Introduction
Esophageal squamous cell carcinoma (ESCC) remains
the most common type of esophageal cancer worldwide. Even in
resectable esophageal cancer, the 5-year survival rate remains only
14–45% (1,2). Treatment failure of ESCC is due to the
high incidence of local-regional failure and early systemic
dissemination of the disease (3).
Chemotherapy is one of the most important treatment methods for
advanced esophageal carcinoma. Several prospective and
retrospective studies in patients with locally advanced thoracic
esophageal cancers or in elderly patients with early stage
esophageal cancer, particularly epidermoid, who respond to
chemoradiotherapy, have suggested that there is no benefit from the
addition of surgery following chemoradiotherapy compared with the
continuation of additional chemoradiotherapy. Following
chemoradiotherapy, with salvage therapy if required, treatment
outcomes among patients with resectable thoracic ESCC were
comparable or superior to the treatment outcomes following surgery
alone (4–7). Therefore, the role of chemotherapy is
becoming increasingly important in the treatment of ESCC.
Patients with ESCC demonstrate different sensitivity
for chemotherapy. Therefore, in order to improve the prognosis of
esophageal carcinoma, there is an urgent requirement to predict the
sensitivity of chemotherapy drugs and tailor individual treatment.
Paclitaxel is one of the most effective drugs for esophageal
carcinoma treatment as it targets cells in the G2/M phase
transition. As a critical regulatory protein, cyclin A may promote
G2/M phase transition and mitosis (8). Our previous studies have revealed that
overexpression of cyclin A in ESCC is associated with cell
proliferation and survival (9–11). As
the clinical application of immunohistochemistry is relatively
cost-effective (2), in this study,
we explore the possibility of evaluating paclitaxel efficacy by
examining the correlation between cyclin A expression and efficacy
of paclitaxel-based chemotherapy in patients with ESCC.
Patients and methods
Patients and specimens
All specimens collected for this retrospective study
were obtained from patients who had not received chemotherapy or
radiotherapy prior to biopsy. A total of 48 ESCC patients were
diagnosed by endoscopic biopsy pathology between 2006 and 2009 at
The People’s Hospital of Taizhou (Taizhou, Jiangsu, China). Those
deemed unsuitable for surgery based on their performance status,
bulky local disease, Karnofsky score (≥70 points) or personal
choice received chemotherapy. Of the 48 patients, 33 were male and
15 were female. The age of the patients ranged from 47 to 78 years
and the median age was 58 years. A total of 16, 19 and 13 cases
were upper, middle and lower thoracic ESCC, respectively. Staging
was determined according to the American Joint Committee on Cancer
tumor-node-metastasis (TNM) classification (12). A total of 7, 13 and 28 cases were
stage I–II, III and IV, respectively; while 10 cases from normal
esophageal mucosa were used as a control group. With regards to
cancer cell differentiation, 10, 16 and 22 cases were well-,
moderately and poorly differentiated, respectively.
Treatment methods
Paclitaxel (180 mg/m2) was infused on day
1 and cisplatin (20 mg/m2) was infused on days 1–3 of a
21-day cycle, yielding a total of 4 chemotherapy cycles. The
patients were also administered conventional anti-allergy
pretreatment prior to chemotherapy.
Short-term efficacy evaluation
All patients received an endoscopy examination,
esophageal barium X-ray and a neck and chest computed tomography
(CT) scan prior to treatment and 1 month following treatment. The
evaluation of the response rate was based on WHO treatment efficacy
evaluation criteria for solid tumors, which divides efficacy into
the subgroups: CR, complete remission; PR, partial remission; SD,
no change; PD, progressive disease; CR+PR, overall response rate
(13).
Follow-up cases
During follow-up, all patients were contacted via
telephone and the last date of follow-up was March 1 2010. A total
of 3 cases were lost; thus, the follow-up rate was 93.8%. The
follow-up time ranged from 6 to 50 months and the average time was
16 months. The survival time was calculated from the diagnostic
date of esophageal carcinoma to the date of mortality or the last
date of follow-up.
Antibody
The antibody used in this study was rabbit
polyclonal antibody anti-human cyclin A (Thermo Fisher Scientific,
Fremont, CA, USA). The final diluted concentration for anti-cyclin
A in TBS containing 1% bovine serum albumin (BSA) was 1:100.
