Introduction
The oxidored-nitro domain containing protein 1
(NOR1) gene was previously cloned in our laboratory at
the Third Xiangya Hospital (Hunan, China) using suppression
subtractive hybridization and cDNA microarrays (1,2). By
examining the human genome working sequence, it has been identified
that that the NOR1 gene is located on 1p34.3 and
contains 10 exons and 9 introns. Additionally, the PROSITE database
identified two possible cAMP and cGMP-dependent protein kinase
phosphorylation sites, two tyrosine phosphorylation sites and four
N-myristoylation sites in NOR1. This gene, has an
important oxidored-nitro domain and shares 38% homology with
bacterial nitroreductase (NTR); therefore, the gene was named
NOR1, as approved by The HUGO Gene Nomenclature
Committee (2). Cell cytotoxicity
assays have demonstrated that the NOR1 gene has similar
functions to NTR, which is able to enhance
5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954)-induced cell
killing (3); however, the signaling
mechanisms by which NOR1 enhances CB1954-induced cell
killing remain unknown.
N-nitroso compounds (NOCs), including nitrosamines,
either present in food or formed endogenously, have been suggested
as etiological factors in hepatic carcinoma, stomach cancer,
nasopharyngeal carcinoma (NPC) pathogenesis and various other types
of cancer (4–6). Humans are exposed through ingestion or
inhalation to preformed NOCs in the environment and through the
endogenous nitrosation of amino precursors in the body. In
vivo mechanisms suggest that the formation of NOCs may involve
chemical and enzymatic nitrosation, which is particularly dependent
on the presence of nitrate reductase and nitroreductase. As a
consequence, endogenous nitrosation may occur at various sites
within the body, including the oral cavity, liver, stomach, urinary
bladder and at other sites of infection or inflammation (7).
Growth factor receptor-bound protein 2 (Grb2) is an
adaptor protein that links the associated downstream molecules on
receptor tyrosine kinases (8–10).
Earlier studies have demonstrated that in addition to the
overexpression of receptor tyrosine kinases, overexpression of
downstream proteins, including Grb2, may also induce the
upregulation of signaling pathways associated with cell
transformation. In human breast cancer cell lines (MCF-7,
MDA-MB-361 and MDA-MB-453), overexpression of Grb2 correlated with
increased complex formation between Grb2 and SOS (11–13).
Overexpression of Grb2 protein in the fibroblast cell line NIH 3T3,
potentiated the activation of mitogen-activated protein kinase
(MAPK) (14). Additionally,
overexpression of Grb2 has been demonstrated in chemical
carcinogen-induced liver tumorigenesis. In N-nitrosodimethylamine
(NDMA)-induced A/J mice at 1 year of age, overexpression of Grb2
was identified in liver lesions, preneoplastic foci, adenomas and
carcinomas. These results suggested that the upregulation of Grb2
is an early event within liver carcinogenesis (15).
In a previous study, using cDNA microarray and
quantitative real-time PCR analysis of HepG2 cells, we revealed
that NOR1 produced a 4.8-fold increase in Grb2 mRNA
levels (16). Grb2 is known to
transduce activated tyrosine kinase signaling to Ras, which
subsequently facilitates the activation of downstream signaling
pathways, including Ras and MAPK. In our study, we identified that
NOR1-enhanced CB1954-induced cell killing and
overexpression of NOR1 upregulated Grb2 expression in
HepG2 cells (3,16). Thus, Grb2 may play a role in
NOR1-enhanced CB1954-induced cell killing. In this
study, we examined the involvement of Grb2 and the downstream MAPK
in NOR1-enhanced CB1954-induced cell cytotoxicity in the
hepatic carcinoma cell line HepG2.
Materials and methods
Cell lines, reagents, plasmids and
antibodies
A NOR1 stably-transfected cell line was
previously built at our laboratory (16). Genistein and PD98059 were purchased
from Sigma Chemical Co. (St. Louis, MO, USA). The small hairpin RNA
(shRNA) Grb2-encoding construct used for Grb2 knockdown
(pU6+27-shGrb2) and its control (empty vector,
pU6+27-shControl) were purchased from Panomics, Inc.
(Fremont, CA, USA). Antibodies specific for Erk1/2 and
phosphorylated Erk1/2 were purchased from Cell Signaling Technology
Inc. (Beverly, MA, USA). Antibodies specific for Grb2 and β-actin
were purchased from BD Transduction Laboratories (San Diego, CA,
USA) and Sigma Chemical Co., respectively. Anti-mouse and
anti-rabbit secondary antibodies conjugated with horseradish
peroxidase were purchased from Amersham Pharmacia Biotech
(Piscataway, NJ, USA).
