Introduction
Glioblastoma (GBM) is the most common and malignant
type of tumor of the central nervous system (1–3), and
is characterized by its rapid proliferation and widespread invasion
from tumor sites to surrounding normal brain tissues. Despite
recent advances in treatments and understanding of the molecular
mechanism, the median survival time for patients with GBM is only
10–12 months (4–5). In addition, the high growth rate of
tumor cells contributes to a pathophysiological consequence:
hypoxia, which is a source of stress for cancer cells. Cancer cells
undergo genetic and adaptive changes in a hypoxic environment that
contribute to aggressive tumor behavior (6). The aggressive tumor behavior is
dependent on the capacity of cancer cells to invade and migrate,
and is closely linked to the activity of matrix metalloproteinases
(MMPs) which regulate cell invasion, migration and extracellular
matrix degradation (7–9). The increase in expression and activity
of matrix metalloproteinases-2 (MMP2) and -9 (MMP9) has been
correlated with an increased grade of glioma (10–12).
Inhibitors of geranylgeranyltransferase I (13) block post-translational
geranylgeranylation of the small G protein RhoA and inhibit the
ability of RhoA to translocate from the cytosol to the plasma
membrane (14,15). Interacted with the plasma membrane,
RhoA modulates downstream signaling pathways to regulate the cell
cycle and survival (16,17). In addition, RhoA plays an essential
role in cancer cell migration and invasion (18–20).
However, it is not fully understood whether RhoA is capable of
regulating MMPs and cell migration and invasion in a hypoxic
condition. In this study, we demonstrate that inactivation or
reduced expression of RhoA decreases cell migration and invasion
induced by hypoxia in human glioma cells through the inhibition of
the c-Jun NH2-terminal kinase (JNK) signaling pathway and MMP2
activity. Our results suggest that RhoA and JNK play an essential
role in human glioma cell migration and invasion induced by
hypoxia.
Materials and methods
Antibodies and reagents
We used antibodies specific to hypoxia inducible
factor-1α (HIF-1α), phospho-JNK (Thr183/185), JNK, phospho-c-Jun
(Ser63/73), c-Jun and RhoA (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). Anti-β-actin was purchased from Abmart (Shanghai,
China). Antibodies against Na/K ATPase and
geranylgeranyltransferase inhibitor-2147 (GGTI-2147) were purchased
from Sigma (St. Louis, MO, USA). The membrane protein extraction
kit was purchased from BioVision (Mountain View, CA, USA).
Cell culture
Human glioma U251 cells were purchased from Shanghai
Institutes for Biological Sciences (SIBS) of Chinese Academy of
Sciences (CAS). Cells were maintained in Dulbecco’s modified
Eagle’s medium (DMEM)/nutrient mixture F12 (Gibco) supplemented
with 10% FBS (Hyclone, Logan, UT, USA) and were incubated at 37°C
in a humidified incubator with 5% CO2 and 95% air.
Hypoxic and normoxic conditions
Hypoxic conditions were created in an airtight
chamber deoxygenated with the constant infusion of a hypoxic gas
mixture (37°C, 5% CO2, 1% O2, 94%
N2). The O2 content was monitored with an
O2 analyzer. Similarly, normoxic conditions were
maintained in a standard incubator (37°C, 5% CO2, 20%
O2, balanced N2).
Knockdown of RhoA protein
RhoA protein was knocked down using small
interfering RNA (siRNA) against RhoA mRNA. The siRNA was obtained
from Santa Cruz Biotechnology (sc-29471). A negative control siRNA
(sc-44230, Santa Cruz) was used as a control. The sequences of
these siRNAs were not disclosed by the companies. U251 cells were
transfected with 1 μg siRNA against RhoA mRNA or a control siRNA
using Lipofectamine™ 2000 (Invitrogen) in Opti-MEM serum-free
medium (Invitrogen) according to the manufacturer’s instructions.
U251 cells were exposed to hypoxia or normoxia. Then RhoA activity
and protein levels of RhoA were analyzed.