Immunohistochemical staining
Sliced sections (4 μm) of paraffin-embedded
specimens were prepared on microscope slides pre-coated with
saline. Once the paraffin was removed by xylene, the slides were
washed in a graded series of ethanol and the sections were placed
in Tris-buffered saline (TBS) for 10 min. Sections were then
incubated with a blocking solution for 5 min to block endogenous
peroxidase activity and placed in TBS. Sections were then placed in
0.01 mM Tris buffer (pH 6.0) and heated at 121°C for 20 min in an
autoclave oven. Following this, the sections were incubated with
TBS consisting of 1% BSA for 20 min to block non-specific binding
of the immunoreagents. Once the cells were washed in TBS, the
sections were incubated with 1:100 diluted primary antibodies at
4°C overnight. Following further washing in TBS, an
immunoperoxidase staining was performed by a MaxVision antibody
complex method using the MaxVision kit (Fujian Maixin Biological
Technology, Fujian, China). Finally, the localization of cyclin A
was visualized using diaminobenzidine tetrahydrochloride (DAB) and
the sections were lightly counterstained in Harris’ hematoxylin
solution for microscopic examination.
The immunostained specimens were analyzed by two
independent pathologists. At least 10 visual fields were observed
and nuclear staining was used to indicate a positive result. A
total of 1,000 cells in the tumor and nontumor sections were
evaluated at a medium magnification (x200) to determine the
proportion of tumor cells and the staining intensity of the nuclei
in entire sections. Immunohistochemical expression of cyclin A was
determined according to the a+b criteria (14). Category a was defined as the
percentage of carcinoma cells with cyclin A expression. The
percentage of positively stained tumor cells was determined
semi-quantitatively by assessing the whole tumor section, and each
sample was assigned to one of the following categories: 0, 0%
cyclin A expression; 1, 1–25%; 2, 26–50%; 3, >50%. Category b
was defined by the intensity of cyclin A staining. The intensity of
immunostaining was defined as 0 (negative), 1 (weak), 2 (moderate)
and 3 (strong). The carcinomas were regarded to have a positive
response to cyclin A when the total scores of a+b were >3.
Statistical analysis
The Chi-square test was used to examine the
difference between the two groups, and the Cox proportional hazards
model was used to analyze the association of overall survival in
cyclin A positive and negative expression groups. Variables
significantly associated with survival in univariate Cox models
were included in a multivariate Cox model. The results were
quantified by calculating the hazard ratios (HRs) with 95%
