Introduction
The treatment of malignant glioma is associated with
a high failure rate. The majority of patients progress following
initial therapy. The current standard of care for newly diagnosed
glioblastoma includes maximal safe surgical resection followed by
radiotherapy with concomitant and adjuvant chemotherapy
(temozolomide) (1). Despite this
approach, the median survival rate is estimated to be 14.6 months
and only approximately 25% of patients are alive at 2 years from
diagnosis. At the time of progression, salvage therapies offer
extremely modest efficacy. The majority of salvage therapies are
associated with low response rates and a marginal benefit on
progression-free and overall survival (OS) rates. Numerous agents
have been tried in this setting, offering low response rates
ranging from 5 to 20% and median OS between 5 and 7.5 months
(2–5).
The introduction of bevacizumab, a humanized
monoclonal antibody against vascular endothelial growth factor
(VEGF), to the therapy of recurrent glioblastoma (GBM) resulted in
a significant improvement in response rates. By removing available
circulating VEGF (frequently overexpressed by malignant gliomas),
bevacizumab inhibits the proliferation of endothelial cells and the
formation of new blood vessels (6),
frequently resulting in remarkable radiographic responses.
Stark-Vance, who pioneered the use of this antibody in glioma,
paved the way for larger studies utilizing bevacizumab in
combination with irinotecan in GBM (7). A phase II study led by Vredenburgh
confirmed the initial encouraging findings and reported an overall
response rate of 57% in patients with recurrent GBM treated with
bevacizumab in combination with irinotecan. Of these patients, 46%
were progression-free at 6 months (8,9).
Single-agent bevacizumab has also been utilized in several
prospective studies for recurrent GBM. Reported progression-free
survival (PFS) was between 4 and 4.2 months. Between 29 and 42% of
patients were free from progression at 6 months and the reported
median OS was between 7.8 and 9.2 months (10,11).
Combination therapies utilizing an anti-VEGF
platform studied in a prospective fashion include bevacizumab with
the following agents: irinotecan (9,10),
etoposide (12), erlotinib
(13), temzolomide (14), carboplatin plus irinotecan (15) and carboplatin plus etoposide
(16). The majority of combinations
offered similar benefits but toxicity was generally more pronounced
when cytotoxic regimens were added to anti-VEGF therapy. Only one
small retrospective study used carboplatin alone in combination
with bevacizumab in patients with recurrent malignant glioma
(17). A total of 9 patients were
treated with this combination (5 with WHO grade III glioma and 4
with WHO grade IV glioma). The PFS rate at 6 months was 40% for
grade III and 50% for grade IV patients. Due to the extremely small
number of subjects in this retrospective study, it is difficult to
draw conclusions concerning the efficacy of this regimen.
Carboplatin, a platinum analog, has been used in
therapy for a variety of types of cancer, including ovarian,
bladder, head and neck, endometrial, small- and non-small cell lung
and germ cell tumors. The drug covalently binds to DNA
preferentially at the N-7 position of guanine and is not cell-cycle
specific. Following intravenous administration, carboplatin is
widely distributed throughout the body and crosses the blood-brain
barrier, which makes it particularly attractive for glioma therapy.
Carboplatin dosing is based on the target area under the curve
(AUC) and, typically, doses between AUC of 4 and 7 mg/ml/min are
prescribed. The major toxicity of carboplatin is myelosuppression.
Carboplatin monotherapy has been shown to be modestly effective in
therapy of recurrent malignant glioma (5,18). The
results of combination therapies utilizing carboplatin for this
indication have also been encouraging (16,19).
As previously mentioned, only one small
retrospective case series evaluated the combination of carboplatin
with bevacizumab in recurrent malignant glioma. We present the
largest retrospective series of patients treated with this
combination at one institution to date.
Materials and methods
We performed a retrospective chart review of
patients treated at the University of Washington Medical Center
between 2008 and 2010. The local Institutional Review Board
approved the study. Patients eligible for the inclusion in the
study cohort had to have WHO grade III or IV glioma that progressed
following initial or prior therapy. Information was gathered,
including patients' demographics, tumor type and grade, prior
treatments, number of progressions, Karnofsky Performance Status
(KPS), treatment schedule, number of doses of the treatment drugs,
time-to-progression, OS rate and toxicity.