Cell invasion assays
Cell invasion assays were performed using a
Transwell system that incorporated a polycarbonate filter membrane
with a diameter of 6.5 mm and pore size of 8 μm (Corning, NY, USA).
To assess invasion, filters were coated with 10 μg BD Matrigel™ (BD
Biosciences, Franklin Lakes, NJ, USA). Following the manufacturer’s
instructions, cells (1×105 cells) in 100 μl serum-free
medium were added to the upper chamber and the lower chamber was
filled with DMEM containing 10% FBS as a chemoattractant. After 24
h incubation at 37°C, the non-invasive cells were removed from the
upper chamber, and filters were fixed with 100% methanol and
stained with a 0.1% crystal violet solution for 10 min. The number
of invading cells was manually counted as the sum of 5 randomly
selected fields at ×20 magnification using an Olympus IX70
fluorescence microscope (Center Valley, PA, USA).
Western blotting
Cells were lysed in western blotting lysis buffer
[50 mM Tris-HCl (pH 8), 150 mM NaCl, 1% NP-40, 2 mM EDTA (pH 8), 10
mM NaF, 1 mM Na3VO4, 1 mM PMSF, aprotinin and
leupeptin each at 10 pg/ml, 0.5% deoxycholic acid and 0.1% SDS] for
30 min on ice. The lysates were clarified by centrifugation and
protein concentrations were measured. Proteins were separated in
SDS-polyacrylamide gel and transferred to nitrocellulose.
Nitrocellulose was blocked in 3% goat serum for 1 h at room
temperature and then incubated with primary antibodies followed by
incubation with horseradish peroxidase-conjugated secondary
antibodies. Both antibodies were diluted in washing buffer
containing 1% BSA. The blots were developed by the enhanced
chemiluminescence technique (ECL kit, Amersham Biosciences,
Sunnyvale, CA, USA). Representative results from at least 3
independent experiments are shown. Densitometry was performed using
Image J software.
Gelatin zymography
Gelatin (Biotech, Shanghai, China) was dissolved in
distilled water at a concentration of 1%, autoclaved, cooled and
stored at 4°C. The transfected cells were incubated in serum-free
DMEM for 24 h. The conditioned medium was collected and centrifuged
for 5 min at 10,000 × g to discard unsoluble materials. Total
protein content was determined by a colorimetric assay using BCA
protein dye. 10% SDS-polyacrylamide gel containing 0.1% gelatin was
polymerized and the medium was separated in the gel without prior
boiling. The gel was washed in 2.5% Triton X-100 in water for 1 h
to remove residual SDS and then incubated in developing buffer [50
mM Tris-HCl (pH 7.5), 5 mM CaCl2, 150 mM NaCl and 0.02%
sodium azide] for 24 h at 37°C to promote the activity of
proteinases. The gel was stained with 0.5% Coomassie Brilliant Blue
for 1 h and then destained with 30% methanol and 10% acetic acid.
Proteolysis was detected as a white zone on a dark field. The
intensity of the bands was quantified using Image J (version
1.34).