Cell culture and cell cytotoxicity
The human hepatocellular carcinoma cells, HepG2,
were maintained in RPMI-1640 supplemented with 10% fetal calf serum
(FCS) in a humidified culture incubator at 37°C with 5%
CO2 and 95% air. Cell cytotoxicity assays were conducted
as previously described (3). HepG2
cells grown to ~80% confluence were washed with PBS and treated
with a signal transduction inhibitor and/or CB1954. Measurements
were collected from 10–12 individual microscopic fields in each
experiment and data were summarized from 3–5 experiments.
Stable transfection of pU6+27
plasmids into HepG2 cells
HepG2 cells were initially seeded in 6-well tissue
culture plates at 1×105 cells/well in 1.5 ml RPMI-1640
containing 10% FCS. Cells were grown at 37°C in a CO2
incubator until ~75% confluence was reached. Stable transfection of
HepG2 cells with pU6+27-shGrb2 or
pU6+27-shControl plasmid was conducted with 3 μg of
linearized vectors using Lipofectamine™ 2000. After two days, cells
were incubated in the presence of neomycin (0.5–1 mg/ml). Once all
untransfected HepG2 cells were killed by neomycin, HepG2 cells
resistant to neomycin were isolated, grown and examined using
western blot analysis. Clones expressing minimal endogenous Grb2
(HepG2-shGrb2) were selected and propagated for further
experiments.
Western blot analysis
Cells were lysed in ice-cold lysis buffer containing
150 mM NaCl, 20 mM Tris (pH 7.5), 1 mM MgCl2, 1 mM PMSF,
1% Triton X-100, 0.5% sodium deoxycholate, 2 mM sodium
orthovanadate, 25 mM sodium fluoride, 1% aprotinin and 10 μg/ml
leupeptin. The protein concentration of the supernatant was
determined using the Bio-Rad DC Protein Assay (Bio-Rad
Laboratories, Hercules, CA, USA). Equal concentrations of total
protein (5–10 μg) were aliquoted and prepared for electrophoresis
by adding 2X SDS-PAGE loading buffer and boiling. Samples were
electrophoresed on 10% polyacrylamide gels (Bio-Rad Laboratories)
and transferred to nitrocellulose membranes (Schleicher &
Schuell Bioscience, Inc., Keene, NH, USA) for western blot
analysis. Membranes were blocked in Tris-buffered saline containing
20 mM Tris (pH 7.6) and 150 mM NaCl, with 0.1% Tween 20 (TBST)
containing 5% non-fat dry milk (Bio-Rad Laboratories). Primary
antibodies (anti-Grb2, 1:5000; anti-phosphorylated MAPK, 1:5000;
anti-MAPK, 1:1000; anti-β-actin, 1:7500) diluted in TBST were then
added. Membranes were washed and incubated with secondary
antibodies conjugated with horseradish peroxidase. Protein bands
were detected via enhanced chemiluminescence (Kirkegaard &
Perry Laboratories, Gaithersburg, MD, USA) and images were scanned
using an AlphaImager densitometer (Alpha Innotech Corp., San
Leandro, CA, USA).
Statistical analysis
Multiple group comparisons were conducted using
ANOVA and the Student's t-test for pairwise comparisons. Group
differences resulting in P<0.05 were considered to be
statistically significant.
Results
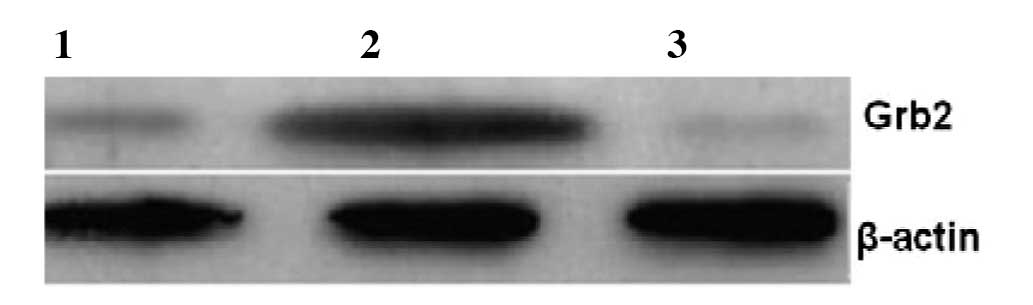
Western blot analysis demonstrates that
NOR1 overexpression enhances the expression of Grb2
In our previous study, we revealed that
overexpression of NOR1 increased the expression of Grb2
mRNA by 4.8-fold in HepG2 cells. To examine whether NOR1
alters the protein level of Grb2, we used
NOR1-overexpressed HepG2 cells as the experimental
group, empty plasmid vector pcDNA3.1(+)-transfected HepG2 cells and
wild-type (WT) HepG2 cells as the control groups. Western blot
analysis demonstrated that NOR1 increases the expression
of Grb2 protein by approximately 3-fold (Fig. 1).
