotic rate of
stably transfected ING4 cells notably increased to 31.51±3.02%
(P<0.05), whereas there were no obvious differences in cell
apoptosis in control group cells (Fig.
4).
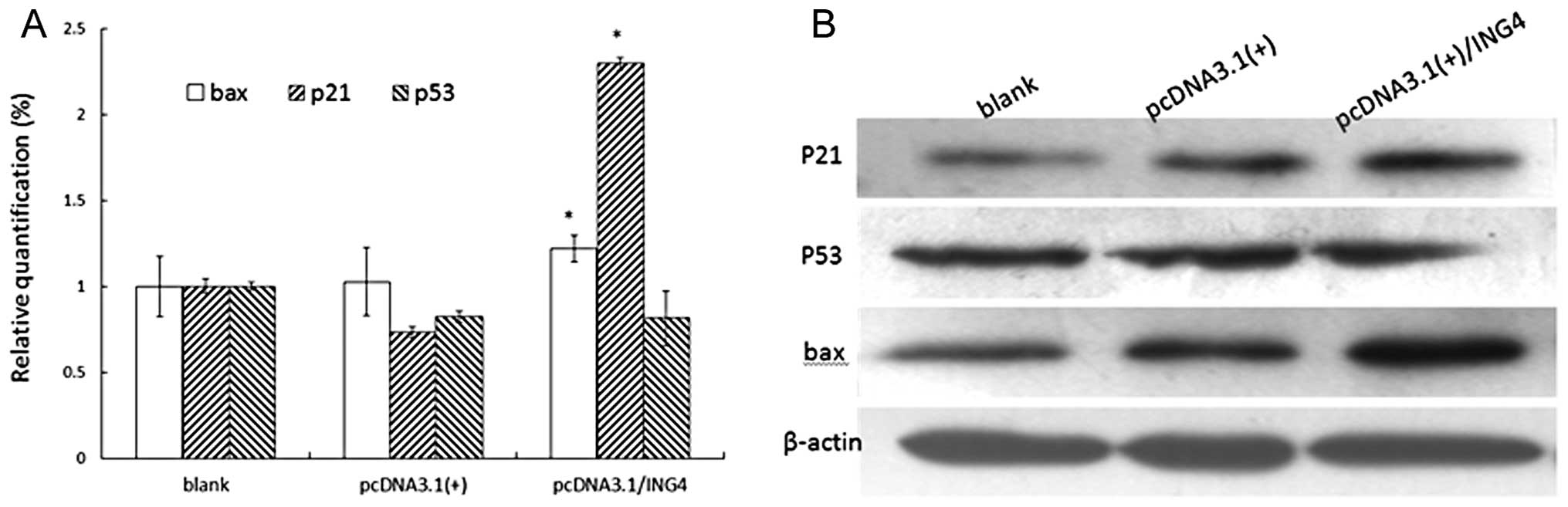
ING4 induces changes in the expression of
certain genes involved in the cell cycle and apoptosis
To further elucidate the basic molecular mechanism
by which ING4 might influence cell fate, the cell cycle and
apoptosis-related genes were investigated. In this study, we
investigated the overexpression of ING4 at mRNA level, and protein
expression of p21, p53 and bax by real-time PCR and western blot
analysis. As shown in Fig. 5, p21
and bax mRNA and protein levels were relatively upregulated in
MCF-7 cells under the impact of overexpression of exogenous ING4.
However, p53 mRNA and protein levels did not change markedly
(Fig. 5).
Discussion
ING4 family proteins are known for their tumor
suppression activity. The type II tumor suppressor protein ING4
specifically displays multiple anti-cancer effects by inhibiting
cell growth and promoting apoptosis, angiogenesis and cell cycle
regulation. Previous studies have shown that ING4 is widely
expressed in normal cell lines, while it is downregulated in
glioblastoma (11) and melanoma
cells (12), as well as in head and
neck squamous cell carcinoma (13).
ING4 overexpression negatively regulates cell growth, with
significant growth arrest at the G2/M stage of the cell cycle, and
also enhances apoptosis triggered by serum starvation in a
p53-dependent manner (14). Studies
exploring ING4 have revealed the frequent deletion or mutational
inactivation of ING4 in multiple cancers including prostate,
ovarian and breast carcinomas (15).