Statistical analysis
The figures show data obtained in at least three
independent experiments as indicated. Quantitative data were
expressed as the means ± SE. Analysis of variance and Student’s
t-test were used to analyze the difference between the means of
test samples and controls, and P<0.05 was considered to indicate
a statistically significant result (*P<0.05,
**P<0.01).
Results
Hypoxia increases glioma cell invasion
and MMP2 activity
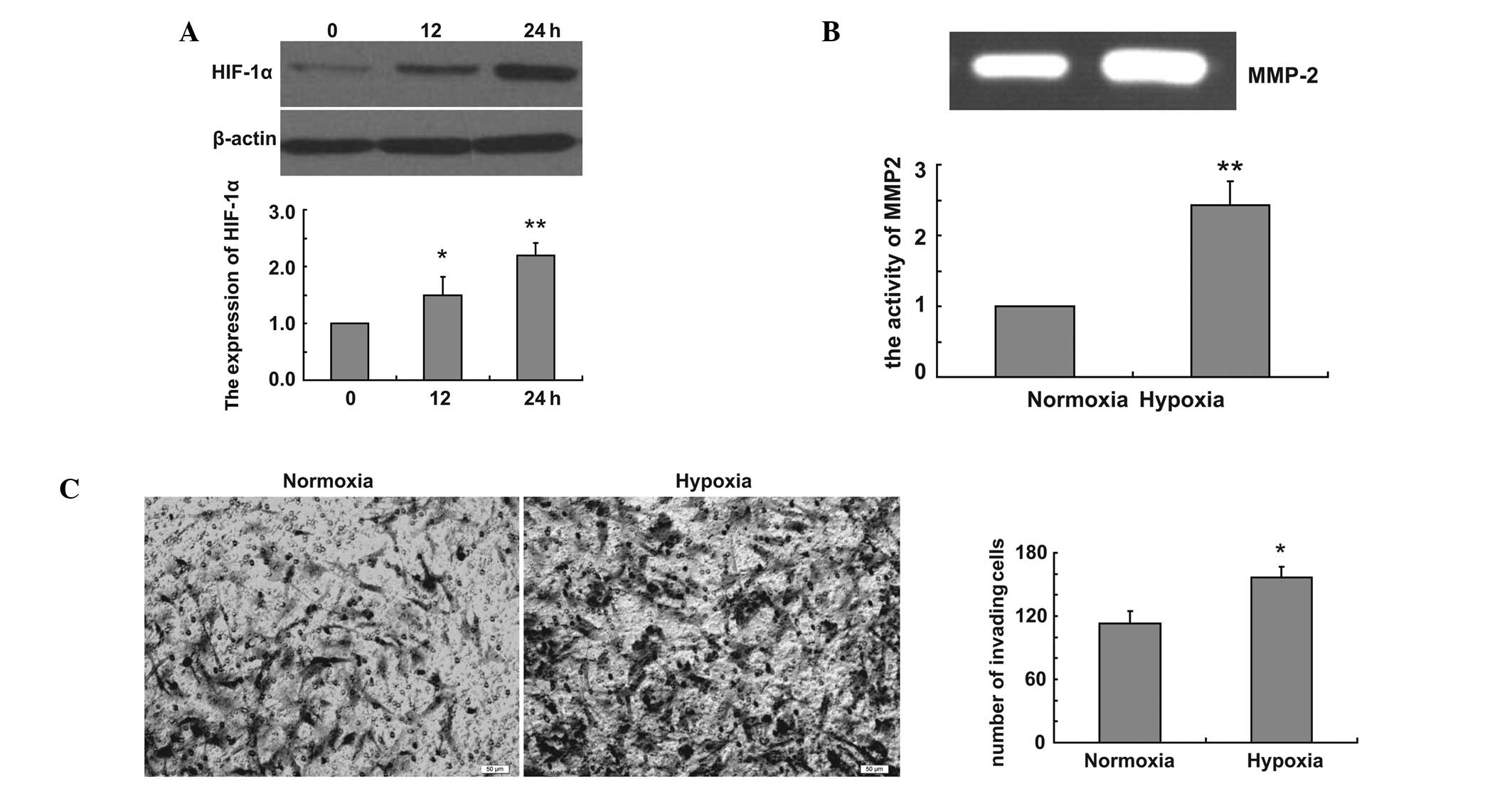
We firstly created hypoxic conditions and examined
the expression of HIF-1α in U251 cells in hypoxic conditions. Our
data revealed that hypoxia induced the expression of HIF-1α in
hypoxic conditions (Fig. 1A). To
examine the effect of hypoxia on glioma cell invasion, Transwell
assay was performed using U251 cells. U251 cells were incubated in
the Transwell chamber under normoxic and hypoxic conditions for 24
h, and the cells were stained with Coomassie Brilliant Blue. The
cells that migrated to the lower chamber were counted from four
randomly selected areas per well. Our data demonstrated that
hypoxia increased the invasion of U251 cells (Fig. 1B). We examined whether MMP
expression contributed to the increased invasion under hypoxia. The
enzymatic activity of MMP2 was measured at 24 h following hypoxia
by gelatin zymography. Our data revealed that hypoxia enhances U251
cell invasion by inducing MMP2 expression (Fig. 1C).
