Introduction
Distant metastases upon presentation of small cell
lung cancer (SCLC) is a frequent clinical problem. Clinically,
treatment for extensive disease (ED)-SCLC consists of systemic
chemotherapy and radiotherapy for symptomatic metastatic sites. It
is generally accepted that the life expectancy of SCLC patients
depends on the extent of disease and the response to chemotherapy.
Performance status (PS), gender, disease extent and response to
chemotherapy have been evaluated as prognostic factors (1). Foster et al revealed that the
number of metastatic sites was also a prognostic factor in ED-SCLC
patients, regardless of the location of the metastatic site
(2). The four most common sites of
metastasis in SCLC at the time of diagnosis appear to be the liver,
bone, brain and lung, with involvement of these sites identified in
SCLC patients with newly diagnosed metastatic disease. Using the
database of SCLC patients, we examined whether specific organ
metastasis at the time of presentation had prognostic significance
in SCLC patients.
Patients and methods
Patients
We retrospectively analyzed 251 patients who were
diagnosed with SCLC in our divisions at the University of Tsukuba
and the Tsukuba Medical Center Hospital between January 1999 and
May 2010. This retrospective study conformed to the Ethical
Guidelines for Clinical Studies issued by the Ministry of Health,
Labor and Welfare of Japan. Analysis of the medical records of the
lung cancer patients was approved by the ethics committee of the
University of Tsukuba Hospital, Ibaraki, Japan. The diagnosis of
SCLC was confirmed in all patients using pathological and/or
cytological specimens. Pathological diagnosis of SCLC was defined
by the WHO classification (3).
Prior to treatments, a staging procedure was conducted for all
patients using computed tomography (CT) or magnetic resonance
imaging (MRI) of the head, bone scintigraphy and ultrasonography
and/or CT of the abdomen. Patients were staged according to the
staging system of the Veterans Administration Lung Cancer Study
Group into one of two categories; limited-stage disease (LD) or
extensive-stage disease (ED) (4,5).
Patients with LD-SCLC had metastatic disease restricted to the
ipsilateral hemithorax within a single radiation port, while
patients with ED-SCLC had widespread metastatic disease (4,5).
Statistical analysis
The statistical significance between the two groups
was determined using the Mann-Whitney U test and the Chi-square
test. Logistic regression analysis was applied in order to examine
the significance of the seven variables, including liver, bone,
brain, lung, extrathoracic lymph node and adrenal metastasis as
well as pleural and/or pericardial fluid, for poor PS and
unfavorable response to chemotherapy. The Kaplan-Meier method was
used to assess the survival curves and the log-rank test was used
to evaluate the statistically significant differences between the
two groups. The length of survival was defined in months as the
interval from the date of initial therapy or supportive care until
the date of mortality or last follow-up. Cox’s proportional hazards
model was used to study the effects of clinicopathological factors
on survival (6). All statistical
analyses were performed using SPSS version 10.1 for Windows (SPSS,
Chicago, IL, USA) and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The diagnosis of all 251 SCLC patients was
pathologically and/or cytologically confirmed. The characteristics
of these patients are shown in Table
I. Among the 251 SCLC patients, 222 (88.4%) were male and 29
were female. The mean age was 71 years (range, 41–86 years). A
total of 73 (29.1%) patients had pleural and/or pericardial fluids
and among the 152 (60.6%) patients that had distant metastasis, 51
(20.3%), 46 (18.3%), 39 (15.5%), 25 (10.0%) and 15 (6.0%) patients
also had liver, bone, brain, lung and adrenal gland metastasis,
respectively.
 | Table I.Characteristics of 251 patients with
SCLC. |
Table I.
Characteristics of 251 patients with
SCLC.
