Introduction
Gynecological malignancy may develop into bowel
obstruction at primary presentation, but obstruction occurs more
frequently with recurrence and disease progression as the
consequence of resistance to and failure of anticancer therapy.
This often results in poor quality of life and prolonged
hospitalization at the end of life. Several prognostic factors for
malignant bowel obstruction (MBO) have been reported to date,
including age, performance status, nutritional status, ascites,
palpable mass, extraabdominal metastasis and previous anticancer
treatment (1–5). Although there have been increased
efforts in the integration of palliative care, clinicians are in
need of clinical data to guide towards more evidence-based
practice. A number of previous studies have investigated the
effects and outcomes of palliative surgery for MBO; however, the
role of surgical treatment for terminal patients is controversial
(6). Differences in outcome
measures, including symptom control, quality of life and survival,
without the establishment of a standardized assessment, makes it
difficult to interpret each result, leading to further confusion.
Moreover, patients who did not have surgery are usually not
analyzed in the same way and there are few data which have been
investigated by prospective study or multivariate analysis, despite
the frequent occurrence of MBO in patients with gynecological
malignancy; consequently, most previous studies have been unable to
define criteria that would allow the selection of patients who are
unlikely to benefit from surgery (7,8). The
purpose of this study was to evaluate the outcomes of patients with
gynecological malignancy who received palliative care with and
without surgical procedure for MBO in our institute and to explore
prognostic factors to aid the selection of patients who would
benefit from palliative surgery.
Patients and methods
Patients
Of all the patients with gynecological malignancy
treated at our institute, medical records of patients who presented
with MBO due to disease progression or recurrence between 2005 and
2010 were reviewed. Cases with symptoms of bowel obstruction which
were temporary and restorable with short medical treatment were
excluded. All patients underwent a physical examination, a plain
film and a CT scan evaluation. Nasogastric or long intestinal tubes
for decompression and symptom relief were frequently attempted
during the course of initial conservative treatment. Water-soluble
contrast medium was also administered via tubes when considered
appropriate to detect sites of strangulation. The study protocol
was approved by the ethics committee of the National Defense
Medical College, Saitama, Japan. Patient consent was obtained
either from the patient or the patient’s family
Surgical procedures
Bowel surgery to palliate MBO was considered only
for patients with relatively good performance status, predicted
life expectancy longer than 60 days, expectation of surgically
possible decompression by image scan or contrast radiography
leading to resumption of oral diet and strong desire for surgery,
including stoma formation. Laparoscopy was not performed during the
study period. Patients whose obstruction was present at diagnosis
of primary disease and corrected at primary surgery were excluded.
Bowel surgery to release bowel obstruction performed together with
cytoreductive surgery was not defined as palliative surgery and was
also excluded from the present study.
Outcomes
Successful palliation following surgery was defined
as the ability to tolerate solid food for at least 60 days, as
defined in several previous studies (4,9). The
treatment-free interval was calculated from the time when prior
treatment was completed to the time of diagnosis for MBO, which was
defined as the first time that bowel obstruction was diagnosed by
radiographic or physical examination following gastrointestinal
symptoms due to obstruction. Overall survival was measured from the
date of diagnosis for MBO to the date of mortality or last
follow-up.
Statistical analysis
Associations between clinical variables were
analyzed using Chi-square or Fisher’s exact tests. Survival curves
were estimated using the Kaplan-Meier method and P-values were
generated using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of the patients
During the study period, 53 patients who required
hospitalization and palliative care for MBO at our institute were
identified. Table I provides a
summary of patient characteristics at diagnosis of MBO. The ovary
was the most common primary site and accounted for >60% of the
cohort, including Fallopian tube and primary peritoneum.
Approximately half of the cases (25/53) had massive ascites
diagnosed by image scan. Of all the cases, 20 (38%) had bowel
surgery for MBO as a palliative procedure (operative group) and 33
(62%) did not (non-operative group).
 | Table ICharacteristics of patients with
palliative care for MBO due to gynecological malignancy (n=53). |
Table I
Characteristics of patients with
palliative care for MBO due to gynecological malignancy (n=53).