Inhibition of JNK decreases the ratio of
p-c-jun/c-jun and p-JNK/JNK, MMP2 activity and glioma cell invasion
under hypoxic conditions
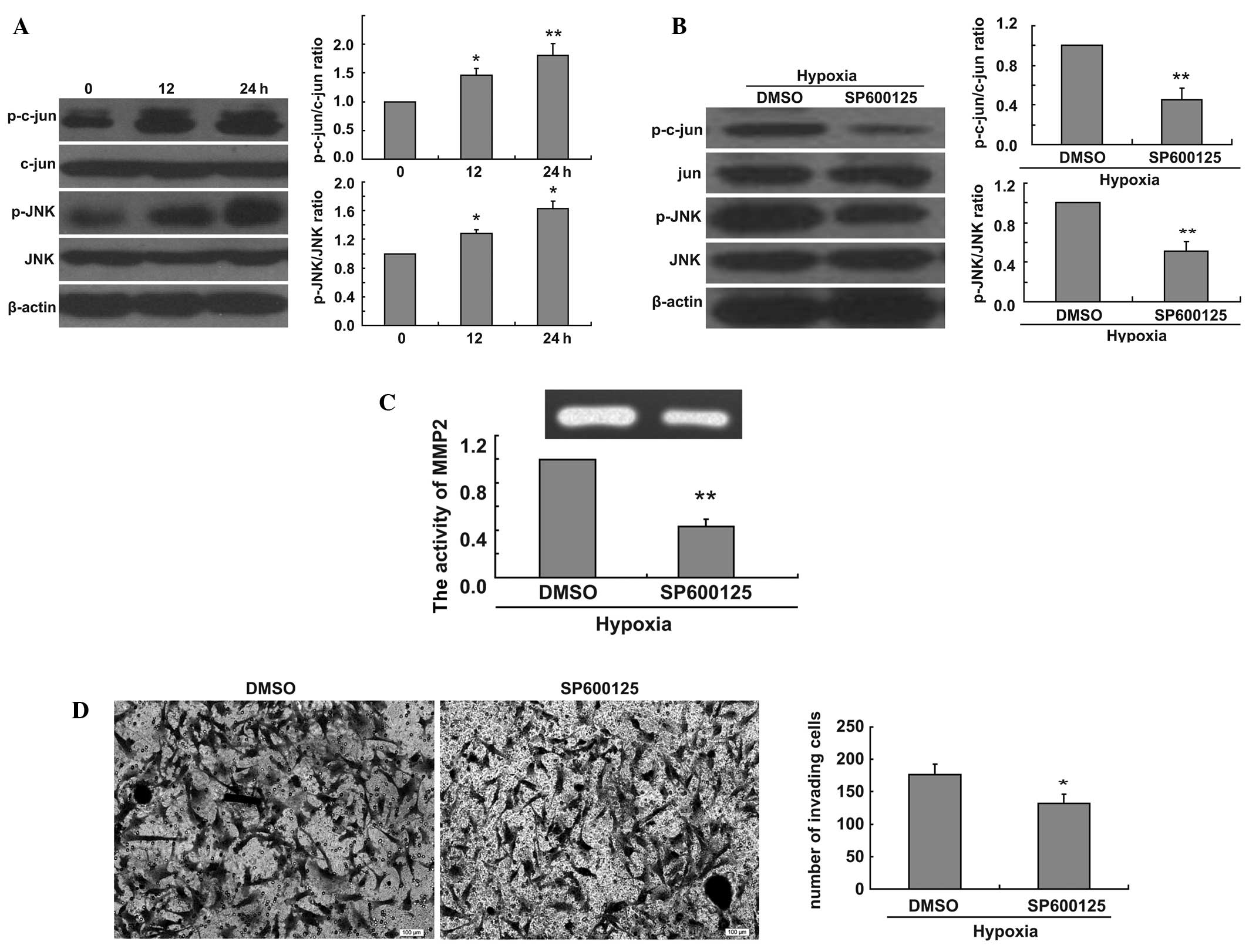
To elucidate the molecular mechanisms of the effect
of hypoxia on the invasion of U251 cells, we measured the ratio of
p-jun/jun and p-JNK/JNK at 0, 12 and 24 h in hypoxic conditions.