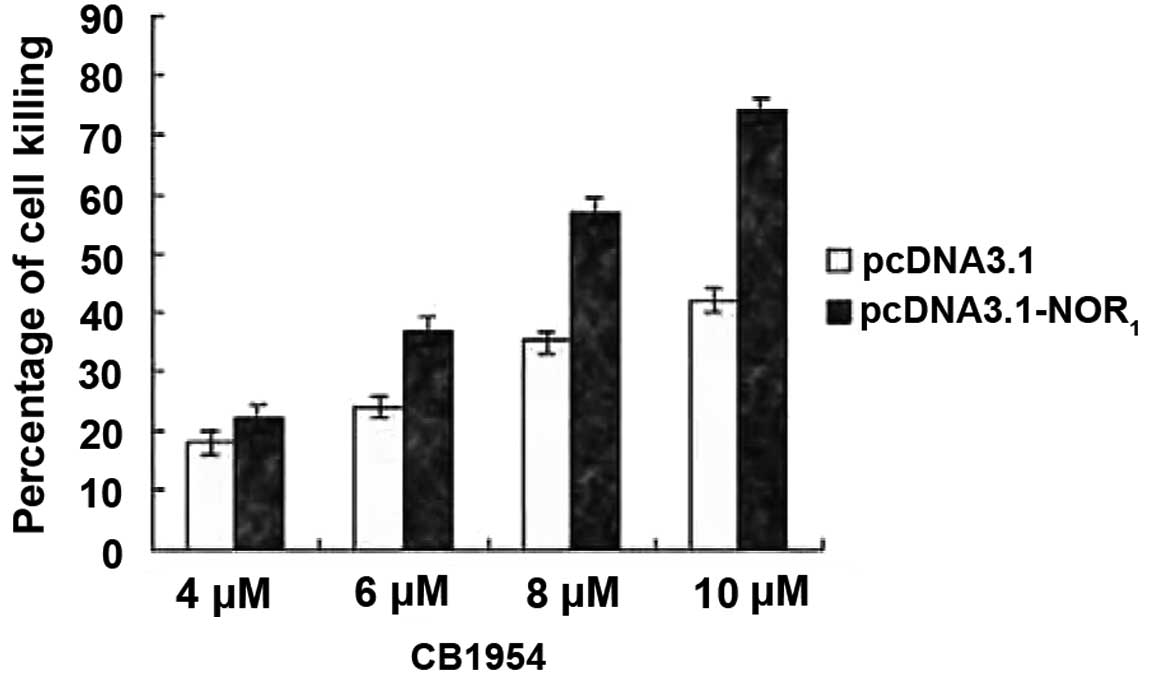
NOR1 overexpression enhances
CB1954-induced cell killing in HepG2 cells
To examine whether NOR1 overexpression
increases CB1954-induced cell killing of HepG2 cells, the HepG2
cell line was transfected with the recombinant plasmid
pcDNA3.1(+)-NOR1 or plasmid pcDNA3.1(+). When the HepG2
cells reached ~80% confluence, they were incubated with various
concentrations of prodrug CB1954 (4–10 μmol/l). Cell viability was
determined after 2 days using Trypan Blue exclusion. As shown in
Fig. 2, expression of the
NOR1 gene in the HepG2 cell line markedly enhanced
CB1954-induced cell killing (P<0.05).