All patients selected for the study were treated
with a combination of bevacizumab and carboplatin. Bevacizumab
(Avastin®; Genetech, South San Francisco, CA, USA) was
administered at a dose of 10 mg/kg every 14 days and carboplatin
was dosed using AUC of 4–6 mg/ml/min, depending on the patient's
prior treatment history and bone marrow reserves. One cycle
consisted of 2 doses of carboplatin (every 28 days) and 3 doses of
bevacizumab (every 14 days) and lasted 6 weeks. Radiographic
assessment included review of MRI scans of all patients at multiple
time points. T1-weighted post-contrast and T2-fluid attenuated
inversion recovery (FLAIR) images were used for analysis. Responses
were determined using modified McDonald/RANO criteria. Steroid
dosing was recorded for all patients and taken into account during
response assessment.
Results
Of the 19 eligible patients, 58% were male and 42%
were female. The median age at diagnosis was 53 years. Median KPS
at the time of therapy initiation was 80. At the time of disease
progression, the median KPS was 60. At initial diagnosis, 14
patients had GBM, two anaplastic oligodendroglioma (AO), one
oligoastrocytoma (OA) and two were classified as high-grade glioma
(HGG) at the time of recurrence. A total of 13 patients were
treated at the time of the first progression and six at the time of
the second progression. In total, 90 doses of carboplatin and 172
doses of bevacizumab were administered. The mean
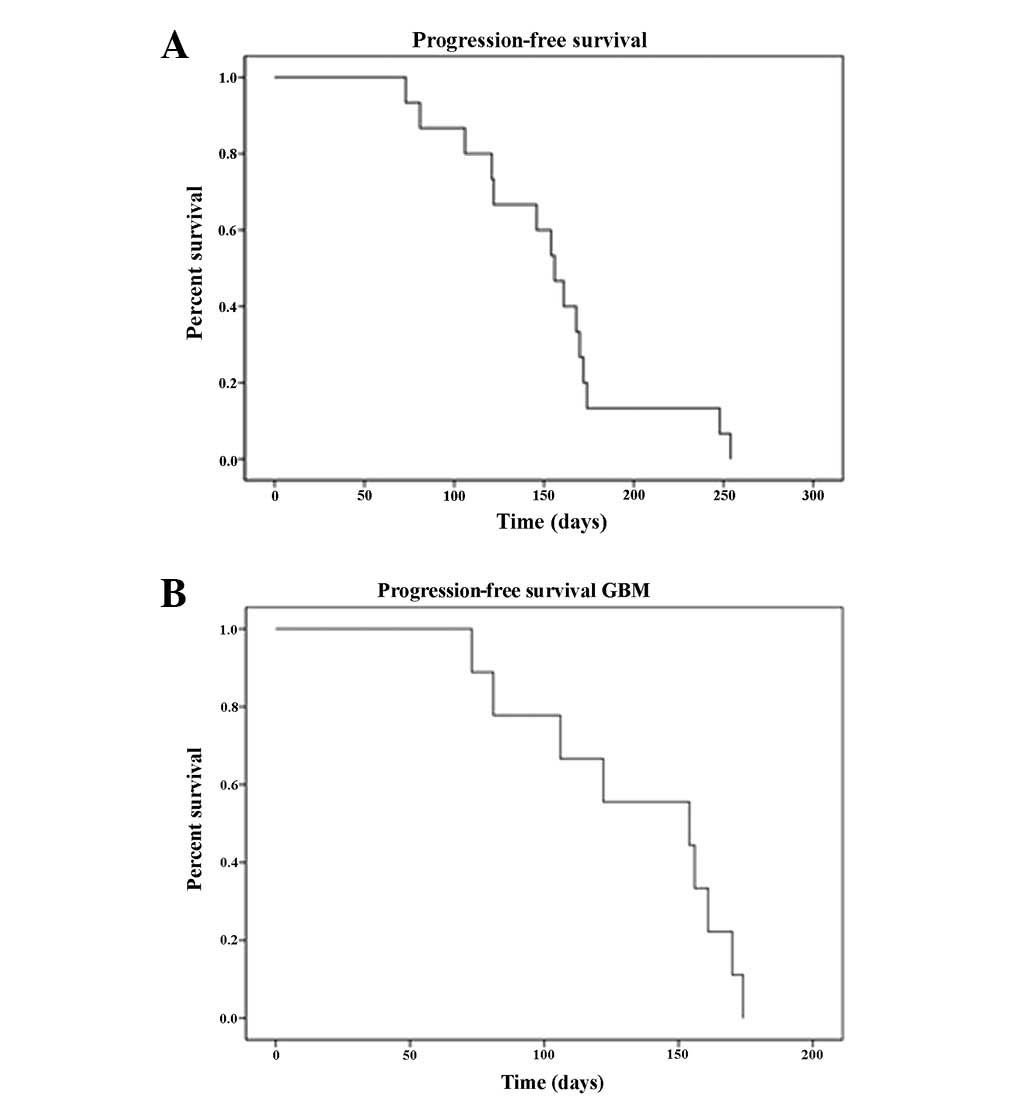
time-to-progression was 19 weeks (95% CI, 15.3–22.6) for the GBM
cohort (median, 22 weeks) and 21.9 weeks (95% CI, 18.2–25.6) for
the entire cohort (median, 22.2 weeks). Survival curves are
depicted in Fig. 1. The median OS
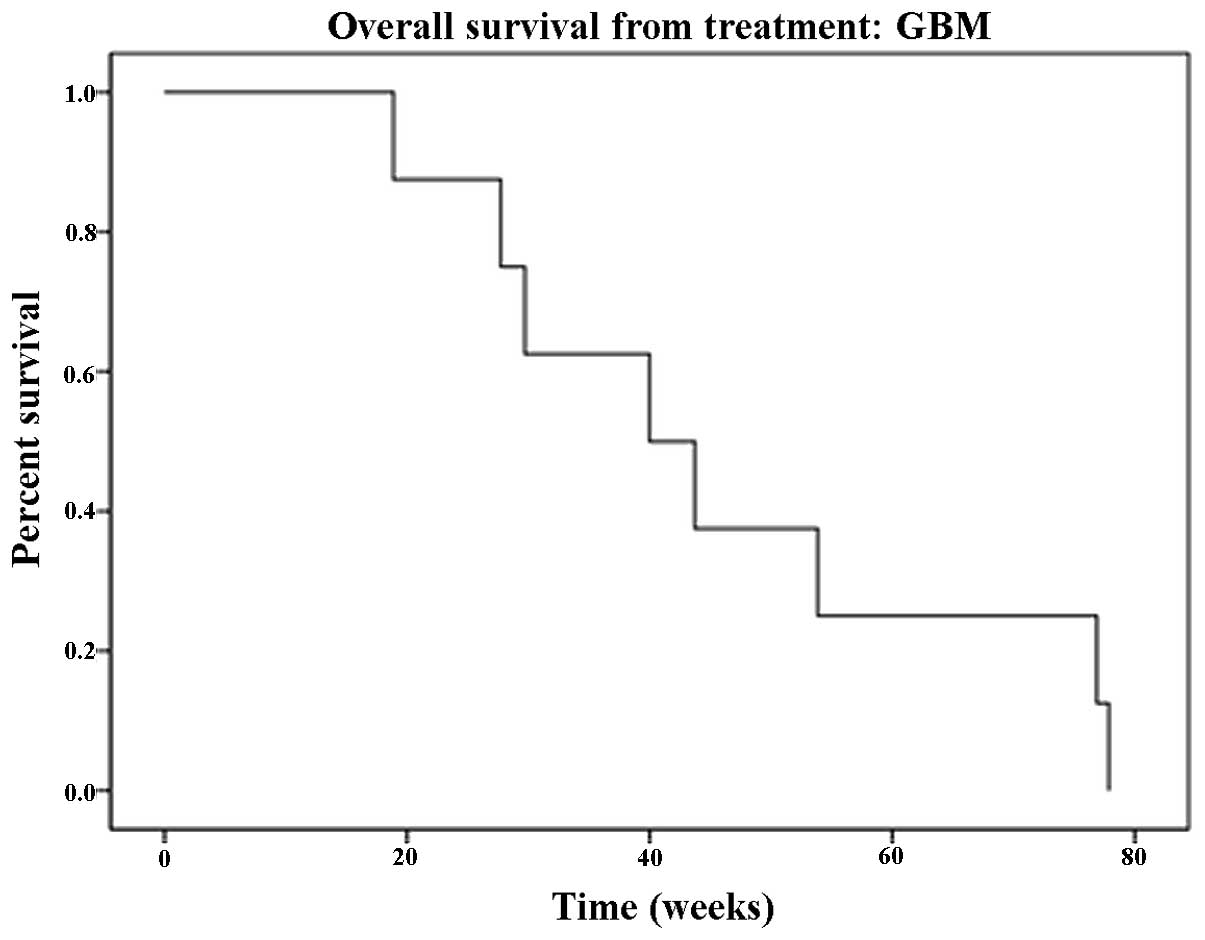
rate for GBM patients was 40 weeks (mean, 46.07 weeks; 95% CI,
30.7–61.3; Fig. 2). The calculated
median OS from the time of initial diagnosis was 111 weeks (95% CI,
68.8–153.1) for GBM patients. At 6 months 40% of GBM patients were
progression-free and 8 were alive at the time of data analysis; for
the 7 deceased patients, the median survival was 43.7 weeks.
Hematological toxicity was observed in 8 (42%) patients. More than
half of the patients (58%) experienced grade I or II toxicity. Of
the patients who experienced hematological toxicity, three patients
