Introduction
Herpes simplex virus-thymidine kinase/ganciclovir
(HSV-TK/GCV) is the most widely used suicide gene/prodrug system.
During gene therapy, the target gene is transfected into target
cells. However, there are no safe or effective strategies for gene
transfection in clinical practice, which significantly limits the
application of gene therapy. Although gene transfection via viral
vectors has been demonstrated to have a high transfection
efficiency, viral vectors have a risk of inducing an immune
response or other toxic reactions in the host (1,2).
Ultrasound has been applied in gene therapy and in recent years, an
ultrasound contrast agent has been identified as an adjuvant to
increase the efficiency of ultrasound-mediated gene transfection.
The application of ultrasound treatment and an ultrasound contrast
agent for gene transfection is a more favorable strategy for gene
therapy, as it has a high safety potential and the ability to
directly target cells (3,4). In this study, we constructed
eukaryotic expression vectors expressing the thymidine kinase (TK)
gene, which contained either the AFP promoter (specific in hepatic
cancer cells) or the kinase insert domain receptor (KDR) promoter
(specific in vascular endothelial cells). In combination with a
microbubble contrast agent, the two vectors were intratumorally
injected into mice, ultrasound treatment was conducted and prodrugs
[GCV and 5-fluorocytosine (5-FC)] were administered. The aim of
this study was to investigate the specific therapeutic effect of
HSV-TK/GCV and cytosine deaminase (CD)/5-FC on hepatic cancer in
vivo.
Materials and methods
Animals and cell lines
Male C57BL/6 nude mice approximately 4–5 weeks old
and weighing 17–19 g were purchased from the Experimental Animal
Center of Sun Yat-sen University (Guangdong, China). The human
hepatic cancer cell line (HepG2) was kindly provided by the
Surgical Laboratory of The First Affiliated Hospital of Sun Yat-sen
University. The study was approved by the First Affiliated Hospital
of Sun Yat-sen University
Materials and instruments
Transcranial Doppler ultrasound (output sound
intensity, 0–3 W/cm2; radiation frequency, 1 MHz; duty
cycle, 10–100%) and a plasmid extraction purification kit were
obtained from Qiagen (Valencia, CA, USA). Double Stain Apoptosis
Detection kit (Hoechst 33342/PI) was purchased from Byeotime
Institution of Biotechnology (Jiangsu, China) and pEGFP-KDR-TK and
pEGFP-C1-AFP-TK were provided by Dr Li JB from Shenzhen Hospital of
Peiking University (Beijing, China). SonoVue ultrasound contrast
(59 mg/bottle of white freeze-dried powder in sulfur hexafluoride
with 2.5 μm microbubble) was purchased from Bracco Imaging BV
(Milan, Italy). The fluorescence microscope, freezing microtome and
GCV were purchased from Lizhu Keyi Pharmaceuticals Co., Ltd (Wuhan,
China) and 5-FC was purchased from Sigma (St. Louis, MO, USA).
SonoVue solution was prepared by gently agitating dissolved SonoVue
in 5% PBS.
Preparation of subcutaneous hepatic
cancer model in nude mice
A total of 24 mice were housed in an aseptic
environment. At week 7, HepG2 cells (0.2 ml; 1x107
cells) were subcutaneously injected into the lateral part of the
back. After 1 week, the subcutaneous nodules were identified. When
the tumor volume reached 100 mm3, animals were selected
for the following experiments. The drugs were mixed in a 1-ml
syringe and injected into the upper outer, lower outer, lower inner
and upper inner quadrants of the tumor. The tumor was then palpated
and ultrasound treatment was conducted. The probe was placed on the
tumor and close to the skin, and the couplant was positioned
between the probe and the skin. The probe frequency was 1 MHz, the
sound intensity was 2 W/cm2, the time for ultrasound
treatment was 2 min and the duty cycle was 50%.
Grouping
The nude mice were randomly assigned into 1 of 4
groups (n=6 per group). Group A (control group) were administered
100 μl of normal saline. Group B were administered 100 μl of normal
saline, 50 μg of pEGFP-KDR-TK and 50 μg of pEGFP-C1-AFP-TK. Group C
were administered 100 μl of normal saline, 50 μg of pEGFP-KDR-TK
and 50 μg of pEGFP-C1-AFP-TK, and ultrasound was conducted. Group D
were administered 100 μl of normal saline, 50 μg of pEGFP-KDR-TK,
50 μg pEGFP-C1-AFP-TK and 5% SonoVue, and ultrasound was
conducted.