| Patient
characteristics | No. of patients
(%) |
|---|
| Age (years) | |
| Median | 71 |
| Range | 41–86 |
| Gender | |
| Male | 222 (88.4) |
| Female | 29 (11.6) |
| Distant
metastasis | |
| Present | 152 (60.6) |
| Absent | 99 (39.4) |
| Clinical stage | |
| Limited | 78 |
| Extensive | 125 |
| Metastatic
site | |
| Pleural and/or
pericardial fluids | 73 (29.1) |
| Liver | 51 (20.3) |
| Bone | 46 (18.3) |
| Brain | 39 (15.5) |
| Lung | 25 (10.0) |
| Adrenal
gland | 15 (6.0) |
| Extrathoracic
lymph node | 15 (6.0) |
| Pancreas | 3 (1.2) |
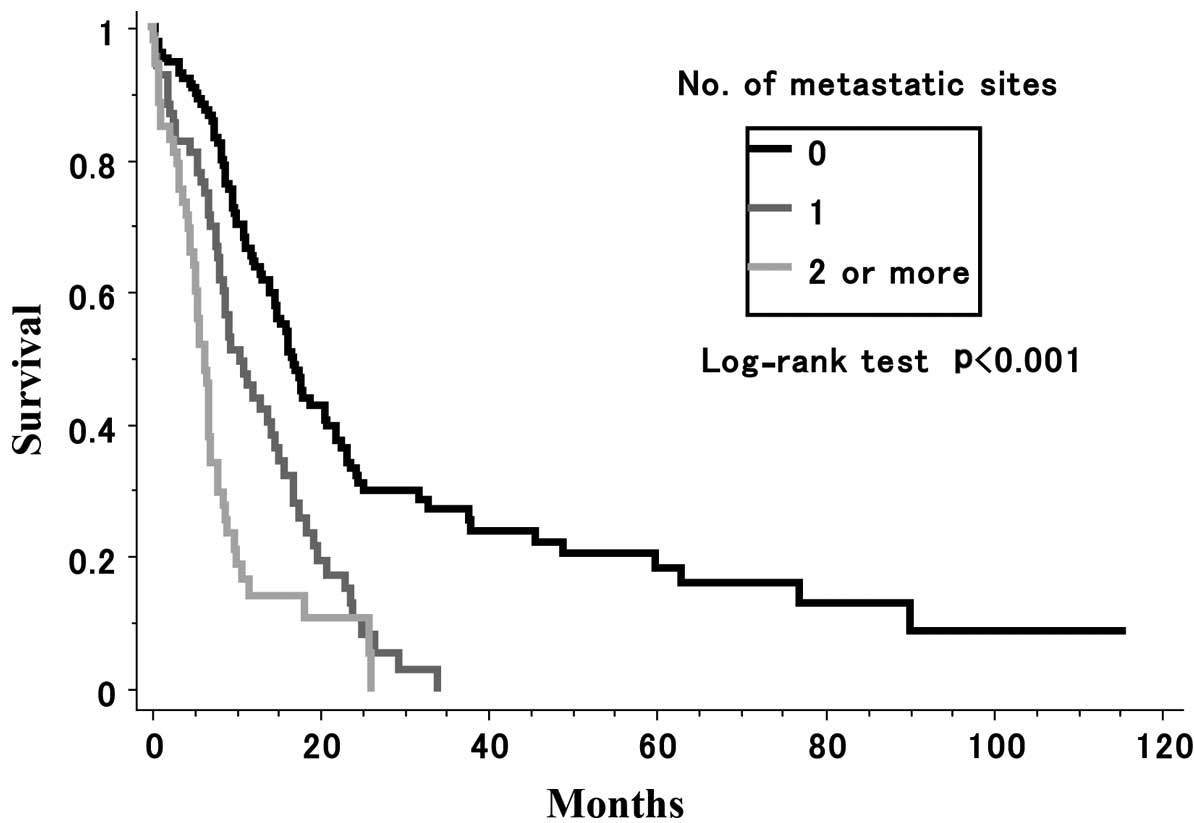
Number of metastatic sites infuences
survival rate
A total of 69 patients had one metastatic site, 28
had two, 20 had three, four had four and one had five (data not
shown). A statistically significant difference was observed in the
survival rate among patients without metastasis, those with one
metastatic site and those with two or more metastatic sites
(P=0.0001) (Fig. 1).
A total of 16 patients had sole liver metastasis,
while 35 patients had liver metastasis accompanied by metastasis in
another site. Additionally, 12, 22, and six patients had sole bone,
brain, and lung metastasis, respectively. A total of 34, 17 and 19
patients had bone, brain and lung metastasis accompanied by
metastasis in another site, respectively. A statistically
significant difference was observed in the one-year survival
between the patients with sole liver metastasis and those without
metastasis (P=0.0009). Similar results were observed between those
with sole bone (P=0.0013), brain (P=0.0163) and lung (P=0.169)
metastasis, and those without metastasis, respectively (Table II).
 | Table II.Metastatic sites and comparison of
the one-year survival between patients with sole specific organ
metastasis and patients without metastasis. |
Table II.