| Characteristic | Value |
|---|
| Age, median (range),
years | 58 (35–85) |
| Origin of primary
disease, n (%) | |
| Cervix | 9 (17) |
| Corpus | 11 (21) |
| Ovary | 29 (55) |
| Fallopian tube | 1 (2) |
| Primary
peritoneal | 3 (6) |
| ECOG performance
status, n (%) | |
| 1 | 18 (34) |
| 2 | 23 (43) |
| 3 | 11 (21) |
| 4 | 1 (2) |
| History of anticancer
therapya, n (%) | |
| Surgery | 49 (92) |
| Chemotherapy | 50 (94) |
| Radiotherapy | 17 (32) |
| Time from primary
therapy to diagnosis of MBO, median (range), days | 780 (30–4486) |
| Treatment-free
interval between last anticancer therapy and diagnosis of MBO,
median (range), days | 51 (6–512) |
| Presence of massive
ascites, n (%) | |
| Yes | 25 (47) |
| No | 28 (53) |
| Palliative procedure
performed for MBO, n (%) | |
| Pharmacological
treatment only | 33 (62) |
| Laparotomy | 20 (38) |
Surgical procedures and outcomes
Table II describes
the palliative surgical procedures performed for MBO in the
operative group and their outcomes. Colostomy was performed in 11
(55%) of 20 patients and ileostomy was in 7 (35%) patients.
Postoperative complications were observed in 7 (35%) of the 20
patients. Of the complications, infections, including wound
dehiscence, abscess formation and sepsis, were relatively frequent
and successfully resolved by antibiotic treatment. Although no
patients underwent open-and-shut laparotomy only without any
corrective surgery taking place, 2 (11%) patients’ symptoms were
not relieved without the resumption of oral diet by surgical
correction and 1 patient tolerated an oral diet following surgery
but succumbed to disease progression within a month. Successful
palliation, as defined in Patients and methods, was achieved in 14
(70%) of the 20 cases, with a median period of 146 days (range,
61–294). The median survival time following surgery was 143 days
(range, 24–294) and after diagnosis of MBO was 146 days (range,
40–334).
 | Table IIPalliative surgical procedures
performed for MBO and outcomes (n=20). |
Table II
Palliative surgical procedures
performed for MBO and outcomes (n=20).
| Characteristic | Value |
|---|
| Surgical procedure
performeda, n | 20 |
| Transverse
colostomy | 10 |
| Descending
colostomy | 1 |
| Ileostomy | 7 |
| Bypass | 7 |
| Time from diagnosis
of MBO to surgery, median (range), days | 9 (1–63) |
| Operating time,
median (range), minutes | 97 (32–257) |
| Blood loss, median
(range), gram | 97 (5–4620) |
| Time to resume diet
following surgery, median (range), days | 5 (2–23) |
| Postoperative
complicationsa, n
(%) | 7 (35) |
| Abscess | 4 |
| Wound infection
and dehiscence | 3 |
| Sepsis | 1 |
| Deep venous