(37.5%) experienced grade III neutropenia, four (50%) grade III
lymphopenia and one (12.5%) grade III thrombocytopenia. Grade IV
hematological toxicity was not observed. One patient (5.2%)
suffered from brain hemorrhage and one patient developed
meningoencephalitis following herpes zoster infection. At the time
of evaluation, the response rate was 52.6% [complete response (CR)
plus partial response (PR); n=10]. Of the patients, 21% (n=4) had
stable disease (SD) and in 26.3% (n=5) the response could not be
fully assessed due to inadequate scans, missing data or lack of
measurable disease at the start of therapy (Table I). Progressive disease was not
observed prior to completion of the first cycle of therapy. A
response following 1 cycle of therapy was achieved by 66% of
responding patients, 25% following 2 cycles and 8.3% following 3
cycles. The majority of patients who failed this therapy
experienced confined, ipsilateral radiographic progression on T1
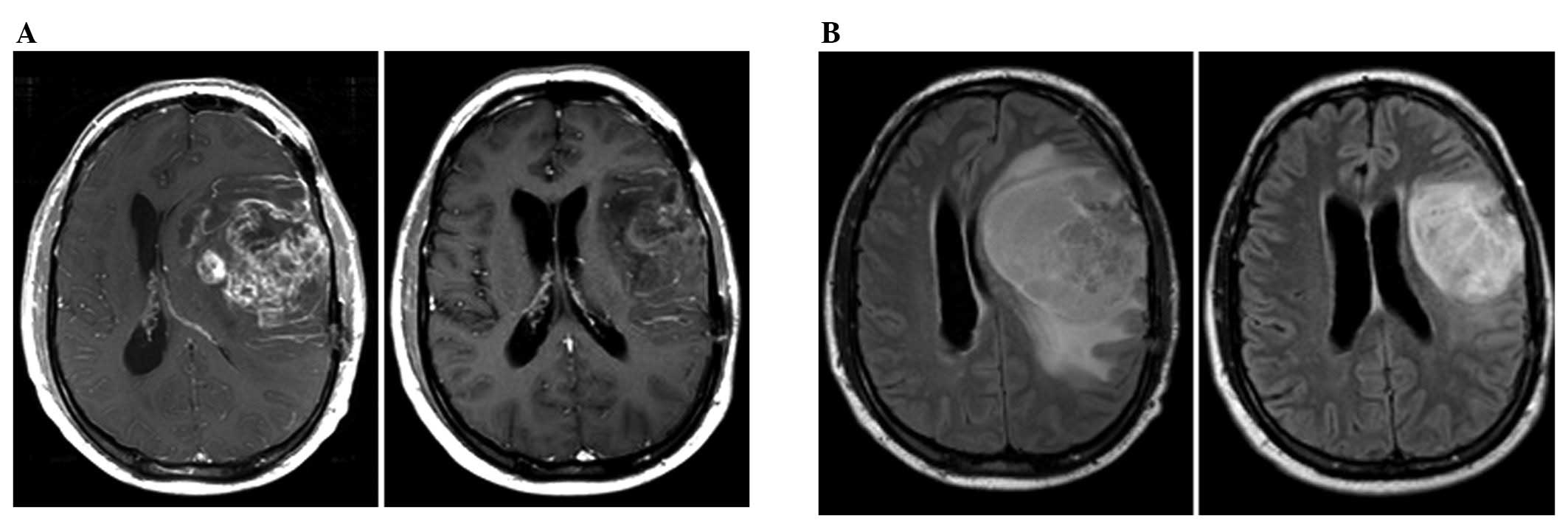
post-gadolinium and FLAIR images. A summary of the data is shown in
Table I and an example of a
radiographic response is shown in Fig.