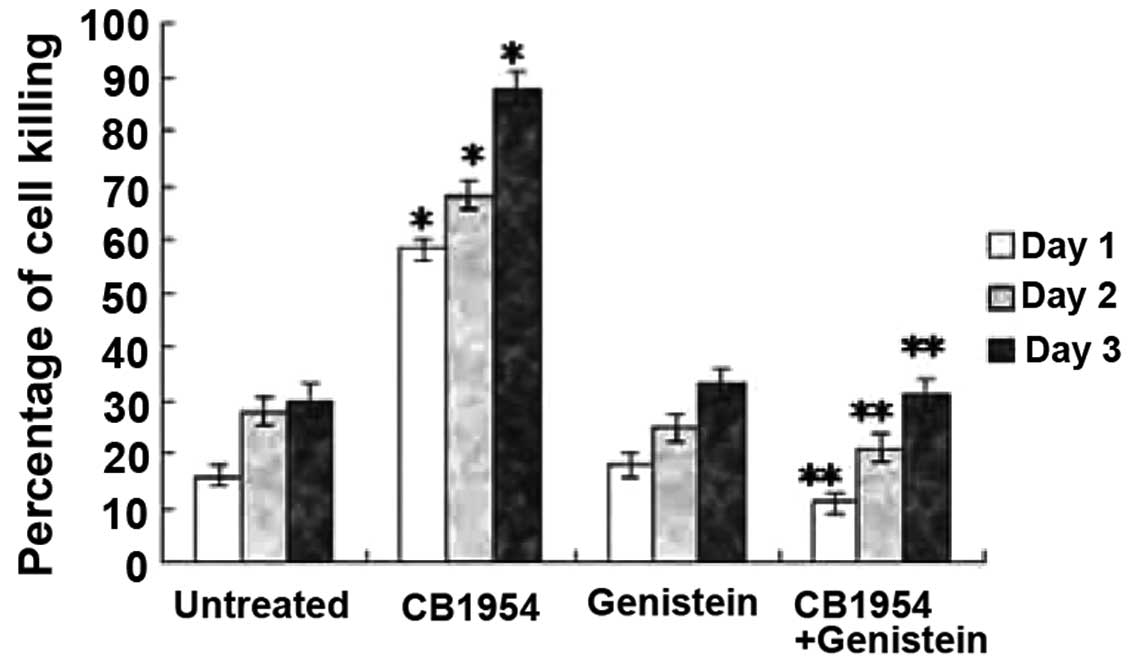
Genistein inhibits
NOR1-enhanced CB1954-induced cell killing
Since the NOR1 gene has two tyrosine
phosphorylation sites and NOR1 gene overexpression
enhanced the expression of Grb2 at mRNA as well as protein level,
and as Grb2 plays an important role in tyrosine kinase receptor
involved signal transduction, we anticipated that NOR1
enhanced CB1954-induced cell killing may depend on tyrosine kinase
activity. Therefore, we assayed levels of cell killing in
pcDNA3.1(+)-NOR1-HepG2 cells treated with the tyrosine
kinase inhibtor genistein in the absence or presence of CB1954. As
we anticipated, treatment with genistein alone did not alter cell
killing; however, in the presence of CB1954, genistein treatment
reduced cell killing comparable to that of untreated control cells
(Fig. 3). These results indicate
that NOR1 enhanced CB1954-induced cell killing is
dependent on tyrosine kinase activity.
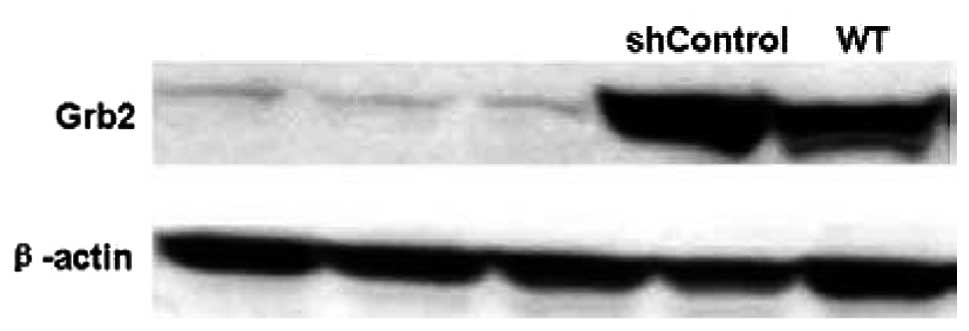
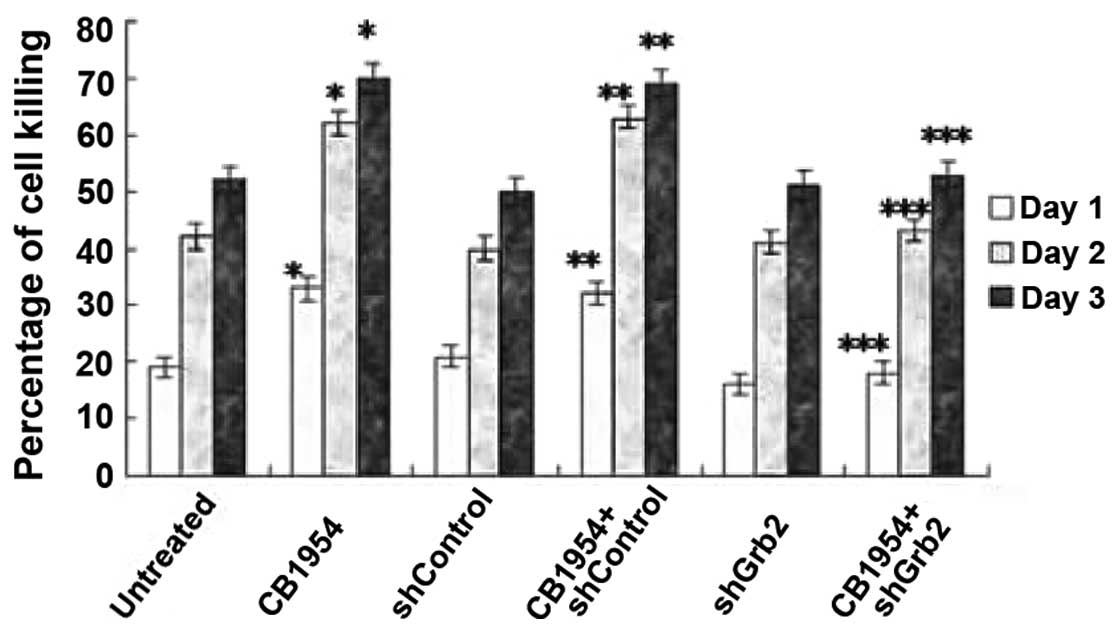
Silencing Grb2 protein expression using