Hypoxia was found to increase the ratio of p-c-jun/c-jun and
p-JNK/JNK (Fig. 2A). We thus
evaluated the effect of JNK signaling pathway inhibition on the
hypoxia-induced increase of U251 cell invasion. We found that the
addition of SP600125 decreased the ratio of p-c-jun/c-jun and
p-JNK/JNK in U251 cells in hypoxic conditions (Fig. 2B). To fully confirm the involvement
of the JNK signaling pathway in the increase of cell invasion
induced by hypoxia, we treated U251 cells with SP600125 for 24 h
under hypoxic conditions and collected cell culture medium samples.
We examined MMP2 activity using gelatin zymography assays. Our data
revealed that the JNK inhibitor decreased MMP2 activity induced by
hypoxia (Fig. 2C). To further
demonstrate the role of the JNK signaling pathway in cell invasion,
we performed Matrigel invasion tests with U251 cells treated with
SP600125. We found that the inhibition of the JNK signaling pathway
decreased the invasion of U251 cells in hypoxic conditions
(Fig. 2D).
Inactivition or knockdown of RhoA
inhibits the JNK signaling pathway and decreases MMP2 activity and
glioma cell invasion in hypoxic conditions
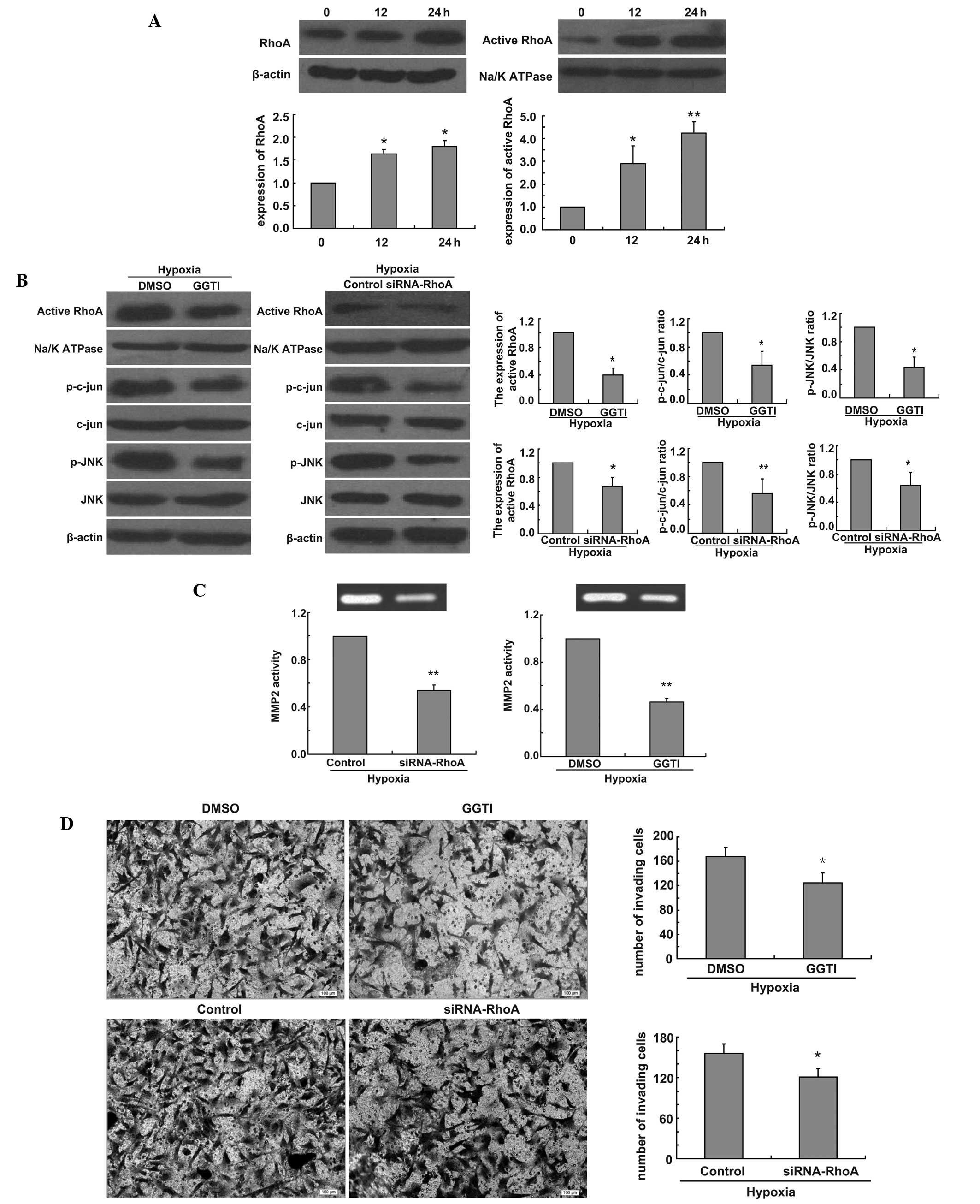
Finally, to evaluate the link between RhoA and the
JNK signaling pathway, we also examined the expression of RhoA and
active RhoA in U251 cells in hypoxic conditions. We found that
hypoxia had the same time-dependent effect on the expression of
active RhoA and RhoA (Fig. 3A). To
investigate whether the production of p-jun and p-JNK could be
blocked by functional inactivation of RhoA, U251 cells were
incubated under hypoxia for 24 h after U251 cells were treated with
GGTI-2147 or transfected RhoA siRNA. Then western blot analysis was
performed. Our data revealed that the expression of active RhoA
decreased and the ratio of p-jun/jun and p-JNK/JNK also decreased
(Fig. 3B). To investigate whether
the activity of MMP2 was affected by GGTI-2147 or RhoA knockdown,
