Introduction
Pancreatic cancer is a highly aggressive malignancy
with extremely poor prognosis that is considered to be partly due
to the chemotherapy-resistant characteristics of specific
pancreatic cancer cell subgroups. CD133 and CXCR4 are potential
markers of pancreatic cancer stem cells (CSCs), which form tumor
tissues and promote tumor progression and metastasis (1). It has been suggested that microRNAs
(miRs) are critical regulators of tumor progression and drug
resistance in pancreatic cancer cells (2). Low miR-21 expression in tumor tissues
has been associated with beneficial adjuvant treatment in
pancreatic cancer cases, and it has been revealed that anti-miR-21
increases anticancer drug activity in vitro (3).
We used the methods described in our previous study
(4) to enrich rare cells from the
pleural fluid and to analyze the expression of the CSC marker
CD133, and the epithelial marker, cytokeratin 18 (CK18). The plasma
levels of miR-21, miR-25, miR-103, miR-151 and cancer antigen 19-9
(CA19-9) in the serum, and the clinical pathological parameters of
the patients, were also studied. Following therapy, the condition
of the patient was monitored until mortality.
Materials and methods
Enrichment of cancer cells from pleural
effusion
The patient, who provided informed consent, was
enrolled using institutional review board-approved protocols.
Pleural effusion (10 ml) was collected from the patient into acid
citrate dextrose venous collection tubes (Becton Dickinson,
Franklin Lakes, NJ, USA) and transferred into a 50-ml centrifuge
tube. Malignant cancer cells were enriched from the pleural fluid
using CD45-coated immunomagnetic beads (Cyttel Biosciences,
Beijing, China) following the method described in our previous
study (4). The cell pellet was then
transferred onto glass slides for further analysis.
Immunofluorescence (IF) staining
Double IF staining was conducted using 100 μl
anti-CK18-FITC (green) and anti-CD133-PE (red; Miltenyi Biotec,
Bergisch Gladbach, Germany). The cell pellet was transferred onto
glass slides and incubated with the labeled primary antibodies
(1:100 dilution in 2% BSA) for 60 min at room temperature. The
nuclei were counterstained with DAPI and a blinded review of
three-color fluorescence images by three examiners (magnification,
×400) confirmed the identity of
CD133+CK18+cells.
Plasma RNA isolation
Total RNA from the plasma of the pancreatic cancer
patient and from the age- and gender-matched healthy donors was
isolated using TRIzol for RNA (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Circulating miRNA was
isolated using a mirVana PARIS kit (Applied Biosystems, Foster
City, CA, USA) according to the manufacturer’s instructions. RNA
concentration was determined using a NanoDrop ND-1000
spectrophotometer (Thermo Scientific, Worcester, MA, USA) on a
denaturing 15% polyacrylamide gel.
Quantitative reverse transcriptase
polymerase chain reaction (qRT-PCR)
miR-16, miR-21, miR-103 and miR-151 were quantified
in triplicate by qRT-PCR using TaqMan MicroRNA Assay kits (Applied
Biosystems). A total of 2.5 μl synthetic C. elegans
miRNA, cel-miR-39 (2×10−3 pmol/μl synthetic RNA
oligonucleotides; Qiagen, Hilden, Germany), was applied to each
sample as an internal control. The method used for the
identification of miR-16, miR-21, miR-103 and miR-151 in the plasma
was described previously (5). The
relative abundance of the miRNAs was determined using the following
equation: Relative miRNA abundance = −ΔΔCt = −[(Sample
Cttarget − Sample Ctcell-miR-39) − (Control
Cttarget − Control Ctcell-miR-39)].
Case report
A 60 year-old male was admitted to the Northern
Jiangsu People’s Hospital and Clinical Medical College of Yangzhou
University (Yangzhou, China) having suffered symptoms of abdominal
distention and anorexia for one month. The patient had been healthy
and had no history of malignant or other common diseases. The
patient did not smoke, but had been addicted to alcohol for 20
years with a 250 g average daily intake of alcohol. Serum alkaline
phosphatase (ALP), γ-glutamate-transpeptidase (γ-GT) and CA19-9
levels were 195 U/l, 291 U/l and 1200 U/ml, respectively; all of
which are significantly elevated compared with normal levels.