shRNA Grb2 inhibits CB1954-induced cell killing
pcDNA3.1(+)-NOR1-HepG2 cells were WT or
stably transfected by pU6+27-shGrb2 or
pU6+27-shControl. Three clones resistant to neomycin and
expressing pU6+27-shGrb2 were screened for Grb2
expression using western blot analysis (Fig. 4). The effects of Grb2 downregulation
on CB1954-induced cell killing of pcDNA3.1(+)-NOR1-HepG2
cells were examined. In the absence of CB1954, shGrb2 did not alter
HepG2 cell killing; however, in the presence of CB1954, shGrb2
decreased the level of cell killing to that of the untreated
control group (Fig. 5). The
shControl did not alter cell motility in either the presence or
absence of CB1954 (Fig. 5). These
results indicate that Grb2 mediates cell killing induced by CB1954
in pcDNA3.1(+)-NOR1-HepG2 cells.
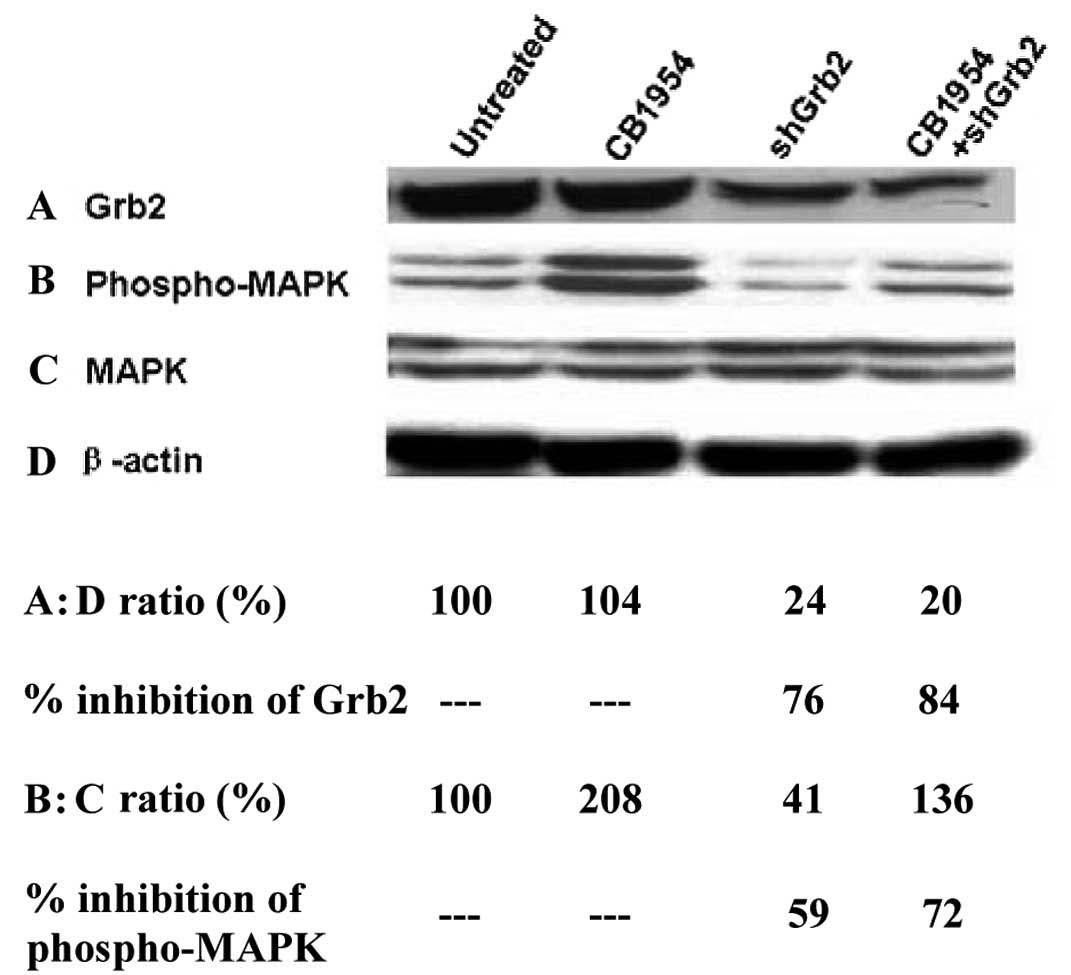
Grb2 mediates the activation of MAPK by
CB1954
Western blot anlysis demonstrated that shGrb2
treatment reduced Grb2 protein expression (Fig. 6A) and CB1954 increased
phosphorylated-MAPK levels (Fig.