the enzymatic activity of MMP2 was measured at 24 h following
hypoxia by gelatin zymography after U251 cells were treated with
GGTI-2147 or transfected RhoA siRNA. Our data showed that the
activity of MMP2 was reduced in GGTI-2147-treated cells or RhoA
siRNA-transfected cells under hypoxia compared with control cells
under hypoxia (Fig. 3C). To
determine whether active RhoA was involved in glioma cell invasion,
we compared the invasive capacity of GGTI-treated or RhoA knockdown
cells and control cells. Directed invasion of U251 cells was
significantly reduced by the inhibition of active RhoA or the loss
of RhoA under hypoxia, respectively, when compared with controls in
hypoxic conditions (Fig. 3D).
Discussion
In this study, we demonstrated that knockdown or
inactivation of RhoA decreased the invasion of glioma cells and the
activity of MMP2 under hypoxic conditions, and we identified a
molecular mechanism involving JNK-c-jun-MMP2 activity in effect in
hypoxic conditions. These data point to a potential antitumoral
effect mediated by the inhibition of RhoA and JNK-c-jun-MMP2
activity in glioma cells under hypoxic conditions.
Hypoxia is an important aspect of the glioma
microoenvironment, and has been correlated with poor prognosis,
increased angiogenesis, tumor growth and resistance to radiotherapy
and chemotherapy (21). The role
that hypoxia plays in glioma cell migration and invasion is well
established (22). We also
demonstrated that hypoxia increases cell invasion in the human
glioma cell line. This suggests a direct effect of hypoxia on
invasion of tumor cells. We hypothesized that the increase in
glioma cell invasion induced by hypoxia in our experiment may
result from increased activity of MMPs. This is supported by our
finding that hypoxia increases the activity of MMP2. Indeed,
abnormal expression of MMPs is thought to play a critical role in
tumor cell invasion in several cancers (23–25)
including glioma (26).