3.
 | Table ISummary of selected characteristics
of patients enrolled in this study. |
Table I
Summary of selected characteristics
of patients enrolled in this study.
| Subject number | Gender | Age at diagnosis
(years) | Tumor type | KPS at initiation
of therapy | No. of doses
(carboplatin/bevacizumab) | Response |
|---|
| 1 | M | 41 | GBM | 80 | 10/20 | PR |
| 2 | F | 60 | GBM | 70 | 5/9 | PR |
| 3 | M | 47 | GBM | 100 | 3/7 | SD |
| 4 | M | 61 | GBM | 80 | 5/11 | CR |
| 5 | M | 27 | GBM | 70 | 4/11 | Possible PD |
| 6 | M | 67 | GBM | 60 | 2/3 | SD |
| 7 | F | 35 | HGG | 70 | 4/7 | Possible SD |
| 8 | M | 53 | GBM | 90 | 7/15 | PR |
| 9 | F | 22 | GBM | 90 | 8/15 | SD |
| 10 | F | 61 | GBM | 50 | 1/10 | Possible CR |
| 11 | M | 47 | GBM | 60 | 4/8 | PR |
| 12 | M | 64 | GBM | 80 | 5/6 | Possible SD |
| 13 | M | 74 | GBM | 70 | 3/1 | PR |
| 14 | F | 63 | AO | 80 | 7/14 | PR |
| 15 | M | 39 | OA | 70 | 5/10 | CR |
| 16 | F | 38 | HGG | 90 | 3/13 | Possible SD |
| 17 | F | 23 | AO | 80 | 5/12 | CR |
| 18 | F | 55 | GBM | 90 | 5/15 | PR |
| 19 | M | 58 | GBM | 50 | 5/UNK | SD |
Discussion
According to the studies published to date, there is
no strong evidence suggesting that the addition of cytotoxic
therapy to anti-VEGF agents translates into improved efficacy
(20,21), although the vascular normalization
theory offers an attractive mechanism supporting a combination
approach utilizing an anti-VEGF agent with a traditional cytotoxic
agent. The current retrospective series is the largest reported to
date utilizing the combination of carboplatin with bevacizumab. The
choice of agents for the treatment of recurrent malignant glioma at
our institution was dictated by the available evidence, properties
and pharmacokinetics of the agents and toxicity profile. It was
revealed that carboplatin paired with bevacizumab offers a high
response rate (52.6% in the present study) that compares favorably
with the results of other published studies. The median OS for GBM
patients in our study was 10 months and 40% of these patients were
progression-free at 6 months. These results also compare favorably
with published prospective data. While direct comparisons are not
possible due to differences in study designs, numbers of patients,
treatment regimens and tumor histologies, the reviewed data
indicate that carboplatin with bevacizumab offers moderate
activity, comparable or possibly superior to single-agent anti-VEGF
therapies (Table II). To confirm
this hypothesis, a prospective randomized study of this combination
is required. Notably, the results of a recently published phase II
study combining carboplatin with irinotecan and bevacizumab did not
confirm the superiority of that particular combined approach over
the single-agent bevacizumab (15).