Metastatic sites and comparison of
the one-year survival between patients with sole specific organ
metastasis and patients without metastasis.
| Metastatic
site | One-year survival
(%) | No. of patients
with sole metastatic sites | P-value |
|---|
| None | 54.3 | | |
| Liver | 18.8 | 16 | 0.0009 |
| Bone | 25.0 | 12 | 0.0013 |
| Brain | 40.9 | 22 | 0.0163 |
| Lung | 33.4 | 6 | 0.0169 |
Metastasis affects response to
chemotherapy
According to logistic regression analysis, liver
metastasis, brain metastasis and pleural and/or pericardial fluid
are correlated with a poor PS of 2–3 (Table III). A logistic regression analysis
also demonstrated that liver and brain metastasis are risk factors
of unfavorable response to chemotherapy (stable disease and
progressive disease; Table IV).
 | Table III.Logistic regression analysis for the
factors of a poor PS (PS 2–3). |
Table III.
Logistic regression analysis for the
factors of a poor PS (PS 2–3).
| Independent
factor | Hazard ratio | 95% CI | P-value |
|---|
| Liver | 2.311 | 1.107–4.823 | 0.0256 |
| Bone | 1.505 | 0.683–3.313 | 0.3103 |
| Brain | 2.634 | 1.244–5.579 | 0.0114 |
| Lung | 0.819 | 0.310–2.164 | 0.6887 |
| Adrenal gland | 1.330 | 0.401–4.409 | 0.6404 |
| Extra-thoracic
lymph node | 1.096 | 0.355–3.377 | 0.8737 |
| Pleural and/or
pericardial fluid | 3.066 | 1.660–5.663 | 0.0003 |
 | Table IV.Logistic regression analysis for the
factors of an unfavorable response to chemotherapy (stable disease
and progressive disease). |
Table IV.
Logistic regression analysis for the
factors of an unfavorable response to chemotherapy (stable disease
and progressive disease).
| Independent
factor | Hazard ratio | 95% CI | P-value |
|---|
| Liver | 5.304 | 2.201–12.785 | 0.0002 |
| Bone | 0.528 | 0.178–1.563 | 0.2484 |
| Brain | 2.695 | 1.073–6.769 | 0.0348 |
| Lung | 1.571 | 0.508–4.856 | 0.4329 |
| Adrenal gland | 2.885 | 0.734–11.388 | 0.1291 |
| Extrathoracic lymph
node | 1.339 | 0.369–4.859 | 0.6568 |
| Pleural and/or
pericardial fluid | 1.535 | 0.721–3.270 | 0.2664 |
Multivariate analysis
In a multivariate analysis using Cox’s proportional
hazards model, presence of liver metastasis (P=0.0001), bone
metastasis (P=0.0401), brain metastasis (P=0.0177) and pleural
and/or pericardial fluids (P=0.0020) were unfavorable prognostic
factors. However, lung (P=0.7028), adrenal gland (P=0.2405) and
extrathoracic lymph node metastasis (P=0.0672) were not
statistically significant prognostic factors (Table V).
 | Table V.Multivariate analysis of prognostic
factors in patients with SCLC. |
Table V.
Multivariate analysis of prognostic
factors in patients with SCLC.
| Factor | Multivariate
analysis (Cox’s proportional hazards model)
|
|---|
| Hazard ratio | 95% CI | P-value |
|---|
| Liver | 0.414 | 0.273–0.627 | 0.0001 |
| Bone | 0.643 | 0.422–0.980 | 0.0401 |
| Brain | 0.608 | 0.403–0.917 | 0.0177 |
| Lung | 0.903 | 0.534–1.527 | 0.7028 |
| Adrenal gland | 0.689 | 0.369–1.284 | 0.2405 |
| Extrathoracic lymph
node | 0.586 | 0.331–1.039 | 0.0672 |
| Pleural and/or
pericardial fluid | 0.608 | 0.443–0.833 | 0.0020 |
Discussion
The incidence of distant metastasis at the time of
initial diagnosis of SCLC in this study was 60.6%, and the most
common metastatic sites were the liver, bone, brain, lung and
adrenal glands. In this study, we examined whether specific organ
metastasis at the time of presentation had prognostic significance
in SCLC patients. For decades, the prognostic significance of bone
marrow involvement in SCLC patients has been reported (7,8).