Intratumoral injection and ultrasound treatment were
conducted once a day for 3 consecutive days. Subsequently, the mice
received an intraperitoneal injection of GCV (40 mg/kg/day) and
5-FC (40 mg/kg/day) for 10 consecutive days. The tumor volume,
tumor inhibition rate and apoptosis of cancer cells (using Hoechst
staining) were determined.
Observations
The tumor volume was measured regularly. The vernier
caliper was used to measure the maximal and minimal diameter of the
tumor (mm) which were expressed as a and b, respectively. The tumor
volume was calculated as: V mm3 = 1/2 × a ×
b2. Measurements were conducted once every 3 days, and
were collected a total of 4 times. The tumor growth curve was
delineated and the tumor growth inhibition rate was calculated:
Inhibition rate = (mean tumor volume control - mean tumor volume
treatment)/mean tumor volume control × 100. After 12 days, mice
were sacrificed (n=3 per group) and the tumors were collected and
stored in liquid nitrogen. The survival times of the 3 remaining
cancer-bearing mice were also recorded and analyzed for 90
consecutive days.
Hoechst and hematoxylin and eosin
(H&E) staining
Frozen sections were obtained and observed under a
fluorescence microscope (excitation wavelength, 350 nm; emission
wavelength, 460 nm) and cells with a blue nuclei were presented. A
total of 3 fields were randomly selected (magnification, ×400), and
the total number of cells and the number of apoptotic cells were
counted. The proportion of apoptotic cells in all cells was
calculated: Apoptosis index (AI) = number of apoptotic cells/number
of total cells × 100. The sections undergoing H&E staining were
observed under a light microscope.
Statistical analysis
SPSS version 13.0 was used for statistical analysis,
and qualitative data were expressed as the mean ± standard
deviation. A one way analysis of variance was employed for
comparisons among groups and Fisher’s Least Significant Difference
(LSD) test was conducted for comparisons between two groups.
P<0.05 was considered to indicate a statistically significant
difference. Survival analysis was conducted using the Kaplan-Meier
method.
Results
Tumor growth
The tumor volume and inhibition rate 3, 6, 9 and 12
days after treatment are shown in Table
I. Statistical analysis revealed that no marked difference was
observed in the tumor inhibition rate and tumor volume between any
two groups (P>0.05). However, the tumor inhibition rate in
groups D and C was markedly different compared with that in groups
A and B (P<0.05).
 | Table ITumor volume and inhibition rate in
cancer-bearing mice following treatment. |
Table I
Tumor volume and inhibition rate in
cancer-bearing mice following treatment.
| Tumor volume
(mm3)
| Inhibition rate
(%) |
|---|
| Group | Day 3 | Day 6 | Day 9 | Day 12 |
|---|
| A | 875.8±27.4 | 1087.5±30.1 | 1187.1±45.8 | 1451.2±48.0 | 0 |
| B | 886.6±29.5 | 971.9±41.6 | 1140.5±42.2 | 1396.5±46.8 | 3.5±1.8 |
| C | 847.8±24.1 | 969.4±59.3 | 1035.1±66.0 | 1205.6±47.1 | 9.7±3.7a |
| D | 834.9±25.2 | 953.6±70.5 | 987.4±85.9 | 1127.3±63.8 | 10.6±4.9a |
Survival time of cancer-bearing mice
After treatment, the survival times were 46.33±19,
45.68±24, 50.27±11 and 48.40±17 days in groups A, B, C and D,
respectively. No marked difference was identified in the survival
time between the two groups (P>0.05).