 | Table IISummary of selected studies using
bevacizumab alone or in combination with other chemotherapeutics
for recurrent high-grade glioma. |
Table II
Summary of selected studies using
bevacizumab alone or in combination with other chemotherapeutics
for recurrent high-grade glioma.
| Regimen | No. patients | Median PFS
(months) | Median OS
(months) | Study design | Author (ref.) |
|---|
| Bev + carbo | 19 (all
subjects) | 5.5 (all
subjects) | 10 (GBM) | Retrospective | Present study |
| 14 (GBM) | 5.4 (GBM) | | | |
| 5 (HGG) | | | | |
| Bev + carbo | 9 (all
subjects) | 7.2 (GBM) | Not attained | Retrospective | Thompson et
al (17) |
| 4 (GBM) | 4.2 (WHO gr.
III) | | | |
| 5 (WHO gr.
III) | | | | |
| Bev + carbo +
VP-16 | 6 (GBM) | 4.7 | 7.4 | Retrospective | Francesconi et
al (16) |
| Bev + carbo +
CPT-11 | 40 (GBM) | 5.9 | 8.3 | Prospective | Reardon et
al (15) |
| Bev | 85 (GBM) | 4.2 | 9.2 | Prospective | Friedman et
al (10) |
| Bev | 48 (GBM) | 4.0 | 7.8 | Prospective | Kreisl et al
(11) |
In addition to the encouraging efficacy of the
studied combination, a rapid response to therapy (66% of patients
responded following only one cycle of therapy) and tolerable
toxicity with no instances of grade IV hematological events was
also observed. Patients maintained a good quality of life while
receiving therapy. The combination was active at first and second
recurrence in bevacizumab-naïve patients. The majority of the
radiographic progressions observed were ipsilateral to the initial
disease site and did not indicate the conversion of glioma into a
more aggressive, diffuse phenotype that is occasionally described
post anti-VEGF therapy (22).
In conclusion, acknowledging the limitations of the
current study, we consider that the combination of carboplatin and
bevacizumab is active and well-tolerated in recurrent HGG patients.
Optimization of carboplatin dose intensity and schedule with regard
to the administration of anti-VEGF agents (‘vascular normalization
window’) may result in improved efficacy in future studies with
this combination.
Abbreviations:
|
GBM
|
glioblastoma
|
|
VEGF
|
vascular endothelial growth factor
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
HGG
|
high-grade glioma
|
Acknowledgements
This manuscript is dedicated to the
late Dr Alexander Spence, a co-author of this work and a dear
colleague and friend. We would like to thank Dr Sandra Johnston for
providing statistical assistance.
References
|
1.
|
R StuppWP MasonMJ van den BentM WellerB
FisherMJ TaphoornK BelangerRadiotherapy plus concomitant and
adjuvant temozolomide for glioblastomaN Engl J
Med352987996200510.1056/NEJMoa04333015758009
|
|
2.
|
D FultonR UrtasunP ForsythPhase II study
of prolonged oral therapy with etoposide (VP16) for patients with
recurrent malignant gliomaJ
Neurooncol27149155199610.1007/BF001774788699237
|
|
3.
|
HS FriedmanWP PetrosAH FriedmanLJ SchaafT
KerbyJ LawyerM ParryIrinotecan therapy in adults with recurrent or
progressive malignant gliomaJ Clin Oncol1715161525199910334539
|
|
4.
|
WK YungRE AlbrightJ OlsonR FredericksK
FinkMD PradosM BradaA phase II study of temozolomide vs.
procarbazine in patients with glioblastoma multiforme at first
relapseBr J Cancer83588593200010.1054/bjoc.2000.131610944597
|
|
5.
|
RE WarnickMD PradosEE MackKL ChandlerF
DozJE RabbittMK MalecA phase II study of intravenous carboplatin
for the treatment of recurrent gliomasJ
Neurooncol196974199410.1007/BF010510507815106
|
|
6.
|
Y WangD FeiM VanderlaanA SongBiological
activity of bevacizumab, a humanized anti-VEGF antibody in
vitroAngiogenesis7335345200410.1007/s10456-004-8272-215886877
|
|
7.