confidence intervals (CIs). Survival curves were calculated using
the Kaplan-Meier method and differences between the curves were
determined using the log-rank test.
In all tests, P<0.05 was considered to indicate a
statistically significant difference. Statistical calculations were
conducted using the SPSS version 16.0 System (SPSS, Chicago, IL,
USA).
Results
Expression pattern of cyclin A in normal
human esophageal mucosa and ESCC
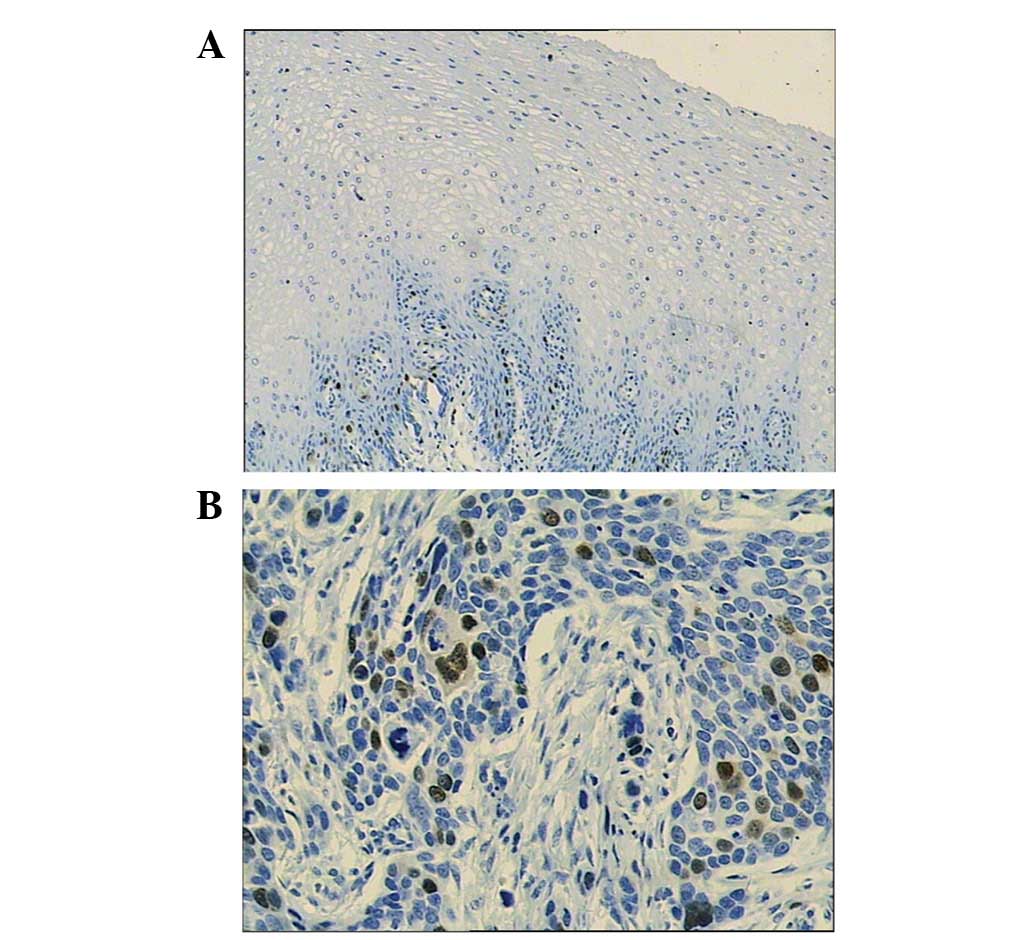
Cyclin A was expressed mainly in the nuclei of
cancer cells and in the basal cells of normal esophageal mucosa
(P<0.05; Fig. 1). The positive
cyclin A expression rate was 64.6% (31/48) in 48 cases with ESCC,
and 20% (2/10) in 10 cases with normal esophageal mucosa.
Comparisons between cyclin A expression pattern in ESCC and normal
tissues have been revealed in our previous studies (10,11).
Cyclin A staining and clinicopathological
factors
The correlations between cyclin A expression and
clinicopathological factors of ESCC are summarized in Table I. Statistically, the expression of
cyclin A was not significantly associated with the age, gender,
tumor size or clinical stage of the patients; however, the
expression levels of cyclin A were significantly higher in poorly
differentiated ESCC cases compared with well-differentiated ESCC
cases (χ2=5.274; P<0.05). This results is consistent
with our previous studies in post-surgery ESCC samples (10,11).
 | Table I.Correlation between cyclin A
expression and clinicopathological factors. |
Table I.
Correlation between cyclin A
expression and clinicopathological factors.
| Clinicopathological
factors | Positive
(%)
n=31 | Negative
(%)
n=17 | χ2 | P-value |
|---|
| Age (years) | | | | |
| ≥60 | 25 (71.4) | 10 (28.6) | | |
| <60 | 6 (46.2) | 7 (53.8) | 2.647 | 0.104 |
| Gender | | | | |
| Male | 21 (63.6) | 12 (36.4) | | |
| Female | 10 (66.7) | 5 (33.3) | 0.041 | 0.839 |
| Tumor size
(cm) | | | | |
| <5 | 14 (56.0) | 11 (44.0) | | |
| ≥5 | 17 (73.9) | 6 (26.1) | 1.680 | 0.195 |
| TNM stage | | | | |
| I–III | 12 (60.0) | 8 (40.0) | | |
| IV | 19 (67.9) | 9 (32.1) | 0.315 | 0.575 |
|
Differentiation | | | | |
|
Well/moderate | 13 (50.0) | 13 (50.0) | | |
| Poor | 18 (81.8) | 4 (18.2) | 5.274 | 0.022 |
Cyclin A staining and short-term
treatment efficacy
Of the 48 ESCC patients, the number of CR, PR, SD
and PD cases were 6, 15, 24 and 3, respectively. The overall
response rate (CR+PR) was 43.8% (21/48). According to the
expression levels of cyclin A, the response rate was 54.8% (17/31)
and 23.5% (4/17) in the positive and negative group, respectively
(χ2=4.373; P=0.037; Table
II).
 | Table II.Correlation between cyclin A
expression and short-term treating efficacy. |
Table II.