thrombosis | 1 |
| Short bowel
syndrome | 1 |
| Symptoms
unrelieved, n (%) | 2 (10) |
| Postoperative
mortality within 30 days, n (%) | 1 (5) |
| Duration capable of
postoperative oral diet, median (range), days | 100 (0–294) |
| Successful
palliation following surgery (>60 days), n (%) | 14 (70) |
| On discharge taking
oral diet, n (%) | 15 (75) |
| Median survival
time following surgery (range), days | 143 (24–294) |
| Median survival
time following diagnosis of MBO (range), days | 146 (40–334) |
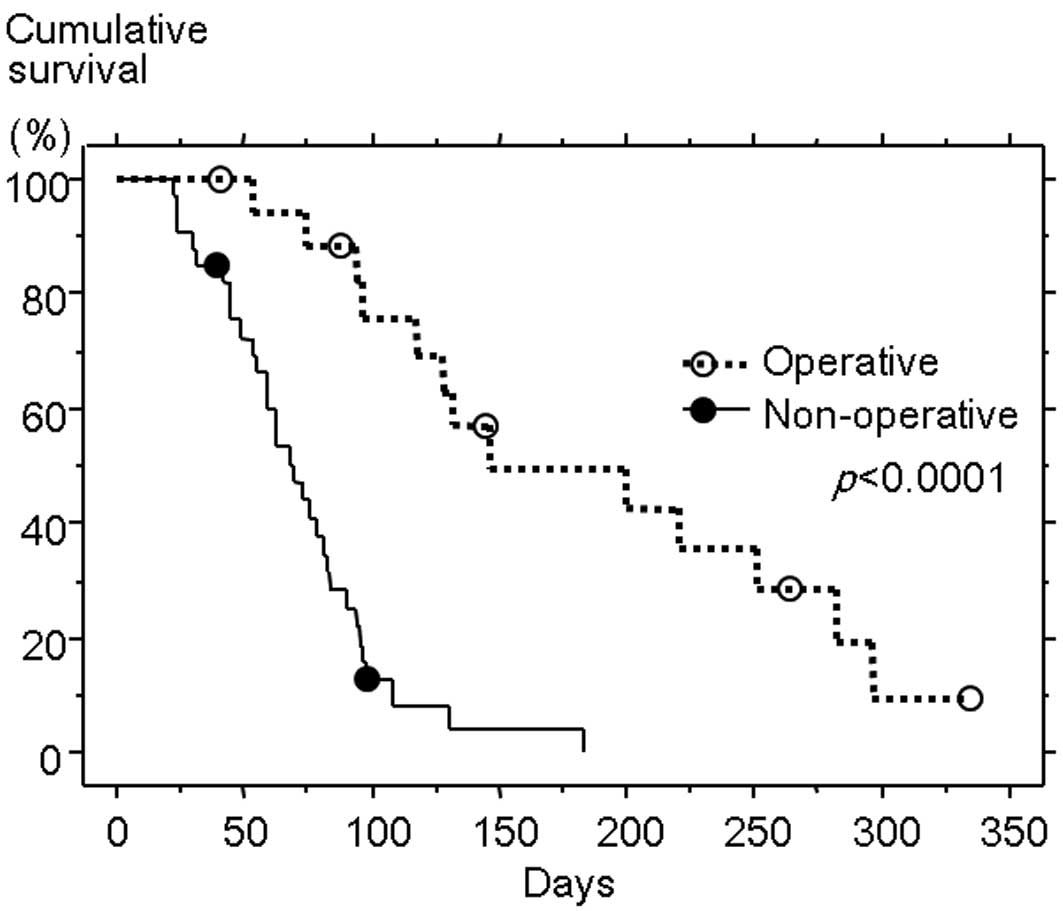
Survival analysis
The Kaplan-Meier test for survival analysis
(Fig. 1) confirmed significantly
longer survival following the diagnosis of MBO in the operative
than the non-operative group (median survival time, 146 versus 69
days; P<0.0001). Comparisons of characteristics between the two
groups are shown in Table III. As
expected, the performance status at diagnosis of MBO was superior
in the operative group. Other variables used to assess potential
medical conditions, including age, presence of massive ascites and
laboratory values, were not significantly different between the
groups; however, a longer interval from the last anticancer therapy
to diagnosis of MBO was observed in the operative than the
non-operative group (median, 57 versus 30 days; P<0.05). Further
analysis comparing the details of the previous anticancer treatment
revealed that there was no significant difference in the number of
chemotherapeutic regimens or courses performed, the number of
previous laparotomy or bowel surgeries performed or previous
radiotherapy of the abdomen or pelvis. Three patients had stoma at
the onset of MBO in the non-operative group and 1 in the operative
group (not statistically significant). Based on these results, we
performed a further comparative analysis of the characteristics of
the operative group (Table IV),
comparing patients who achieved successful palliation following
surgery (successful group; n=14) with those who did not
(unsuccessful group; n=6). The interval from the last anticancer
therapy to diagnosis of MBO was also longer in the successful than
the unsuccessful group (median, 83 versus 32 days; P<0.05),
although the performance status did not show a marked difference.
Time from diagnosis of MBO to surgery, site of stoma and
postoperative complications also had no impact on surgical outcome
according to the comparison of these two groups.
 | Table IIIComparison of characteristics of
patients between the operative (n=20) and non-operative groups
(n=33). |
Table III
Comparison of characteristics of
patients between the operative (n=20) and non-operative groups
(n=33).