6B) in pcDNA3.1(+)-NOR1-HepG2 cells. However,
whether or not their activation is dependent on Grb2 was unknown.
Therefore, we determined whether CB1954-mediated activation of MAPK
is altered by shGrb2. In the presence of CB1954, shGrb2 decreased
Grb2 protein level by 4-fold (Fig.
6A). Compared with CB1954 treatment alone, shGrb2 also
decreased CB1954 mediated activation of MAPK by approximately
3-fold (Fig. 6B and C). These
results indicate that Grb2 mediates CB1954-induced activation of
MAPK.
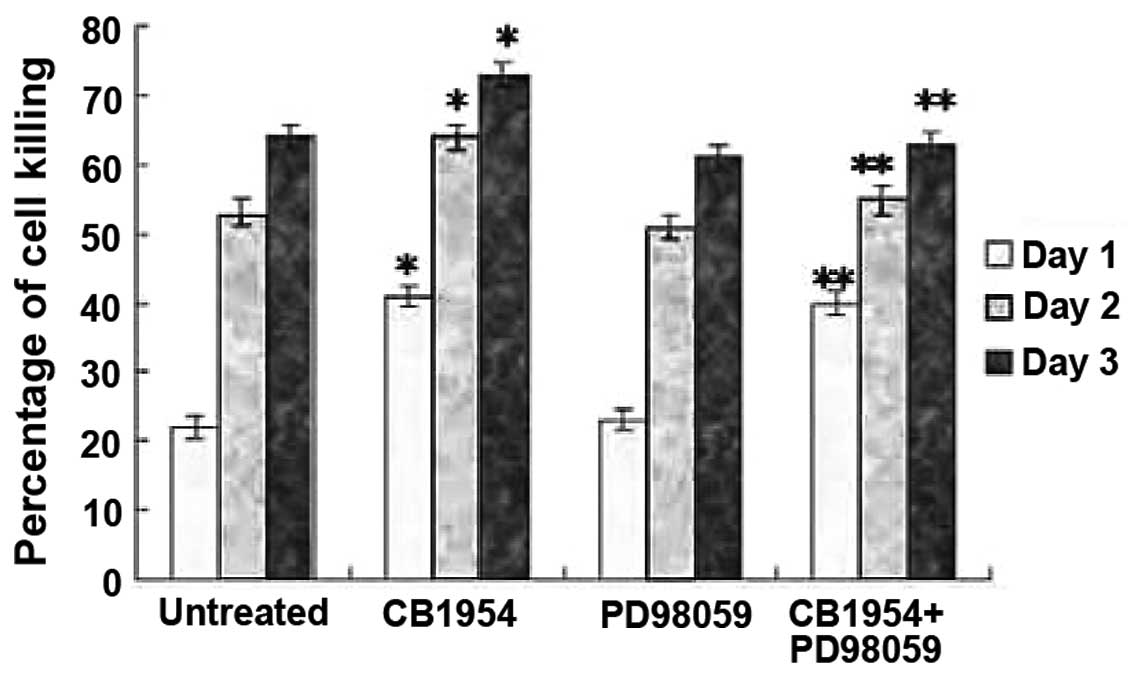
PD98059 inhibits CB1954-induced cell
killing of HepG2 cells
To determine whether MAPK mediates CB1954-induced
cell killing, pcDNA3.1(+)-NOR1-HepG2 cells were treated
with PD98059. PD98059 inhibits methyl ethyl ketone (MEK) activity
and consequently inhibits the activation of its downstream kinase,
MAPK. In the presence of CB1954, PD98059 inhibited the cell killing
of HepG2 (Fig. 7). These data
indicate that CB1954-induced HepG2 cell killing is mediated by the
MEK/MAPK pathway.