Having established that glioma cell invasion and
activity of MMP2 may be induced by hypoxia, we investigated the
signaling mechanisms involved in the increased MMP2 activity and
tumor cell invasion under hypoxic conditions. However, both P38/Akt
and PI3K/Akt were found to modulate the activity of MMP2 in glioma
cells (27). Furthermore, it was
found that JNK inhibition reduced MMP2 activity in osteosarcoma
cells (30). N-terminal
phosphorylation of c-Jun by JNK is thought to increase
transcription to target gene promoters, including the MMP2 promoter
(28,29). In our study, we found that activity
of MMP2 was reduced by the inhibition of JNK in glioma cells in
hypoxic conditions. This is supported by our finding that JNK
inhibition by treatment with SP600125 reduced MMP2 activity and
expression of p-c-jun, and decreased cell invasion induced by
hypoxia. It suggests the involvement of JNK in the glioma invasion
process under hypoxic conditions.
It was found that geranylgeranylation played a
predominant role in the regulation of the JNK-MMP2-osteosarcoma
cell invasion cascade through the downstream target GTPase RhoA
(30). We then sought to identify
whether RhoA may play the same role in glioma cell invasion in
hypoxic conditions. We found that inhibition of RhoA
geranylgeranylation or knockdown of RhoA inhibited the JNK-c-jun
signaling pathway and decreased the activity of MMP2 and glioma
cell invasion in hypoxic conditions, which indicates that
RhoA-GTPase plays an important role in glioma cell invasion in
hypoxic conditions.
In summary, our data reveal a molecular mechanism by
which the inhibition of RhoA geranylgeranylation or knockdown of
RhoA results in inhibition of the JNK-c-jun signaling pathway,
decreased MMP2 activity and cell invasion in glioma cells in
hypoxic conditions. These in vitro findings may contribute
to our understanding of the mechanisms that impact glioma cell
invasion in hypoxic conditions and provide a novel therapeutic
strategy in the treatment of glioma.
References
|
1
|
Loeper S, Romeike BF, Heckmann N, Jung V,
Henn W, Feiden W, Zang KD and Urbschat S: Frequent mitotic errors
in tumor cells of genetically microheterogeneous glioblastomas.
Cytogenet Cell Genet. 94:1–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu M, Chen Q, Li D, Li X, Li X, Huang C,
Tang Y, Zhou Y, Wang D, Tang K, et al: LRRC4 inhibits human
glioblastoma cells proliferation, invasion, and proMMP-2 activation
by reducing SDF-1 alpha/CXCR4-mediated ERK1/2 and Akt signaling
pathways. J Cell Biochem. 103:245–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DM, Cavenee WK and DePinho RA: Malignant glioma: genetics
and biology of a grave matter. Gene Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJB, et al: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. New Engl J Med.
352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hockel M, Schlenger K, Aral B, et al:
Association between tumor hypoxia and malignant progression in
advanced cancer of the uterine cervix. Cancer Res. 56:4509–4515.
1996.PubMed/NCBI
|
|
7
|
Woodhouse EC, Chuaqui RF and Liotta LA:
General mechanisms of metastasis. Cancer. 80:1529–1537. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Noorden CJ: Proteases and protease
inhibitors in cancer. Acta Histochem. 100:344–354. 1998.PubMed/NCBI
|
|
9
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choe G, Park JK, Jouben-Steele L, Kremen
TJ, Liau LM, et al: Active matrix metalloproteinase 9 expression is
associated with primary glioblastoma subtype. Clin Cancer Res.
8:2894–2901. 2002.PubMed/NCBI
|
|
11
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forsyth PA, Wong H, Laing TD, Rewcastle
NB, Morris DG, et al: Gelatinase-A (MMP-2), gelatinase-B (MMP-9)
and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved
in different aspects of the pathophysiology of malignant gliomas.