|
Characteristics | Operative
(n=20) | Non-operative
(n=33) | P-value |
|---|
| Age, median
(range), years | 58 (35–84) | 59 (40–85) | N.S. |
| Primary site, n
(%) | | | N.S. |
| Cervix | 2 (10) | 7 (21) | |
| Corpus | 6 (30) | 5 (15) | |
| Ovary | 12 (60) | 17 (52) | |
| Fallopian
tube | 0 | 1 (3) | |
| Peritoneum | 0 | 3 (9) | |
| ECOG performance
status, n (%) | | | <0.001 |
| <2 | 15 (75) | 3 (9) | |
| ≥2 | 5 (25) | 30 (91) | |
| History of
anticancer therapya, n
(%) | | | N.S. |
| Surgery | 19 (95) | 31 (94) | |
| Chemotherapy | 19 (95) | 31 (94) | |
| Radiotherapy | 7 (35) | 10 (30) | |
| Time from primary
therapy to diagnosis of MBO, median (range), days | 820 (43–4486) | 720 (30–2430) | N.S. |
| Treatment-free
interval between last anticancer therapy and diagnosis of MBO,
median (range), days | 57 (6–512) | 30 (8–360) | <0.05 |
| Presence of massive
ascites, n (%) | | | N.S. |
| Yes | 8 (40) | 17 (52) | |
| No | 12 (60) | 16 (48) | |
| Laboratory values,
median (range) | | | N.S. |
| Hemoglobin,
g/dl | 10.0
(6.7–13.0) | 9.3 (6.3–14.0) | |
| Serum albumin,
g/dl | 2.8 (1.8–3.6) | 2.9 (1.6–4.3) | |
| White blood cell
count (WBC), /μl | 5400
(2790–21390) | 5300
(1410–16220) | |
| Total lymphocyte
count (TLC), /μl | 767 (362–2195) | 705 (106–2201) | |
| C-reactive
protein (CRP), mg/dl | 5.3 (0.1–23.7) | 3.4 (0.3–27.3) | |
 | Table IVComparison of characteristics of
patients between successful (n=14) and unsuccessful (n=6)
palliation groups following surgery for MBO. |
Table IV
Comparison of characteristics of
patients between successful (n=14) and unsuccessful (n=6)
palliation groups following surgery for MBO.
|
Characteristics | Successful
(n=14) | Unsuccessful
(n=6) | P-value |
|---|
| Age, median
(range), years | 59 (35–68) | 54 (45–84) | N.S. |
| Primary site, n
(%) | | | N.S. |
| Cervix | 2 (14) | 0 (0) | |
| Corpus | 5 (36) | 1 (17) | |
| Ovary | 7 (50) | 5 (83) | |
| ECOG performance
status, n (%) | | | N.S. |
| <2 | 11 (79) | 4 (67) | |
| ≥2 | 3 (21) | 2 (33) | |
| History of
anticancer therapya, n
(%) | | | N.S. |
| Surgery | 13 (93) | 6 (100) | |
| Chemotherapy | 13 (93) | 6 (100) | |
| Radiotherapy | 6 (43) | 1 (17) | |
| Time from primary
therapy to diagnosis of MBO, median (range), days | 855 (271–4486) | 513 (43–3768) | N.S. |
| Treatment-free
interval between last anticancer therapy and diagnosis of MBO,
median (range), days | 83 (33–512) | 32 (6–74) | <0.05 |
| Presence of massive
ascites, n (%) | | | N.S. |
| Yes | 5 (36) | 3 (50) | |
| No | 9 (64) | 3 (50) | |
| Laboratory values,
median (range) | | | N.S. |
| Hemoglobin,
g/dl | 10.3
(8.5–13.0) | 7.4 (6.7–10.9) | |
| Serum albumin,
g/dl | 2.8 (1.8–3.5) | 2.8 (1.9–3.6) | |
| White blood cell
count (WBC), /μl | 6100
(2790–21390) | 5400
(5100–7300) | |
| Total lymphocyte
count (TLC), /μl | 762 (362–2195) | 759 (383–968) | |
| C-reactive
protein (CRP), mg/dl | 2.5 (0.1–23.7) | 5.7 (4.9–21.0) | |
| Time from diagnosis
of MBO to surgery, median (range), days | 10 (1–40) | 6 (3–63) | N.S. |
| Site of stoma, n
(%) | | | N.S. |
| Colostomy | 7 (50) | 4 (67) | |
| Ileostomy | 5 (36) | 2 (33) | |
| Multiple bowel
procedures, including bypass at palliative surgery, n (%) | 4 (29) | 1 (17) | N.S. |
| Postoperative
complications, n (%) | 6 (43) | 2 (33) | N.S. |
| Median survival
time following surgery (range), days | 192 (67–294) | 64 (24–143) | <0.05 |
| Median survival
time following diagnosis of MBO (range), days | 221 (75–334) | 94 (40–146) | <0.05 |
Discussion
In our cases of MBO due to gynecological malignancy,
the most predominant primary disease was ovarian cancer, accounting
for more than half of the cases. Reflecting the large population,
approximately half of the cases suffered from massive ascites at
the onset of MBO. Ovarian cancers frequently present with
intraabdominal dissemination without impairment of vital organs in
the course of disease progression and recurrence, leading to
peritonitis carcinomatosa with impaired bowel movements. Successful
palliation following surgery was observed in our patients with
ovarian cancer who presented with ascites requiring occasional
paracentesis. Considering that neither the proportion of patients
with ovarian cancer nor those with ascites was significantly
different in the two comparisons of operative versus non-operative
group and successful versus unsuccessful group, such patients could
not be excluded from the candidacy for palliative surgery and
careful assessment should be individually required.