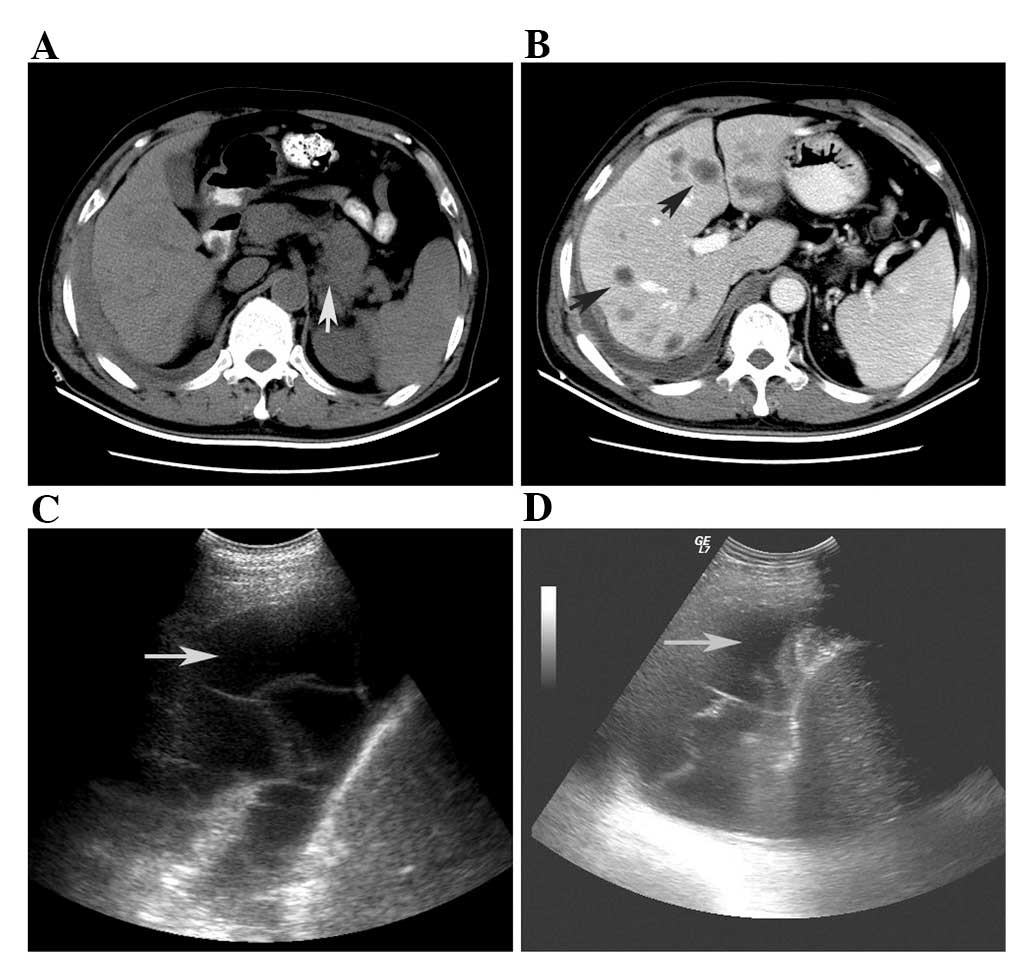
Abdominal ultrasonographic diagnosis indicated
pleural effusion in the right chest (Fig. 1C and D). The features of the pleural
fluid are shown in Table I. A total
of 50 malignant cancer cells/ml were identified in the pleural
fluid, while carcinoembryonic antigen (CEA) was elevated to 103.9
U/l. The 43x48 mm mass in the body-tail of the pancreas was
detected using computer tomography (CT; Fig. 1A). A different nodus size was
detected at low density in the liver. The maximum diameter of the
metastatic tumor was ∼20 mm (Fig.
1B). The patient was administered chemotherapy with one cycle
of gemcitabine and oxaliplatin combined with abdominal cavity
perfusion and gemcitabine. However, the patient was insensitive to
the systemic therapy and succumbed to liver metastasis and other
complications 3 months later.
 | Table I.Laboratory data of pleural effusion
from the pancreatic cancer patient with liver and pleural
metastasis. |
Table I.