Thereafter, several studies indicated that patients with multiple
metastatic sites had a significantly poor survival rate (9–11). Our
results revealed that there was a significant difference in
survival among patients without metastasis compared to those with
one metastatic site and those with two or more metastatic sites.
These results were consistent with previous studies (9–11). In
these studies, the site of involvement did not appear to have an
impact on survival (2,9); however, other researchers have
identified that specific organ metastasis is related to poor
prognosis (12–14). Bremnes et al reported that
liver and brain metastasis were independent prognostic factors in
ED-SCLC (12). Mohan et al
also revealed that brain metastasis was a significant predictor of
survival (13). In a study of 116
SCLC patients, Arinc et al indicated that bone metastasis
was one of the significant prognostic factors in univariate
analysis, but was not identified as a prognostic factor in
multivariate analysis (14). In the
present study, univariate analysis revealed that patients with sole
organ metastasis in the liver, bone, brain and lung had a poorer
prognosis compared to those without metastasis. However, in
multivariate analysis, liver, bone and brain metastasis were
unfavorable prognostic factors, while lung metastasis was not. In
our logistic regression analysis, the presence of liver metastasis,
brain metastasis and pleural and/or pericardial fluid demonstrated
a statistically significant correlation with poor PS. Liver
metastasis and brain metastasis also correlated with an unfavorable
response to chemotherapy. Therefore, these results indicate that
liver metastasis, brain metastasis and pleural and/or pericardial
fluid have certain associations with poor prognosis.
Nussbaum et al identified that multiple brain
metastases were common in patients with SCLC (15). Activity of daily living (ADL) may be
low in a number of patients with brain metastases, and may also
deteriorate in patients with bone metastases due to pain and
fracture. The discontinuation of chemotherapy due to the
deterioration of ADL in these patients has a certain correlation
with poor survival. With regard to liver metastasis, the majority
of SCLC patients had multiple nodules in our previous study
(16). SCLC may cause biliary tract
obstruction by metastasizing to lymph nodes in the porta hepatis or
hepatic parenchyma (17,18). The administration of chemotherapy
may be complicated by metastasis of the liver in the activation or
metabolism of several cytotoxic drugs commonly used in the
treatment of SCLC. Lung metastatic lesions usually respond well to
chemotherapy, and these lesions rarely cause complications unless
hemoptysis and respiratory tract obstruction develop. Further
studies are required to confirm the impact of metastasis of these
organs as observed in the present study.
Recently, Ignatius Ou and Zell reported the
applicability of the proposed International Association for the
Study of Lung Cancer (IASLC) staging for SCLC, in comparison with
the current Union for International Cancer Control (UICC) 6th Tumor
Node Metastasis (TNM) edition (19). They concluded that patients with
pleural and/or pericardial effusion had a poorer prognosis compared
to those with metastatic disease (19). In the UICC TNM 7th edition, patients
with pleural effusion have an intermediate prognosis between LD-
and EL-SCLC (20). Our present
study also confirmed that patients with pleural and/or pericardial
effusion had a poorer prognosis compared to those without.
Despite these significant findings, this study has
limitations. Firstly, the retrospective design of the study meant
that it was complicated by lead time and length time biases.
Secondly, there was a lack of information with regard to the
development of distant metastases in the patients’ clinical
courses. Regardless of these limitations, we recognize that our
findings have certain clinical importance for the management of
future SCLC patients of unselected groups.
In conclusion, the therapeutic approach for treating
SCLC patients with distant metastases is complicated, as our
results suggest that existing liver, bone and brain metastasis
adversely affects the outcome of the disease. When deciding whether
or not to offer an intensive therapy, which may increase
treatment-related mortality, the patient’s medical condition,
including coexistence of such metastases, should be taken into
consideration, although favorable results have been reported in a
number of these patients.