In the present study, significantly longer survival
following the diagnosis of MBO in the operative than the
non-operative group was observed, in accordance with several
previous studies (3,10,11).
By comparing the characteristics of the two groups, the performance
status at diagnosis of MBO was found to be superior in the
operative group as expected, since surgery had been considered only
for patients with a relatively good performance status,
unsurprisingly leading to the longer survival in the operative
group. In addition to performance status, a longer interval from
last anticancer therapy to diagnosis of MBO was observed in the
operative than the non-operative group. In our comparisons in the
operative group, the successful group also showed a longer interval
from the last anticancer therapy to the diagnosis of MBO than the
unsuccessful group, despite no marked difference in performance
status. However, we did not find any difference in the type nor the
number of anticancer therapy. Since our patients did not receive
further chemotherapy following the diagnosis of MBO, the
implication should be different from that of the treatment-free
interval as a good prognostic factor to predict a better response
to further anticancer therapy in recurrent ovarian cancer; however,
patients with a longer treatment-free interval despite having
progressive disease or recurrence may have a superior nutritional
status, be relatively stable in their disease and be better
prepared for palliative therapy, possibly resulting in a good
outcome. Zoetmulder et al also reported that the interval
between last treatment and bowel obstruction, followed by the
presence of ascites, was the most significant prognostic factor for
MBO in ovarian cancer, discussing the implication of a long
interval reflecting differences in the biology of relatively
slow-growing tumors (3). A short
interval between the diagnosis of primary cancer and bowel
obstruction correlated with lower survival probabilities, according
to Fernandes et al (2). In
our cases, the time from primary therapy to diagnosis of MBO was
longer in the operative and successful groups when compared with
their opponents, but was not statistically significant, similar to
the result reported by Zoetmulder et al (3).
Concerning previous anticancer therapy, there was no
significant difference in the history of previous bowel surgery
when operative versus non-operative and successful versus
unsuccessful group comparisons were performed; however, three
patients had stoma at the onset of MBO in the non-operative group,
which were constructed at primary or secondary debulking surgery,
while there was only one case with stoma, which was constructed due
to rectovaginal fistula following recurrence, in the operative
group. Pothuri et al reported their experiences of
reoperation for palliation of recurrent MBO in ovarian cancer and
concluded that patients undergoing repeat surgery for recurrent
bowel obstruction have a low likelihood of successful palliation,
although the details of their previous palliative surgeries were
not described (9). Considering
their results, patients who had a history of bowel surgery to
correct bowel obstruction, especially stoma, may not be suitable
even for the first palliative bowel surgery, as a poor possibility
of surgical correction and high morbidity rate were expected.
Also concerning previous anticancer therapy,
approximately half of our patients (6/14) with successful
palliation had prior radiotherapy (for 2 cervical, 2 corpus and 2
ovarian cancers), compared with 1 of the 6 patients in the
unsuccessful group. Conflicting results have been reported
concerning the prognostic significance of prior radiotherapy on
MBO. Krebs and Goplerud reported that previous radiotherapy of the
abdomen was a poor prognostic indicator of low likelihood of
clinical benefit from surgery to treat MBO (1). They explained that patients treated by
radiotherapy were found to be inoperable at laparotomy or developed
severe postoperative bowel complications, including dehiscence of
bowel anastomosis and formation of enterocutaneous fistulas.
Postoperative complications were observed in 3 of our 7 patients
with prior radiotherapy, including 2 infections and 1 case of short
bowel syndrome. By contrast, Fernandes et al reported that a
lack of previous radiotherapy resulted in lower survival
probabilities in patients with ovarian cancer and bowel obstruction
(2). Although multiple factors
associated with radiotherapy may affect the outcomes of patients
with MBO, 6 (86%) of our 7 patients with prior radiotherapy had
successful palliation following surgery; therefore, irradiated
patients may not always constitute an unfavorable subgroup of
patients when considering surgical intervention.