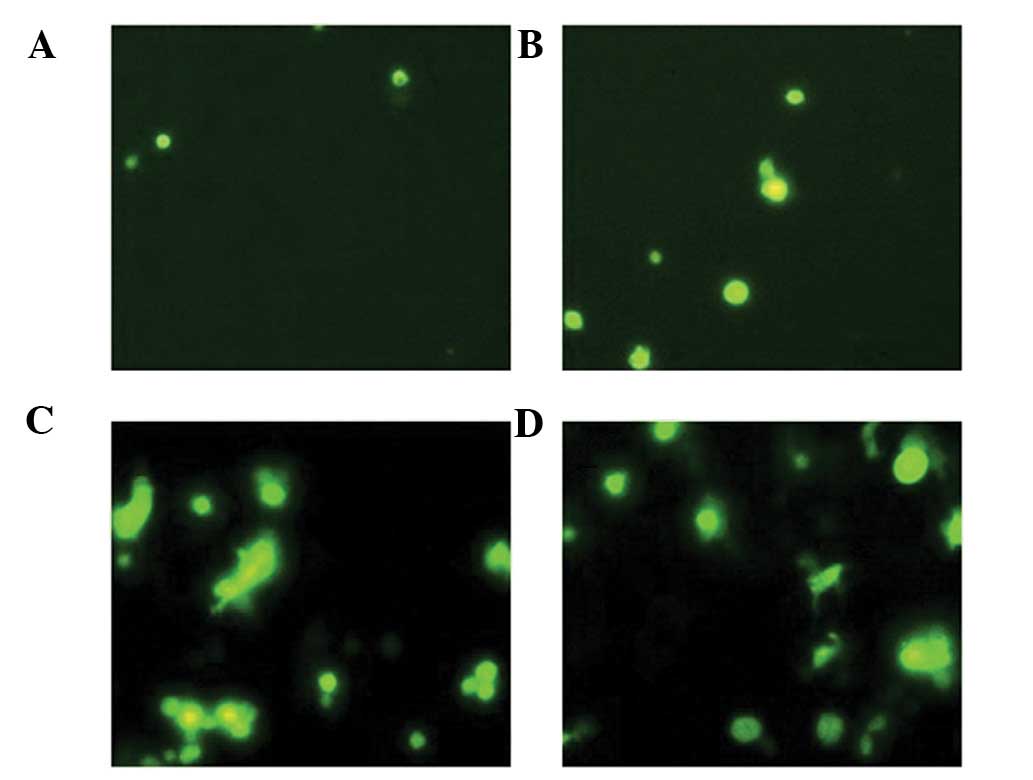
Apoptosis of cancer cells
Compared with groups A and B, the number of
apoptotic cells in groups C and D was markedly higher. The AI was
3±1.1, 8±2.6, 17±3.4 and 19±5.5% in group A, B, C and D,
respectively. A marked difference was observed between groups D and
C and groups A and B (P<0.05). There was no significant
difference between the number of apoptotic cells between groups A
and B (P>0.05) (Fig. 1).
Discussion
Gene therapy has been an effective strategy in the
treatment of cancer. The ultrasound cavitation effect may mediate
gene transfection, which could be enhanced by microbubble contrast
agent pretreatment (5,6). Our previous study (7) demonstrated that ultrasound treatment
in the presence of an ultrasound contrast agent may significantly
increase the killing of vascular endothelial and hepatic cancer
cells by the HSV-TK/GCV and CD/5-FC system. We also revealed that
ultrasound treatment is able to increase vascular endothelial
permeability in mice with subcutaneously transplanted hepatic
cancer and increase the efficiency of TK gene transfection.
In the present study, KDR-TK, AFP-TK and a
microbubble contrast reagent were intratumorally injected into nude
mice. Ultrasound treatment was conducted for 3 consecutive days and
mice were administered with two prodrugs (GCV and 5-FC). This study
aimed to investigate the therapeutic effect of HSV-TK/GCV and
CD/5-FC on hepatic cancer in vivo. The HSV-TK gene encodes
TK which may convert inactive GCV into diphosphorylated GCV. The
latter may be converted into toxic triphosphorylated GCV in the
presence of intracellular enzymes. The triphosphorylated GCV is
able to significantly inhibit DNA polymerase and, under the
regulation of the KDR gene promoter, specifically damage the
vascular endothelial cells in the tumor. CD is able to convert
inactive 5-FC into highly toxic chemotherapeutic 5-FU. This may
specifically inhibit thymidylate synthetase in hepatic cancer cells
under regulation by the AFP gene promoter and inhibit the synthesis
of DNA, RNA and proteins, resulting in cell death. Our results
demonstrated that the tumor volume in the treatment groups was
comparable to that in the control group, but the tumor growth
inhibition rate in the treatment groups was markedly higher than
that in the control group (P<0.05). We also revealed that there
was no marked difference in the survival time between any two
groups, and the number of apoptotic cells in the treatment groups
was significantly higher than that in the control group
(P<0.05). Additionally, we identified that, except at the needle
track, necrosis was not observed in any tumor. These findings
demonstrated that the treatment in this study was not able to
completely remove the hepatic cancer. This treatment has no
significant control of tumor growth, and has no influence on the
survival time of cancer-bearing mice; however, it may increase the
tumor inhibition rate, which may be attributed to the increase in
the number of apoptotic cancer cells (8,9).