Laboratory data of pleural effusion
from the pancreatic cancer patient with liver and pleural
metastasis.
| Variable | Control | Result | Clinical
significance |
|---|
| Color | Colorless | Yellow | Abnormal |
| Trait | Clear | Muddy | Abnormal |
| Pleural chylous
test | Negative | Negative | Normal |
| Acid-fast
staining | Negative | Negative | Normal |
| Cell counting
(number/l) | Negative |
0.5x109 | Normal |
| Total protein
(g/dl) | 6.0–8.0 | 4.4 | Decreased |
| Lactate
dehydrogenase (U/l) | 106–246 | 211 | Normal |
| Adenosine deaminase
(U/l) | 0–25 | 13 | Normal |
| CEA (U/l) | <5 | 103 | Tumor
metastasis |
| Cancer cell
staining (/ml) | Negative | 30 | Malignant diseases
diagnosis |
|
CD133+CK18−
cells | Negative | Positive | Maybe CSCs |
|
CD133+CK18+
cells | Negative | Positive | Maybe CSCs |
|
CK18+CD133−
cells | Negative | Positive | Malignant
epithelial cells |
The plasma miR-21, miR-25, miR-103 and miR-151
levels were notably increased in the serum of this patient, being
8.3, 2.0, 6.8 and 4.4-times higher compared with that of the
average from five age- and gender-matched healthy controls,
respectively (Table II). Moreover,
malignant cancer cells in the pleural fluid were enriched by
CD45-coated immuno-magnetic beads. The number of cancer cells in
the pleural fluid was enumerated as 30/ml using Wright-Giemsa
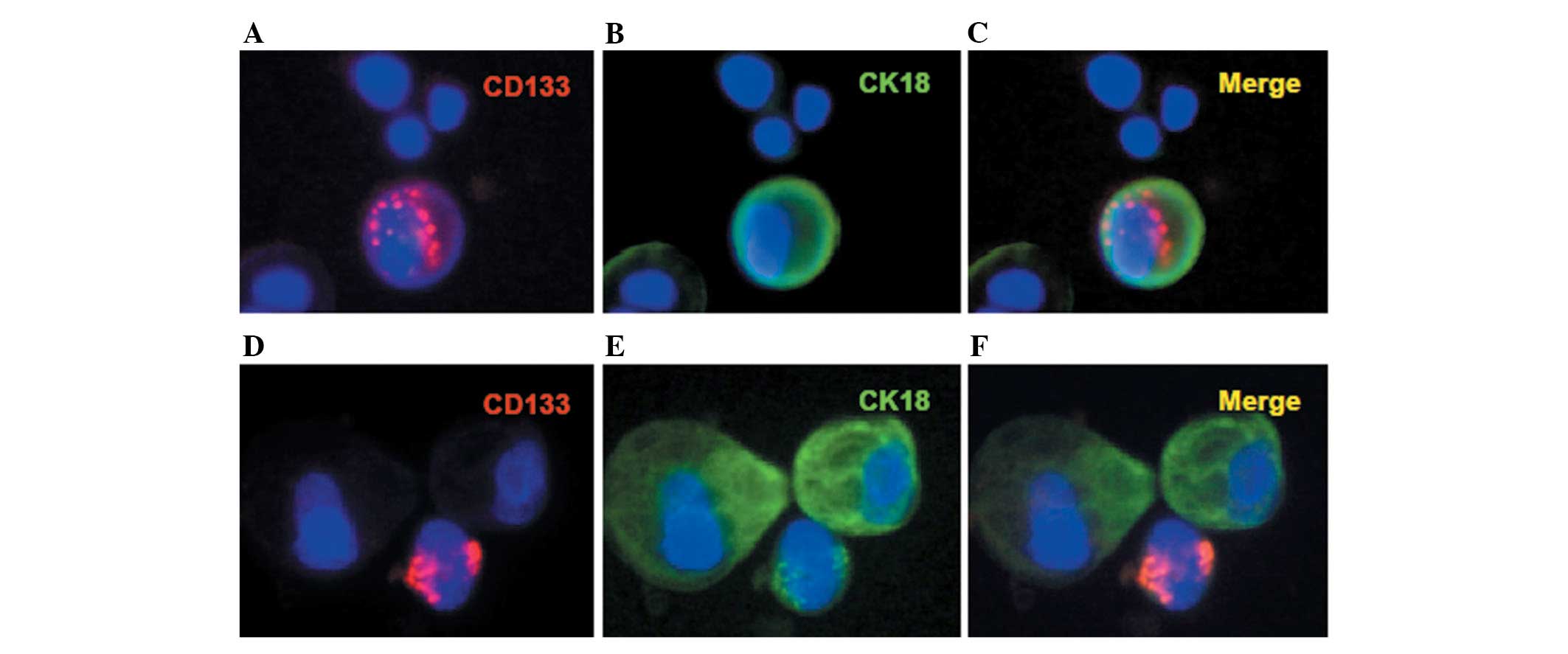
stain. CD133+CK18+,
CD133+CK18− and
CK18+CD133− cancer cells were detected in the
pleural fluid of the patient (Table
I; Fig. 2).
 | Table II.Circulating miRNA-21, miRNA-25,
miRNA-103 and miRNA-151 levels in the pancreatic cancer patient and
in five age- and gender-matched healthy donors. |
Table II.