Discussion
CB1954 is an antitumor prodrug which recently
entered clinical trials in combination with Escherichia coli
(E. coli) NTR as a potential gene-directed enzyme prodrug
therapy (GDEPT) (17–19). Nitroreduction of CB1954 by E.
coli NTR results in the formation of cytotoxic 4-hydroxylamine
and the reduction of a 2-nitro group to either 2-hydroxylamine or
2-amine, which are potent cytotoxins (20).
Certain studies have demonstrated that the human
liver is capable of aerobic reductive bioactivation of CB1954 to
cytotoxic metabolites in vitro, possibly involving multiple
enzymes, including nitrate reductase and NTR (20). The NOR1 gene is the first
member of the nitroreductase family which has been cloned from
human tissue and has a similar function to the reduction of the
nitro of NTR (2). Our previous
study demonstrated that NOR1 overexpression is able to
convert CB1954 into a toxic form by reducing the 4-nitro group of
CB1954, which subsequently enhances cell killing in CNE1
cells. In this study, we revealed that NOR1 also
enhanced CB1954-induced cell killing in HepG2 cells, and
demonstrated that this process may be due to the upregulated
expression of Grb2 and the activiation of MAPK signal
transduction.
The Grb2 gene is highly conserved among numerous
species and the Grb2 protein is an ubiquitously expressed adapter
protein. Overexpression of the Grb2 protein has been identified in
breast cancer cells, hepatic carcinoma cells and other cancer
tissue specimens. The Grb2 protein contains one Src Homology 2
(SH2) domain situated between two Src Homology 3 (SH3) domains, and
is known to use its SH2 domain to bind to phosphorylated tyrosine
residues of the YXNX motif located in target growth factor
receptors (8,21). However, the NOR1 gene
does not contain this motif and our experiments revealed that
NOR1 does not contact Grb2 directly by
coimmunoprecipitation (data not shown). However, the
NOR1 gene may increase Grb2 expression at the mRNA and
protein level; thus, it is possible that NOR1 does not
form a complex with Grb2, but regulates it to mediate Grb2
signaling.
In conclusion, we used a tyrosine kinase inhibtor
(genistein), a MEK inhibtor (PD98059) and shRNA Grb2 to examine the
mechanisms of NOR1 function. We demonstrated that the
NOR1 gene enhanced CB1954-induced cell cytotoxicity
through upregulating the expression of Grb2 and activiating MAPK
signal transduction. Accordingly, inhibitors targeting the
NOR1/Grb2/MAPK pathway have the potential to be
selective therapeutic or chemopreventive modalities against hepatic
cancer development.
Acknowledgements
This study was supported by grants from the National
Natural Sciences Foundation of China (Nos. 3030083, 81072270 and
81101828), the Fundamental Research Funds for the Central
Universities (No. 2011JQ030) and the Hunan Provincial Natural
Science Foundation of China (No. 07JJ3036).
References
|
1
|
Zhang B, Nie X, Xiao B, Xiang J, Shen S,
Gong J, Zhou M, et al: Identification of tissue-specific genes in
nasopharyngeal epithelial tissue and differentially expressed genes
in nasopharyngeal carcinoma by suppression subtractive
hybridization and cDNA microarray. Genes Chromosomes Cancer.
38:80–90. 2003. View Article : Google Scholar
|
|
2
|
Nie XM, Zhang BC, Li XL, Xiang JJ, Xiao
BY, Ma J, Zhou M, et al: Cloning, expression and mutation analysis
of NOR1, a novel human gene down-regulated in HNE1
nasopharyngeal carcinoma cell line. J Cancer Res Clin Oncol.
129:410–414. 2003.PubMed/NCBI
|
|
3
|
Nie XM, Zhou M, Tang K, Zhang BC, Xiong W,
Lu HB, Li XL, et al: Molecular cloning and characterization of a
novel nitroreductase gene, NOR1, possibly involved in
chemical carcinogenesis of NPC. Chin J Biochem Mol Biol.
19:423–428. 2003.
|
|
4
|
Poirier S, Bouvier G, Malaveille C,
Ohshima H, Shao YM, Hubert A, Zeng Y, et al: Volatile nitrosamine
levels and genotoxicity of food samples from high-risk areas for
nasopharyngeal carcinoma before and after nirosation. Int J Cancer.