Our results demonstrate that ultrasound treatment in
the presence of a microbubble contrast agent is an effective method
mediating gene transfection. The contrast agent serves as a carrier
with target genes, which reach the blood vessels at the target
sites via the circulation. Following ultrasound treatment, the
genes are released and gene transfection is enhanced by the
cavitation effect of ultrasound (10). However, currently, the genes and
microbubbles are largely injected via the tail vein, which often
produces unsatisfactory efficacy. This may be attributed to the
small amount of genes and microbubbles. Intratumoral injection also
has the disadvantage of an uneven distribution. These may be the
causes of the varying results obtained between in vitro and
in vivo studies. In addition, our results revealed that
there was no marked difference in the tumor growth inhibition rate
between the ultrasound treatment group and the microbubble and
ultrasound treatment group, which may be correlated with the short
observation time or the small cohort number.
Microbubble injection in combination with ultrasound
may serve as a new strategy for gene therapy as it has been
demonstrated to inhibit the tumor growth rate. However, further
studies are required to investigate the role of microbubble and
ultrasound treatment in the gene therapy of hepatic cancer.
References
|
1.
|
B DegrèveE De ClercqJ BalzariniBystander
effect of purine nucleoside analogues in HSV-tk suicide gene
therapy is superior to that of pyrimidine nucleoside analoguesGene
Ther6162170199910435100
|
|
2.
|
VG ZarnitsynMR PrausnitzPhysical
parameters influencing optimization of ultrasound-mediated DNA
transfectionUltrasound Med
Biol30527538200410.1016/j.ultrasmedbio.2004.01.00815121255
|
|
3.
|
PA DijkmansLJ JuffermansRJ
MustersMicrobubbles and ultrasound: from diagnosis to therapyEur J
Echocardiogr5245256200410.1016/j.euje.2004.02.00115219539
|
|
4.
|
K FerraraR PollardM BordenUltrasound
microbubble contrast agents: fundamentals and application to gene
and drug deliveryAnnu Rev Biomed
Eng9415447200710.1146/annurev.bioeng.8.061505.09585217651012
|
|
5.
|
A AoiY WatanabeS MoriHerpes simplex virus
thymidine kinase-mediated suicide gene therapy using
nano/microbubbles and ultrasoundUltrasound Med
Biol34425434200810.1016/j.ultrasmedbio.2007.09.00418096302
|
|
6.
|
QL LuHD LiangT PartridgeMJ
BlomleyMicrobubble ultrasound improves the efficiency of gene
transduction in skeletal muscle in vivo with reduced tissue
damageGene Ther10396405200310.1038/sj.gt.330191312601394
|
|
7.
|
Q TangHX XuMD lvF NieH YangY
WangUltrasound contrast agent enhancing changes of murine
hepatocellular carcinoma microvascular permeability caused by
ultrasound irradiationChin J Ultra Med224114132006
|
|
8.
|
N SheikovN McDannoldS SharmaK
HynynenEffect of focused ultrasound applied with an ultrasound
contrast agent on the tight junctional integrity of the brain
microvascular endotheliumUltrasound Med
Biol3410931104200810.1016/j.ultrasmedbio.2007.12.015
|
|
9.
|
P SchratzbergerJG KraininG
SchratzbgerTranscutaneous ultrasound augments naked DNA
transfection of skeletal muscleMol
Ther6576583200210.1016/S1525-0016(02)90715-X12409255
|
|
10.
|
XH LiP ZhouLH WangThe targeted gene
(KDRP-CD/TK) therapy of breast cancer mediated by SonoVue and
ultrasound irradiation in
vitroUltrasonics52186191201210.1016/j.ultras.2011.08.00221906771
|