To investigate the effects of ING4 on cell
proliferation in breast cancer cells, we established the stable
breast cancer cell (MCF-7) with constitutive overexpression of
ING4. Furthermore, we detected the cellular behaviors of the stably
transfected cells with exogenous ING4. The results of our study
demonstrate that the overexpression of exogenous ING4 in MCF-7
cells inhibited cell proliferation by inducing G0/G1 arrest,
inhibiting the S phase and enhancing apoptosis. The exogenous ING4
upregulated endogenous p21 and bax, whereas there was no
significant increase of p53 expression in MCF-7 cells.
To explain our experimental results, we referred to
previous studies and assumed that ING4 binds directly to p53, which
modulates its transcriptional activity and also regulates the
expression of p21 and bax (16).
Although the expression level of p53 did not change significantly,
p53 may be involved via the p53-dependent apoptosis pathway, which
is not affected by the amount of p53 present. It has been reported
that crosstalk between signaling pathways may be regulated by
differing levels and modifications of p53, including acetylation
and/or phosphorylation at Lys-382, Ser-15 and Ser-392 residues
(7). Therefore, it is unsurprising
that in the present study the expression levels of p53 remained
constant, whereas those of p21 and bax increased following
treatment. The induced overexpression of p21 led to an increase in
the number of cells in the G1 phase, as shown by flow cytometry
analysis. The p21 protein may interact with proliferating cell
nuclear antigen (PCNA) to regulate S phase DNA replication and
repair. An increase in the levels of p21 inhibits the activity of
cyclin E/CDK2 and cyclin D/CDK4/6, resulting in S phase inhibition
(17). It also has been reported
that overexpression of p21 results in G2 phase arrest (18), but this was not observed in our
study. In addition, p21 is reported to be cleaved by caspase, which
leads to activation of CDK2, and may be functional in the execution
of apoptosis following caspase activation. Bax has been shown to
interact with SH3GLB1 (19), VDAC1
(20), Bcl-2-related protein A1
(21) and others. In this study, we
inferred that overexpression of bax might act as a regulator in its
classical form. Bax is thought to induce the opening of the
mitochondrial voltage-dependent anion channel, resulting in the
release of cytochrome C and other pro-apoptotic factors from the
mitochondria, which subsequently leads to the activation of caspase
via the intrinsic apoptotic pathway.
Together, overexpression of exogenous ING4
significantly induced in vitro cell repression, G1 phase
arrest, S phase inhibition and apoptosis in human breast cancer
MCF-7 cells. This inhibited tumor cell growth elicited by
pcDNA3.1(+)/ING4 was closely associated with upregulation of the
cell cycle and apoptosis-related molecules p21 and bax. ING4 plus
relevant chemotherapy drugs have been widely reported in in
vitro and in vivo studies in cancer cell lines or animal
model experiments (5,22). Our study further suggests that ING4
functions as a tumor-suppressor molecule in the human breast cancer
cell line MCF-7 and may provide new approaches to using ING4 in
breast cancer treatment.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (No. 81172142).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Wang Y and Li G: ING3 promotes UV-induced
apoptosis via Fas/caspase-8 pathway in melanoma cells. J Biol Chem.
281:11887–11893. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moreno A, Palacios A, Orgaz JL, Jimenez B,
Blanco FJ and Palmero I: Functional impact of cancer-associated
mutations in the tumor suppressor protein ING4. Carcinogenesis.
31:1932–1938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J and Li G: Cell cycle regulator ING4
is a suppressor of melanoma angiogenesis that is regulated by the
metastasis suppressor BRMS1. Cancer Res. 70:10445–10453. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie Y, Sheng W, Miao J, Xiang J and Yang
J: Enhanced antitumor activity by combining an adenovirus harboring
ING4 with cisplatin for hepatocarcinoma cells. Cancer Gene Ther.
18:176–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garkavtsev I and Riabowol K: Extension of
the replicative life span of human diploid fibroblasts by
inhibition of the p33ING1 candidate tumor suppressor. Mol Cell
Biol. 17:2014–2019. 1997.PubMed/NCBI
|
|
7
|
Shiseki M, Nagashima M, Pedeux RM,
Kitahama-Shiseki M, Miura K, Okamura S, Onogi H, Higashimoto Y,
Appella E, Yokota J and Harris CC: p29ING4 and p28ING5 bind to p53
and p300, and enhance p53 activity. Cancer Res. 63:2373–2378.