44:1088–1094. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Ausman LM, Greenberg AS, Russell
RM and Wang XD: Nonalcoholic steatohepatitis induced by a high-fat
diet promotes diethylnitrosamine-initiate early
hepatocarcinogenesis in rats. Int J Cancer. 124:540–546. 2009.
View Article : Google Scholar
|
|
6
|
Liu C and Russell RM: Nutrition and
gastric cancer risk: an update. Nutr Rev. 66:237–249. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartsch H, Pignatelli B, Calmels S and
Ohshima H: Inhibition of nitrosation. Basic Life Sci. 61:27–44.
1993.
|
|
8
|
Lowenstein EJ, Daly RJ, Batzer AG, Li W,
Margolis B, Lammers R, Ullrich A, et al: The SH2 and SH3
domain-containing protein GRB2 links receptor tyrosine kinases to
ras signaling. Cell. 70:431–442. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rozakis-Adcock M, Fernley R, Wade J,
Pawson T and Bowtell D: The SH2 and SH3 domains of mammalian Grb2
couple the EGF receptor to the Ras activator mSos1. Nature.
363:83–85. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Skolnik EY, Batzer A, Li N, Lee CH,
Lowenstein E, Mohammadi M, Margolis B and Schlessinger J: The
function of GRB2 in linking the insulin receptor to Ras signaling
pathways. Science. 260:1953–1955. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daly RJ, Gu H, Parmar J, Malaney S, Lyons
RJ, Kairouz R, Head DR, et al: The docking protein Gab2 is
overexpressed and estrogen regulated in human breast cancer.
Oncogene. 21:5175–5181. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nguyen DH, Webb DJ, Catling AD, Song Q,
Dhakephalkar A, Weber MJ, Ravichandran KS and Gonias SL:
Urokinase-type plasminogen activator stimulates the
Ras/extracellular signal-regulated kinase (ERK) signaling pathway
and MCF-7 cell migration by a mechanism that requires focal
adhesion kinase, Src, and Shc. Rapid dissociation of GRB2/Sps-Shc
complex is associated with the transient phosphorylation of ERK in
urokinase-treated cells. J Biol Chem. 275:19382–19388. 2000.
|
|
13
|
Normanno N, Campiglio M, Maiello MR, De
Luca A, Mancino M, Gallo M, D'Alessio A and Menard S: Breast cancer
cells with acquired resistance to the EGFR tyrosine kinase
inhibitor gefitinib show persistent activation of MAPK signaling.
Breast Cancer Res Treat. 112:25–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martínez N, García-Domínguez CA, Domingo
B, Oliva JL, Zarich N, Sánchez A, Gutiérrez-Eisman S, et al:
Sprouty2 binds Grb2 at two different proline-rich regions, and the
mechanism of ERK inhibition is independent of this interaction.
Cell Signal. 19:2277–2285. 2007.PubMed/NCBI
|
|
15
|
Diwan BA, Ramakrishna G, Anderson LM and
Ramljak D: Overexpression of Grb2 in inflammatory lesions and
preneoplastic foci and tumors induced by N-nitrosodimethylamine in
Helicobacter hepaticus-infected and -noninfected A/J mice. Toxicol
Pathol. 28:548–554. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li DQ, Tang H, Gui R and Nie XM: Changes
in global gene expression induced by NOR1 over
expression in HepG2 cells. Prog Biochem Biophys. 35:457–464.
2008.
|
|
17
|
Dachs GU, Tupper J and Tozer GM: From
bench to bedside for gene-directed enzyme prodrug therapy of
cancer. Anticancer Drugs. 16:349–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Djeha HA, Todryk SM, Pelech S, Wrighton
CJ, Irvine AS, Mountain A and Lipinski KS: Antitumor immune
responses mediated by adenoviral GDEPT using nitroreductase/CB1954
is enhanced by high-level coexpression of heat shock protein 70.
Cancer Gene Ther. 12:560–571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Helsby NA, Ferry DM, Patterson AV, Pullen
SM and Wilson WR: 2-Amino metabolites are key mediators of CB 1954
and SN 23862 bystander effects in nitroreductase GDEPT. Br J
Cancer. 90:1084–1092. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang MH, Helsby NA, Wilson WR and Tingle
MD: Aerobic 2- and 4-nitroreduction of CB 1954 by human liver.
Toxicology. 216:129–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clark SG, Stern MJ and Horvitz HR: C.
elegans cell-signalling gene sem-5 encodes a protein with SH2
and SH3 domains. Nature. 356:340–344. 1992. View Article : Google Scholar
|