Br J Cancer. 79:1828–1835. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vogt A, Sun J, Qian Y, Hamilton AD and
Sebti SM: The geranylgeranyltransferase-I inhibitor GGTI-298
arrests human tumor cells in G0/G1 and induces p21 (WAF1/CIP1/SDI1)
in a p53-independent manner. J Biol Chem. 272:27224–27229. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laufs U and Liao JK: Post-transcriptional
regulation of endothelial nitric oxide synthase mRNA stability by
Rho GTPase. J Biol Chem. 273:24266–24271. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noguchi Y, Nakamura S, Yasuda T, Kitagawa
M, Kohn LD, Saito Y and Hirai A: Newly synthesized RhoA, not Ras,
is isoprenylated and translocated to membranes coincident with
progression of the G1 to S phase of growth-stimulated rat FRTL-5
cells. J Biol Chem. 273:3649–3653. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong WW, Dimitroulakos J, Minden MD and
Penn LZ: HMG-CoA reductase inhibitors and the malignant cell: the
statin family of drugs as triggers of tumor-specific apoptosis.
Leukemia. 16:508–519. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Graaf MR, Richel DJ, van Noorden CJ and
Guchelaar HJ: Effects of statins and farnesyltransferase inhibitors
on the development and progression of cancer. Cancer Treat Rev.
30:609–641. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Denoyelle C, Vasse M, Korner M, Mishal Z,
Ganne F, Vannier JP, Soria J and Soria C: Cerivastatin, an
inhibitor of HMG-CoA reductase, inhibits the signaling pathways
involved in the invasiveness and metastatic properties of highly
invasive breast cancer cell lines: an in vitro study.
Carcinogenesis. 22:1139–1148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sawada K, Morishige K, Tahara M, Kawagishi
R, Ikebuchi Y, Tasaka K and Murata YL: Alendronate inhibits
lysophosphatidic acid-induced migration of human ovarian cancer
cells by attenuating the activation of rho. Cancer Res.
62:6015–6020. 2002.
|
|
20
|
Kusama T, Mukai M, Tatsuta M, Nakamura H
and Inoue M: Inhibition of transendothelial migration and invasion
of human breast cancer cells by preventing geranylgeranylation of
Rho. Int J Oncol. 29:217–223. 2006.PubMed/NCBI
|
|
21
|
Sullivan R and Graham CH: Hypoxia-driven
selection of the metastatic phenotype. Cancer Metastasis Rev.
26:319–331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujiwara S, Nakagawa K, Harada H, Nagato
S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S and Ohnishi T:
Silencing hypoxia-inducible factor-1alpha inhibits cell migration
and invasion under hypoxic environment in malignant gliomas. Int J
Oncol. 30:793–802. 2007.PubMed/NCBI
|
|
23
|
Bruland OS, Hoifodt H, Saeter G, Smeland S
and Fodstad O: Hematogenous micrometastases in osteosarcoma
patients. Clin Cancer Res. 11:4666–4673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Forsyth PA, Wong H, Laing TD, Rewcastle
NB, Morris DG, et al: Gelatinase-A (MMP-2), gelatinase-B (MMP-9)
and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved
in different aspects of the pathophysiology of malignant gliomas.
Br J Cancer. 79:1828–1835. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS,
Lee SH, Park IC, Rhee CH and Hong SI: Ionizing radiation enhances
matrix metalloproteinase-2 secretion and invasion of glioma cells
through Src/epidermal growth factor receptor-mediated p38/Akt and
phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res.
66:8511–8519. 2006. View Article : Google Scholar
|
|
28
|
Arias J, Alberts AS, Brindle P, Claret FX,
Smeal T, Karin M, Feramisco J and Montminy M: Activation of cAMP
and mitogen responsive genes relies on a common nuclear factor.
Nature. 370:226–229. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bannister AJ, Oehler T, Wilhelm D, Angel P
and Kouzarides T: Stimulation of c-Jun activity by CBP: c-Jun
residues Ser63/73 are required for CBP induced stimulation in vivo
and CBP binding in vitro. Oncogene. 11:2509–2514. 1995.PubMed/NCBI
|
|
30
|
Fromigué O, Hamidouche Z and Marie PJ:
Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces
osteosarcoma cell invasion. J Biol Chem. 283:30549–30556.
2008.PubMed/NCBI
|