Correlation between cyclin A
expression and short-term treating efficacy.
| Efficacy | Positive
n=31 (%) | Negative
n=17 (%) | χ2 | P-value |
|---|
| CR | 5 (16.13) | 1 (5.88) | 1.054 | 0.305 |
| PR | 12 (38.71) | 3 (17.65) | 2.267 | 0.132 |
| SD | 13 (41.94) | 11 (64.71) | 0.024 | 0.877 |
| PD | 1 (3.22) | 2 (11.76) | 4.296 | 0.039 |
| CR+PRa | 17 (54.84) | 4 (23.53) | 4.373 | 0.037 |
Cyclin A staining and prognosis
Univariate Cox analysis revealed that the
clinicopathological stage, degree of cancer cell differentiation
and expression of cyclin A were impact factors for prognosis in
ESCC patients; while no significant correlation was observed
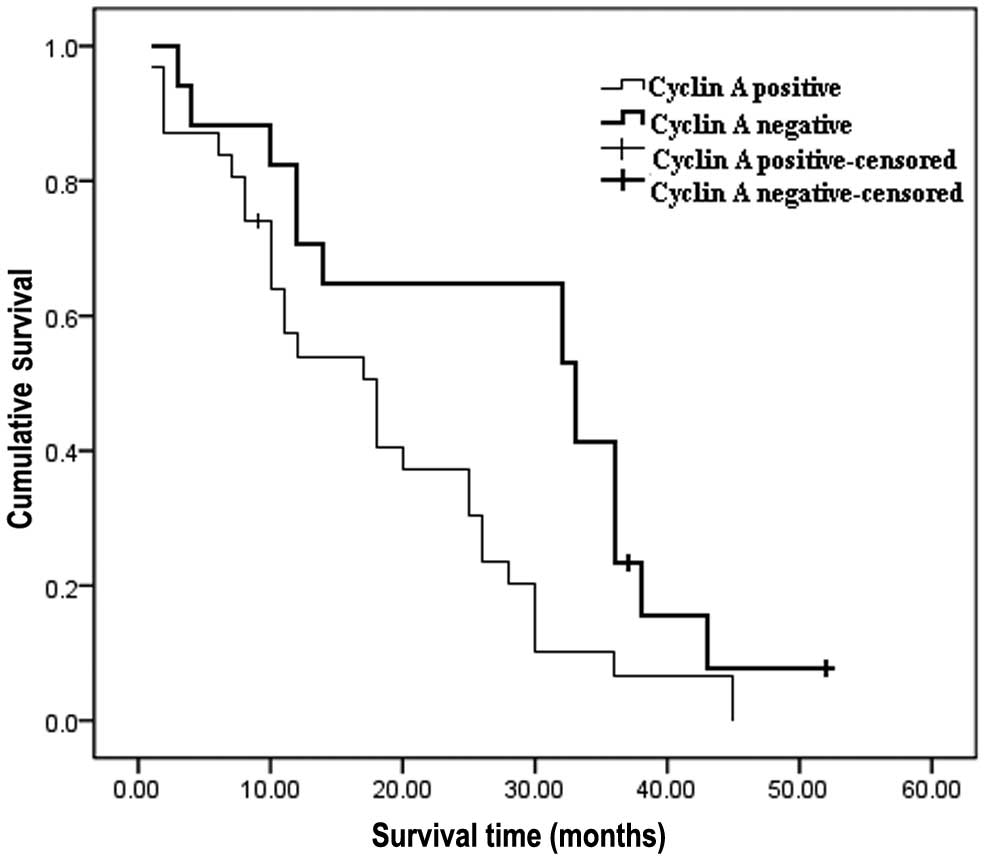
between age, gender and tumor size (Table III). The median survival time for
patients with positive cyclin A expression was 17 months. This was
significantly shorter compared with that for the patients with
negative cyclin A expression, which was 33 months (P=0.016).
Kaplan-Meier survival curves demonstrated that the 1- and 3-year
survival rates in patients with positive cyclin A expression were
significantly lower compared with that of the patients with
negative cyclin A expression (54.9 vs. 82.4%, P=0.037; 6.2 vs.
41.2%, P=0.016, respectively) (Fig.
2).
 | Table III.Univariate analysis of prognostic
impact factors of ESCC patients. |
Table III.