Time from diagnosis of MBO to surgery, site of stoma
and postoperative complications had no impact on surgical outcome
according to the comparison of the successful and unsuccessful
groups. The reason why postoperative morbidity did not correlate
with successful palliation following surgery was probably due to
the complications observed in our cases recovering with appropriate
medications. A wide range of morbidity and mortality rates
following surgery for MBO have been reported in the previous
studies, varying from 5 to 49% for the morbidity rate (4,12).
Careful perioperative management as well as deliberate selection of
patients is important to achieve the palliative goal in these
terminally ill patients. The weakness of this study is that the
small number of patients analyzed may preclude the detection of
statistically significant results, and further clinical data should
be gathered by large prospective studies to enable physicians to
apply clear criteria for the selection of patients who are likely
to benefit from surgery.
In conclusion, our study demonstrated the palliative
benefit by bowel surgery for MBO in selected patients with
gynecological malignancy; therefore, surgery may be considered as a
measure of palliative care for MBO in patients who had a life
expectancy of longer than 2 months without serious
contraindications and poor performance status. In addition, the
interval from last anticancer therapy to diagnosis of MBO may also
serve as a prognostic factor when considering surgical
intervention.
References
|
1.
|
HB KrebsDR GoplerudSurgical management of
bowel obstruction in advanced ovarian carcinomaObstet
Gynecol6132733019836823374
|
|
2.
|
JR FernandesRJ SeymourS SuissaBowel
obstruction in patients with ovarian cancer: a search for
prognostic factorsAm J Obstet
Gynecol158244249198810.1016/0002-9378(88)90131-73341401
|
|
3.
|
FA ZoetmulderTJ HelmerhorstF van
CoevordenPE WolfsJP LeyerAA HartManagement of bowel obstruction in
patients with advanced ovarian cancerEur J
Cancer30A16251628199410.1016/0959-8049(94)E0131-M7833134
|
|
4.
|
C RipamontiE BrueraPalliative management
of malignant bowel obstructionInt J Gynecol
Cancer12135143200210.1046/j.1525-1438.2002.01103.x11975672
|
|
5.
|
H Medina-FrancoMN García-AlvarezLJ
Ortiz-LópezJZ CuairánPredictors of adverse surgical outcome in the
management of malignant bowel obstructionRev Invest
Clin60212216200818807733
|
|
6.
|
A KucukmetinR NaikK GalaalA BryantHO
DickinsonPalliative surgery versus medical management for bowel
obstruction in ovarian cancerCochrane Database Syst
Rev7CD007792201020614464
|
|
7.
|
TW CastaldoES PetrilliSC BallonLD
LagasseIntestinal operations in patients with ovarian carcinomaAm J
Obstet Gynecol139808419817457527
|
|
8.
|
SC RubinWJ HoskinsI BenjaminJL Lewis
JrPalliative surgery for intestinal obstruction in advanced ovarian
cancerGynecol
Oncol341619198910.1016/0090-8258(89)90097-82472311
|
|
9.
|
B PothuriL MeyerM GerardiRR BarakatDS
ChiReoperation for palliation of recurrent malignant bowel
obstruction in ovarian carcinomaGynecol
Oncol95193195200410.1016/j.ygyno.2004.07.02815385131
|
|
10.
|
G MangiliG AlettiL FrigerioM FranchiN
PanacciR ViganòP DE MarziF ZanettoA FerrariPalliative care for
intestinal obstruction in recurrent ovarian cancer: a multivariate
analysisInt J Gynecol
Cancer15830835200510.1111/j.1525-1438.2005.00144.x16174232
|
|
11.
|
DS ChiR PhaëtonTJ MinerSV KardosJP DiazMM
Leitao JrG GardnerJ HuhWP TewJA KonnerA prospective outcomes
analysis of palliative procedures performed for malignant
intestinal obstruction due to recurrent ovarian
cancerOncologist148358392009
|
|
12.
|
DJ FeuerKE BroadleyJH ShepherdDP
BartonSystematic review of surgery in malignant bowel obstruction
in advanced gynecological and gastrointestinal cancer. The
Systematic Review Steering CommitteeGynecol
Oncol75313322199910.1006/gyno.1999.559410600282
|