2003.PubMed/NCBI
|
|
8
|
Garkavtsev I, Kozin SV, Chernova O, Xu L,
Winkler F, Brown E, Barnett GH and Jain RK: The candidate tumour
suppressor protein ING4 regulates brain tumour growth and
angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi X, Hong T, Walter KL, Ewalt M,
Michishita E, Hung T, Carney D, Peña P, Lan F, Kaadige MR, et al:
ING2 PHD domain links histone H3 lysine 4 methylation to active
gene repression. Nature. 442:96–99. 2006.PubMed/NCBI
|
|
10
|
Cai L, Li X, Zheng S, Wang Y, Wang Y, Li
H, Yang J and Sun J: Inhibitor of growth 4 is involved in
melanomagenesis and induces growth suppression and apoptosis in
melanoma cell line M14. Melanoma Res. 19:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hassler M, Seidl S, Fazeny-Doerner B,
Preusser M, Hainfellner J, Rössler K, Prayer D and Marosi C:
Diversity of cytogenetic and pathohistologic profiles in
glioblastoma. Cancer Genet Cytogenet. 166:46–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Martinka M and Li G: Role of ING4 in
human melanoma cell migration, invasion and patient survival.
Carcinogenesis. 29:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gunduz M, Nagatsuka H, Demircan K, Gunduz
E, Cengiz B, Ouchida M, Tsujigiwa H, Yamachika E, Fukushima K,
Beder L, et al: Frequent deletion and down-regulation of ING4, a
candidate tumor suppressor gene at 12p13, in head and neck squamous
cell carcinomas. Gene. 356:109–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Xu LS, Wang ZQ, Wang KS, Li N,
Cheng ZH, Huang SZ, Wei DZ and Han ZG: ING4 induces G2/M cell cycle
arrest and enhances the chemosensitivity to DNA-damage agents in
HepG2 cells. FEBS Lett. 570:7–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tapia C, Zlobec I, Schneider S, Kilic E,
Güth U, Bubendorf L and Kim S: Deletion of the inhibitor of growth
4 (ING4) tumor suppressor gene is prevalent in human epidermal
growth factor 2 (HER2)-positive breast cancer. Hum Pathol.
42:983–990. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soliman MA and Riabowol K: After a decade
of study-ING, a PHD for a versatile family of proteins. Trends
Biochem Sci. 32:509–519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Radhakrishnan SK, Feliciano CS, Najmabadi
F, Haegebarth A, Kandel ES, Tyner AL and Gartel AL: Constitutive
expression of E2F-1 leads to p21-dependent cell cycle arrest in S
phase of the cell cycle. Oncogene. 23:4173–4176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niculescu AB III, Chen X, Smeets M, Hengst
L, Prives C and Reed SI: Effects of p21 (Cip1/Waf1) at both the
G1/S and the G2/M cell cycle transitions: pRb is a critical
determinant in blocking DNA replication and in preventing
endoreduplication. Mol Cell Biol. 18:629–643. 1998.PubMed/NCBI
|
|
19
|
Pierrat B, Simonen M, Cueto M, Mestan J,
Ferrigno P and Heim J: SH3GLB, a new endophilin-related protein
family featuring an SH3 domain. Genomics. 71:222–234. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weng C, Li Y, Xu D, Shi Y and Tang H:
Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
in Jurkat leukemia T cells. J Biol Chem. 280:10491–10500. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Cowan-Jacob SW, Simonen M,
Greenhalf W, Heim J and Meyhack B: Structural basis of BFL-1 for
its interaction with BAX and its anti-apoptotic action in mammalian
and yeast cells. J Biol Chem. 275:11092–11099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tzouvelekis A, Aidinis V, Harokopos V,
Karameris A, Zacharis G, Mikroulis D, Konstantinou F, Steiropoulos
P, Sotiriou I, Froudarakis M, et al: Down-regulation of the
inhibitor of growth family member 4 (ING4) in different forms of
pulmonary fibrosis. Respir Res. 10:142009. View Article : Google Scholar : PubMed/NCBI
|