Univariate analysis of prognostic
impact factors of ESCC patients.
| Impact factor | No. | Median survival
(months) | χ2 | P-value |
|---|
| Age (years) | | | | |
| ≥60 | 35 | 18 | | |
| >60 | 13 | 20 | 0.080 | 0.778 |
| Gender | | | | |
| Male | 33 | 18 | | |
| Female | 15 | 25 | 0.076 | 0.783 |
| Tumor size
(cm) | | | | |
| <5 | 25 | 20 | | |
| ≥5 | 23 | 18 | 0.116 | 0.733 |
| TNM stage | | | | |
| I–III | 20 | 28 | | |
| IV | 28 | 10.5 | 3.980 | 0.046 |
|
Differentiation | | | | |
|
Well/moderate | 26 | 26 | | |
| Poor | 22 | 12 | 5.863 | 0.015 |
| Cyclin A
expression | | | | |
| Positive | 31 | 17 | | |
| Negative | 17 | 33 | 5.749 | 0.016 |
Multivariate Cox analysis revealed that the
clinicopathological stage, degree of cancer cell differentiation
and expression of cyclin A were of statistical significance in ESCC
patients. We demonstrated that advanced clinicopathological stage
(P=0.000; 95% CI, 0.018–0.309), poor cancer cell differentiation
(P=0.008; 95% CI, 0.138–0.739) and positive expression of cyclin A
(P=0.002, 95% CI, 0.119–0.611) were independent prognostic factors
in patients with ESCC following paclitaxel-based chemoradiotherapy
(Table IV).
 | Table IV.Multivariate Cox analysis of
clinicopathological and biological molecular characteristics in
ESCC patients. |
Table IV.
Multivariate Cox analysis of
clinicopathological and biological molecular characteristics in
ESCC patients.
| B | SE | Wald | P-value | Exp (B) | 95% CI for Exp (B)
|
|---|
| Lower | Upper |
|---|
| TNM stage | −2.607 | 0.732 | 12.693 | 0.000 | 0.074 | 0.018 | 0.309 |
|
Differentiation | −1.414 | 0.427 | 7.133 | 0.008 | 0.320 | 0.138 | 0.739 |
| Cyclin A
expression | −1.312 | 0.418 | 9.845 | 0.002 | 0.269 | 0.119 | 0.611 |
Discussion
Since the overall survival rate of ESCC patients
following surgery alone is poor, multimodality approaches have been
developed. However, these are inadequate with regard to improving
the treatment efficacy of esophageal cancer by basing treatment on
surgical approaches alone. Instead, surgeons should be prepared for
a new approach, which comprises biological tumor staging and
targeted therapies combined with neoadjuvant chemoradiotherapy.
Certain studies suggest that the tumor response to induction
chemoradiotherapy aids the identification of patients with good
prognosis, regardless of whether surgery is conducted or not
(3,15). In these patients, surgery may no
longer be recommended as routine treatment (15); therefore, ESCC patients may have a
survival benefit if they are responsive to chemoradiotherapy. To
predict the response prior to chemoradiotherapy is undoubtedly one
of the critical factors to determine the best treatment. Although
not all tumors respond to paclitaxel, it is used widely in the
treatment of ESCC. A previous study has revealed that the response
rate of paclitaxel as a single drug in ESCC patients was only
20–37%, and the overall response rate of paclitaxel in combination
with cisplatin was 42.6% (16). The
characteristics that distinguish drug-resistant tumors from
drug-sensitive tumors are not well defined, and for that reason,
predicting the response prior to chemotherapy is a research
hotspot. Therefore, several studies have focused on predicting
paclitaxel sensitivity in vitro using BRCA1, CDK1 and CDK2
(17,18).
Cyclins, which control key checkpoints of the cell
cycle, play a fundamental role in regulating cell growth and
survival. In humans, several types of cyclin, including three
categories, A-, B- and C-type (C-, D- and E-types), have now been
isolated. Cyclins A and B1–2 reach maximum levels in the S and G2
phases. Cyclin A may be activated during the transition from the G1
to the S phase of the cell cycle; therefore, it is suggested to be
an important regulator of mitosis (19,20).
In this study, we detected the expression of cyclin A in tissue
samples from 48 patients with ESCC prior to treatment with
paclitaxel/cisplatin. We revealed that the expression of cyclin A
was significantly correlated with the degree of cell
differentiation and the response to paclitaxel-based chemotherapy.