Circulating miRNA-21, miRNA-25,
miRNA-103 and miRNA-151 levels in the pancreatic cancer patient and
in five age- and gender-matched healthy donors.
| Sample | miR-21 | miR-25 | miR-103 | miR-151 |
|---|
| Pancreatic cancer
(n=1) (pmol/μl) |
2.5×10−4 |
0.24×10−4 |
0.34×10−4 |
0.71×10−4 |
| Healthy control
(n=5) (pmol/μl) |
0.3×10−4 |
0.12×10−4 |
0.05×10−4 |
0.16×10−4 |
| Multiple of
increase compared with healthy control | 8.3 | 2.0 | 6.8 | 4.4 |
Discussion
Although the only potentially curative treatment for
pancreatic ductal adenocarcinoma is surgical resection, the vast
majority of patients are diagnosed at stages that are too advanced
to undergo curative surgery. Therefore, biomarkers for early
detection and new therapeutic strategies are urgently required.
Pancreatic cancer has unique miRNA expression
patterns, which are different from those of other cancers, and
enable the differentiation of normal pancreatic tissue from benign
inflammatory pancreatic tissue (6).
Overexpression of miR-21 in tumors is markedly associated with
liver metastasis of pancreatic cancer (7). In this patient, the plasma levels of
miR-21 were 8.3 times higher than those of the age- and
gender-matched healthy controls. The levels of the other three
miRNAs examined, miR-25, miR-103 and miR-151, were also 2.0, 6.8
and 4.4 times higher compared with those of the healthy controls,
respectively (Table II). In this
study, multiple liver metastases and pleural fluid in the right
chest were detected by CT and ultrasonographic examination
(Fig. 1). Furthermore, increased
serum CA19-9, ALP and γ-GT levels indicated malignant disease and
liver metastasis. As this patient was not fit for surgery, the
diagnosis of pancreatic cancer by pathology was not acquired.
However, the diagnosis could be confirmed by clinical symptoms,
imaging diagnosis and laboratory examinations.
We employed a method using CD45-coated
immunomagnetic beads to enrich cancer cells from pleural blood
(4,8). A total of 30 cancer cells/ml pleural
effusion were enriched and detected (Table I). CD133+CK18+
double positive cells as well as CD133+CK18−
and CK18+CD133− single positive cells, were
detected in the pleural fluid of this patient. Our results support
the recent findings from the Stanger research group, which
identified that circulating pancreatic cells maintained a
mesenchymal phenotype, exhibited stem cell properties and seeded
the liver in animal models of pancreatic cancer (9).
As CKs are markers of epithelial cells or epithelial
originated tumors, they are often used to judge whether cells in
serous effusions are derived from epithelial cells (10). The detection of CK18+
cells in the pleural fluid implied that these cells may have
originated from epithelial tumor cells. The different states of
CD133+ cancer cells in the pleural fluid may reflect the
heterogeneity of these malignant cancer cells. It has been revealed
that CD133 expression in pancreatic cancer was exclusively
tumorigenic and highly resistant to standard gemcitabine
chemotherapy (1,11). Cyclopamine is an effective method of
reversing gemcitabine resistance in pancreatic cancer with high
levels of cancer stem cell markers (11). A small number of cancers have
CD133-expressing cells in pleural effusion, which may imply
resistance to standard chemotherapy. Since the patient was admitted
to hospital with multiple liver metastasis and pleural effusion,
alleviative chemotherapy with gemcitabine and oxaliplatin combined
with abdominal cavity perfusion chemotherapy and gemcitabine was
administered. However, the patient was insensitive to the
chemotherapy and succumbed to liver metastasis and other
complications three months later. The patient may have benefited
from treatment with cyclopamine or other targeted therapies. The
most highly increased miRNA level in the plasma of this patient was
that of miR-21. Downregulation of miR-21 results in an increased
sensitivity of these cells to gemcitabine (3,12–14).
Measurement of plasma miR-21 levels and serum CA19-9 may be helpful
for early detection of pancreatic cancer (5).