References
|
1.
|
R FerraldeschiS BakaB JyotiC Faivre-FinnN
ThatcherP LoriganModern management of small-cell lung
cancerDrugs6721352152200710.2165/00003495-200767150-0000317927281
|
|
2.
|
NR FosterSJ MandrekarSE SchildPrognostic
factors differ by tumor stage for small cell lung cancer: a pooled
analysis of North Central Cancer Treatment Group
trialsCancer11527212731200910.1002/cncr.2431419402175
|
|
3.
|
W TravisE BrambillaH Muller-HermelinkC
HarrisTumours of the lung, pleura, thymus and heartPathology and
Genetics World Health Organisation Classification of TumoursIARC
PressLyon2004
|
|
4.
|
M ZelenKeynote address on biostatistics
and data retrievalCancer Chemother Rep 34314219734580860
|
|
5.
|
FA ShepherdRJ GinsbergR HaddadImportance
of clinical staging in limited small-cell lung cancer: a valuable
system to separate prognostic subgroups. The University of Toronto
Lung Oncology GroupJ Clin Oncol111592159719938393098
|
|
6.
|
DR CoxRegression models and life tablesJ
Roy Statist Soc Ser B Metho341872201972
|
|
7.
|
WR BezwodaD LewisN LiviniBone marrow
involvement in anaplastic small cell lung cancer. Diagnosis,
hematologic features, and prognostic
implicationsCancer5817621765198610.1002/1097-0142(19861015)58:8%3C1762::AID-CNCR2820580830%3E3.0.CO;2-V3019512
|
|
8.
|
J ZychZ PolowiecE WiatrA BroniekE
Rowinska-ZakrzewskaThe prognostic significance of bone marrow
metastases in small cell lung cancer patientsLung
Cancer10239245199310.1016/0169-5002(93)90184-Y8075969
|
|
9.
|
F TasA AydinerE TopuzH CamlicaP SaipY
EralpFactors influencing the distribution of metastases and
survival in extensive disease small cell lung cancerActa
Oncol3810111015199910.1080/02841869943227510665754
|
|
10.
|
J LiCH DaiP ChenSurvival and prognostic
factors in small cell lung cancerMed
Oncol277381201010.1007/s12032-009-9174-319214813
|
|
11.
|
M TamuraH UeokaK KiuraPrognostic factors
of small-cell lung cancer in Okayama Lung Cancer Study Group
TrialsActa Med Okayama5210511119989588226
|
|
12.
|
RM BremnesS SundstromU AasebøS KaasaR
HatlevollS AamdalNorweigian Lung Cancer Study GroupThe value of
prognostic factors in small cell lung cancer: results from a
randomised multicenter study with minimum 5 year follow-upLung
Cancer39303313200312609569
|
|
13.
|
A MohanA GoyalP SinghSurvival in small
cell lung cancer in India: prognostic utility of clinical features,
laboratory parameters and response to treatmentIndian J
Cancer436774200610.4103/0019-509X.2588716790943
|
|
14.
|
S ArincU GonlugurO DevranPrognostic
factors in patients with small cell lung carcinomaMed
Oncol27237241201010.1007/s12032-009-9198-819399653
|
|
15.
|
ES NussbaumHR DjalilianKH ChoWA HallBrain
metastases. Histology, multiplicity, surgery, and
survivalCancer7817811988199610.1002/(SICI)1097-0142(19961015)78:8%3C1781::AID-CNCR19%3E3.0.CO;2-U8859192
|
|
16.
|
K KagohashiH SatohH IshikawaM OhtsukaK
SekizawaLiver metastasis at the time of initial diagnosis of lung
cancerMed Oncol202528200310.1385/MO:20:1:2512665681
|
|
17.
|
M ObaraH SatohYT YamashitaMetastatic small
cell lung cancer causing biliary obstructionMed
Oncol15292294199810.1007/BF027872179951697
|
|
18.
|
R OgawaT KodamaK KurishimaK KagohashiH
SatohPortal vein tumor thrombus of liver metastasis from lung
cancerActa Medica (Hradec Kralove)52163166200920369711
|
|
19.
|
SH Ignatius OuJA ZellThe applicability of
the proposed IASLC staging revisions to small cell lung cancer
(SCLC) with comparison to the current UICC 6th TNM EditionJ Thorac
Oncol4300310200919156001
|
|
20.
|
FA ShepherdJ CrowleyP Van HoutteThe
International Association for the Study of Lung Cancer lung cancer
staging project: proposals regarding the clinical staging of small
cell lung cancer in the forthcoming (seventh) edition of the tumor,
node, metastasis classification for lung cancerJ Thorac
Oncol210671077200710.1097/JTO.0b013e31815bdc0d
|