The expression of cyclin A in the poorly differentiated ESCC
samples was higher compared with that of the well-differentiated
ESCC samples, indicating that cyclin A expression correlates with
the degree of ESCC malignancy. This suggests that ESCC samples with
positive cyclin A expression were poorly differentiated, and the
cell proliferation cycle was more active. Of the 48 cases of ESCC,
the response rate of 31 cases with positive cyclin A expression was
54.8%, while the response rate of 17 cases with negative cyclin A
expression was 23.5%. This result indicates that ESCC patients with
positive cyclin A expression were sensitive to paclitaxel-based
chemotherapy and may have a higher response rate.
Paclitaxel binds to microtubules and stabilizes the
polymerized structure. Consequently, paclitaxel inhibits
microtubule depolymerization, suppressing tumor growth and holding
proliferative cells in the G2/M phase (21). Nakayama et al reported that
the analysis of cyclin-dependent kinase activity in the clinical
setting may be a powerful approach for predicting paclitaxel
sensitivity (18). Another study
revealed that targeting cyclin B1 sensitized breast cancer cells to
taxol, suggesting that specific cyclin B1 targeting is an
attractive strategy to be used in combination with conventionally
used agents in gynecological cancer therapy (22). Our study demonstrated that cyclin A
expression was correlated with chemosensitivity to paclitaxel-based
chemotherapy in patients with ESCC. This indicates that the cell
cycle is located at the G2/M phase and that when the expression of
cyclin A was positive in tumor cells, patients had a higher
response to paclitaxel, which is consistent with the cell cycle
theory.
Our data also revealed that ESCC patients with
positive cyclin A expression had poor prognosis, while the patients
with negative cyclin A expression had good prognosis. This result
is consistent with previous studies on breast (23), ovarian (24) and laryngeal cancer (25).
Determining whether the tumor is sensitive to
paclitaxel prior to treatment is one of the most efficient ways of
improving response. Our study revealed that ESCC patients
overexpressing cyclin A prior to treatment may be more sensitive to
paclitaxel-based chemotherapy. It would be beneficial to extend
this preliminary result into a larger sample size study in order to
formalize it as a standard procedure for the clinical treatment of
ESCC. Our study indicates the importance of applying ‘personalized
cancer medicine’ in the clinical treatment of cancer, which is
based on gene expression profile, to guide the antitumor drug
selection.
Acknowledgements
This study was supported by the 333
Plan Foundation (Grant No. 2009-24) of Jiangsu Province, China, the
Six Talents Peak Foundation (Grant No. 2011-WS-023) of Jiangsu,
China, and the Key Medical Talent Foundation (Grant No. RC2011212)
of Jiangsu, China. The authors are grateful to Dr Jianming Zhang
for his assistance with the manuscript preparation.
References
|
1.
|
PC EnzingerRJ MayerEsophageal cancerN Engl
J Med34922412252200310.1056/NEJMra03501014657432
|
|
2.
|
S TakenoT NoguchiY TakahashiS FumotoT
ShibataK KawaharaAssessment of clinical outcome in patients with
esophageal squamous cell carcinoma using TNM classification score
and molecular biological classificationAnn Surg
Oncol1414311438200710.1245/s10434-006-9286-3
|
|
3.
|
DH IlsonEsophageal cancer chemotherapy:
recent advancesGastrointest Cancer Res285922008
|
|
4.
|
L BedenneP MichelO BouchéChemoradiation
followed by surgery compared with chemoradiation alone in squamous
cancer of the esophagus: FFCD 9102J Clin
Oncol2511601168200710.1200/JCO.2005.04.711817401004
|
|
5.
|
JA AbramsDL BuonoJ StraussRB McBrideDL
HershmanAI NeugutEsophagectomy compared with chemoradiation for
early stage esophageal cancer in the
elderlyCancer11549244933200910.1002/cncr.2453619637343
|
|
6.
|
H YamashitaK OkumaY SetoA retrospective
comparison of clinical outcomes and quality of life measures
between definitive chemoradiation alone and radical surgery for
clinical stage II–III esophageal carcinomaJ Surg
Oncol100435441200919653240
|
|
7.