|
V Stark-VanceBevacizumab and CPT-11 in the
treatment of relapsed malignant glioma. Abstracts from the World
Federation of Neuro-Oncology Second Quadrennial Meeting and the
Sixth Meeting of the European Association for
Neuro-OncologyNeuro-Oncol7abstract 3422005
|
|
8.
|
JJ VredenburghA DesjardinsJE Herndon IIJ
MarcelloDA ReardonJA QuinnJN RichBevacizumab plus irinotecan in
recurrent glioblastoma multiformeJ Clin
Oncol2547224729200710.1200/JCO.2007.12.244017947719
|
|
9.
|
JJ VredenburghA DesjardinsJE Herndon IIJM
DowellDA ReardonJA QuinnJN RichPhase II trial of bevacizumab and
irinotecan in recurrent malignant gliomaClin Cancer
Res1312531259200710.1158/1078-0432.CCR-06-230917317837
|
|
10.
|
HS FriedmanMD PradosPY WenT MikkelsenD
SchiffLE AbreyWK YungBevacizumab alone and in combination with
irinotecan in recurrent glioblastomaJ Clin
Oncol2747334740200910.1200/JCO.2008.19.872119720927
|
|
11.
|
TN KreislL KimK MooreP DuicC RoyceI
StroudN GarrenPhase II trial of single-agent bevacizumab followed
by bevacizumab plus irinotecan at tumor progression in recurrent
glioblastomaJ Clin
Oncol27740745200910.1200/JCO.2008.16.305519114704
|
|
12.
|
DA ReardonA DesjardinsJJ VredenburghS
GururanganJH SampsonS SathornsumeteeRE McLendonMetronomic
chemotherapy with daily, oral etoposide plus bevacizumab for
recurrent malignant glioma: a phase II studyBr J
Cancer10119861994200910.1038/sj.bjc.660541219920819
|
|
13.
|
S SathornsumeteeA DesjardinsJJ
VredenburghRE McLendonJ MarcelloJE HerndonA MathePhase II trial of
bevacizumab and erlotinib in patients with recurrent malignant
gliomaNeuro Oncol1213001310201020716591
|
|
14.
|
A DesjardinsDA ReardonA CoanJ MarcelloJE
Herndon IIL BaileyKB PetersBevacizumab and daily temozolomide for
recurrent
glioblastomaCancer11813021312201210.1002/cncr.2638121792866
|
|
15.
|
DA ReardonA DesjardinsKB PetersS
GururanganJH SampsonRE McLendonJE Herndon IIPhase II study of
carboplatin, irinotecan and bevacizumab for bevacizumab naïve,
recurrent glioblastomaJ Neurooncol107155164201221986722
|
|
16.
|
AB FrancesconiS DupreM MatosD MartinBG
HughesDK WyldJD LickliterCarboplatin and etoposide combined with
bevacizumab for the treatment of recurrent glioblastoma multiformeJ
Clin Neurosci17970974201010.1016/j.jocn.2009.12.00920541421
|
|
17.
|
EM ThompsonE DosaDF KraemerEA
NeuweltTreatment with bevacizumab plus carboplatin for recurrent
malignant
gliomaNeurosurgery678793201010.1227/01.NEU.0000370918.51053.BC20559095
|
|
18.
|
WK YungL MechtlerMJ GleasonIntravenous
carboplatin for recurrent malignant glioma: a phase II studyJ Clin
Oncol986086419911849986
|
|
19.
|
B JeremicD GrujicicS JevremovicB
StanisavljevicL MilojevicL DjuricL MijatovicCarboplatin and
etoposide chemotherapy regimen for recurrent malignant glioma: a
phase II studyJ Clin Oncol101074107719921318951
|
|
20.
|
RK JainNormalization of tumor vasculature:
an emerging concept in antiangiogenic
therapyScience3075862200510.1126/science.110481915637262
|
|
21.
|
RK JainAntiangiogenic therapy for cancer:
current and emerging conceptsOncology (Williston
Park)19716200515934498
|
|
22.
|
AD NordenGS YoungK SetayeshA MuzikanskyR
KlufasGL RossAS CiampaBevacizumab for recurrent malignant gliomas:
efficacy, toxicity, and patterns of
recurrenceNeurology70779787200810.1212/01.wnl.0000304121.57857.3818316689
|