To the best of our knowledge, this is the first
study of CD133+ cancer cells in the pleural effusion of
a pancreatic cancer patient with liver metastasis. Detection of
CD133+ cancer cells in the pleural effusion of this
patient may imply the chemoresistance and cancer stem feature of
these cells, which may provide physicians with significant
information for tailored treatment and evaluating prognosis. In
addition, the plasma miR-21, miR-25, miR-103 and miR-151 levels
were also increased compared with the healthy controls. This
molecular feature of the patient is consistent with the clinical
pathological parameters. Full elucidation of the molecular and
pathological features of pancreatic cancer may be a novel strategy
for tailored therapy.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. 81172508), the
Foundation of the Health Department Program of Jiangsu Province
(No. 200970) and the Foundation of the Social Development of
Jiangsu Province (No. SBE201270298). We thank Dr Pin Lin and Dr
Xingxiang Xu for excellent experimental assistance.
References
|
1.
|
PC HermannSL HuberT HerrlerDistinct
populations of cancer stem cells determine tumor growth and
metastatic activity in human pancreatic cancerCell Stem
Cell1313323200710.1016/j.stem.2007.06.00218371365
|
|
2.
|
Z WangY LiA AhmadPancreatic cancer:
understanding and overcoming chemoresistanceNat Rev Gastroenterol
Hepatol82733201110.1038/nrgastro.2010.18821102532
|
|
3.
|
JB StokesSJ AdairJK Slack-DavisInhibition
of focal adhesion kinase by PF-562,271 inhibits the growth and
metastasis of pancreatic cancer concomitant with altering the tumor
micro-environmentMol Cancer
Ther1021352145201110.1158/1535-7163.MCT-11-026121903606
|
|
4.
|
C RenC HanJ ZhangDetection of apoptotic
circulating tumor cells in advanced pancreatic cancer following
5-fluorouracil chemotherapyCancer Biol
Ther12700706201110.4161/cbt.12.8.1596021811100
|
|
5.
|
J LiuJ GaoY DuCombination of plasma
microRNAs with serum CA19-9 for early detection of pancreatic
cancerInt J Cancer131683691201110.1002/ijc.2642221913185
|
|
6.
|
JY ParkJ HelmD CoppolaD KimM MalafaSJ
KimMicroRNAs in pancreatic ductal adenocarcinomaWorld J
Gastroenterol17817827201110.3748/wjg.v17.i7.81721412491
|
|
7.
|
C RoldoE MissiagliaJP HaganMicroRNA
expression abnormalities in pancreatic endocrine and acinar tumors
are associated with distinctive pathologic features and clinical
behaviorJ Clin
Oncol2446774684200610.1200/JCO.2005.05.519416966691
|
|
8.
|
C RenP HeJ ZhangZ ZhengY QianX
ZhaoMalignant characteristics of circulating tumor cells and
corresponding primary tumor in a patient with esophageal squamous
cell carcinoma before and after surgeryCancer Biol
Ther11633638201110.4161/cbt.11.7.1495021307656
|
|
9.
|
AD RhimET MirekNM AielloEMT and
dissemination precede pancreatic tumor
formationCell148349361201210.1016/j.cell.2011.11.02522265420
|
|
10.
|
V AscoliS TaccognaCC ScalzoF NardiUtility
of cytokeratin 20 in identifying the origin of metastatic
carcinomas in effusionsDiagn
Cytopathol12303308199510.1002/dc.28401204047656755
|
|
11.
|
J YaoY AnJS WieCyclopamine reverts
acquired chemoresistance and down-regulates cancer stem cell
markers in pancreatic cancer cell linesSwiss Med
Wkly141w13208201121630164
|
|
12.
|
E GiovannettiN FunelGJ PetersMicroRNA-21
in pancreatic cancer: correlation with clinical outcome and
pharmacologic aspects underlying its role in the modulation of
gemcitabine activityCancer
Res7045284538201010.1158/0008-5472.CAN-09-446720460539
|
|
13.
|
S AliA AhmadS BanerjeeGemcitabine
sensitivity can be induced in pancreatic cancer cells through
modulation of miR-200 and miR-21 expression by curcumin or its
analogue CDFCancer
Res7036063617201010.1158/0008-5472.CAN-09-459820388782
|
|
14.
|
T MoriyamaK OhuchidaK MizumotoMicroRNA-21
modulates biological functions of pancreatic cancer cells including
their proliferation, invasion, and chemoresistanceMol Cancer
Ther810671074200910.1158/1535-7163.MCT-08-0592
|