|
H ArigaK NemotoS MiyazakiProspective
comparison of surgery alone and chemoradiotherapy with selective
surgery in resectable squamous cell carcinoma of the esophagusInt J
Radiat Oncol Biol
Phys75348356200910.1016/j.ijrobp.2009.02.08619735862
|
|
8.
|
C Cordon-CardoMutations of cell cycle
regulators. Biological and clinical implications for human
neoplasiaAm J Pathol14754556019957677168
|
|
9.
|
JX HuangW YanZX SongRelationship between
proliferative activity of cancer cells and clinicopathological
factors in patients with esophageal squamous cell carcinomaWorld J
Gastroenterol1129562959200510.3748/wjg.v11.i19.295615902736
|
|
10.
|
JX HuangW XiaoWC ChenRelationship between
COX-2 and cell cycle-regulatory proteins in patients with
esophageal squamous cell carcinomaWorld J
Gastroenterol1659755981201021157974
|
|
11.
|
JX HuangWC ChenM LinClinicopathological
significance of cyclooxygenase-2 and cell cycle-regulatory proteins
expression in patients with esophageal squamous cell carcinomaDis
Esophagus25121129201210.1111/j.1442-2050.2011.01219.x21762277
|
|
12.
|
J HuangEsophageal cancerManual Medical
OncologyY SunYK Shi5th editionThe People’s Medical Publishing
HouseBeijing4664692007
|
|
13.
|
Y SunThe clinical experiment of anti-tumor
drugsManual Medical OncologyY SunYK Shi5th editionThe People’s
Medical Publishing HouseBeijing1611622007
|
|
14.
|
T NozoeD KorenagaM FutatsugiH SaekiT OhgaK
SugimachiCyclin A expression in superficial squamous cell carcinoma
of the esophagus and coexisting infiltrated lymphocyte
follicleCancer
Lett188221229200210.1016/S0304-3835(02)00434-212406568
|
|
15.
|
MC WolfM StahlBJ KrauseCurative treatment
of oesophageal carcinoma: current options and future
developmentsRadiat Oncol655201110.1186/1748-717X-6-5521615894
|
|
16.
|
XD ZhangL ShenJ LiProspective
non-randomized study of chemotherapy combined with radiotherapy
versus chemotherapy alone in patients with metastatic or relapsed
esophageal squamous cell carcinomaZhonghua Zhong Liu Za
Zhi294744772007(In Chinese).
|
|
17.
|
B StordalR DaveyA systematic review of
genes involved in the inverse resistance relationship between
cisplatin and paclitaxel chemotherapy: role of BRCA1Curr Cancer
Drug Targets9354365200910.2174/15680090978816659219442054
|
|
18.
|
S NakayamaY TorikoshiT TakahashiPrediction
of paclitaxel sensitivity by CDK1 and CDK2 activity in human breast
cancer cellsBreast Cancer Res11R12200910.1186/bcr223119239702
|
|
19.
|
T HunterJ PinesCyclins and
cancerCell6610711074199110.1016/0092-8674(91)90028-W
|
|
20.
|
M PaganoR PepperkokF VerdeW AnsorgeG
DraettaCyclin A is required at two points in the human cell
cycleEMBO J1196197119921312467
|
|
21.
|
TJ MitchisonThe proliferation rate paradox
in antimitotic chemotherapyMol Biol
Cell2316201210.1091/mbc.E10-04-033522210845
|
|
22.
|
I AndroicA KrämerR YanTargeting cyclin B1
inhibits proliferation and sensitizes breast cancer cells to
taxolBMC Cancer8391402200810.1186/1471-2407-8-39119113992
|
|
23.
|
P BoströmM SöderströmT PalokangasT
VahlbergY CollanO CarpenP HirsimäkiAnalysis of cyclins A, B1, D1
and E in breast cancer in relation to tumour grade and other
prognostic factorsBMC Res Notes2140148200919615042
|
|
24.
|
BS YoonYT KimS KimPrognostic value of
nuclear DNA quantification and cyclin A expression in epithelial
ovarian carcinomaEur J Obstet Gynecol Reprod
Biol136110115200810.1016/j.ejogrb.2006.10.00817157431
|
|
25.
|
M FraczekZ WozniakD RamseyT ZatonskiT
KrecickiClinicopathologic significance and prognostic role of
cyclin E and cyclin A expression in laryngeal epithelial
lesionsActa
Otolaryngol128329334200810.1080/0001648070148774217917837
|