Introduction
Clear cell adenocarcinoma of the ovary (CCC) is
defined by the World Health Organization as lesions characterized
by clear cells growing in solid/tubular or glandular patterns as
well as hobnail cells (1). The
frequency of CCC in female individuals of Western countries is 8%
(2). The frequency is higher in
Japan at 15% (3). The standard
first-line chemotherapy for ovarian epithelial cancer is
paclitaxel-platinum combination chemotherapy (T/platinum), which
has a response rate of 78% (4).
However, for the treatment of CCC, the response rate is only 22–56%
(5,6). As such, CCC patients have a poorer
prognosis than patients with serous cystadenocarcinoma of the ovary
(3). Alternative regimens for the
treatment of CCC using combinations of camptothecin derivates,
irinotecan hydrochloride and cisplatin (CDDP/CPT) have been
investigated (7–9). CDDP/CPT has also been used as an
alternative regimen for the treatment of recurrent ovarian
carcinoma (10,11). CDDP/CPT is regarded as one of the
common treatments of primary CCC and recurrent ovarian
carcinoma.
Nedaplatin (cis-diammine glycolato platinum, NDP) is
an analog of cisplatin, that shows lower rates of nephrotoxicity
and nausea than CDDP. Cervical carcinoma is frequently treated with
nedaplatin and irinotecan hydrochloride combination therapy
(NDP/CPT). A previous multi-center dose-escalation study was
performed to identify appropriate doses of NDP/CPT for cervical
carcinoma patients (12). NDP/CPT
was found to have a shorter infusion time and lead to a shorter
hospital stay than CDDP/CPT.
NDP has been reported to be effective as a single
agent or as combination therapy for the treatment of ovarian
carcinoma (13,14), and its basic and clinical efficacy
in the treatment of CCC has been demonstrated (15,16).
Thus, based on our experience, we applied the postoperative
chemotherapy regimen for primary CCC from CDDP/CPT to NDP/CPT. This
treatment is also currently used for cases of recurrent ovarian
carcinoma.
In this study, we retrospectively compared the
efficacy and toxicity of NDP/CPT to that of CDDP/CPT in the
treatment of CCC and recurrent ovarian carcinoma.
Patients and methods
Patient characteristics
In total, 115 patients were included in this study,
including patients with primary CCC and recurrent ovarian carcinoma
were administered CDDP/CPT or NDP/CPT at the Jichi Medical
University Hospital, Japan. Subjects had no other known
co-morbidities. Informed consent was obtained from all
patients.
Inclusion criteria consisted of the following: i)
age, ≤75 years; ii) Eastern Cooperative Oncology Group performance
status, 0–2; and iii) white blood cell count, ≥3,000/μl;
neutrophil count, ≥1,500/μl; platelet count,
≥10x104/μl; hemoglobin level, ≥9.0 g/dl;
aspartate aminotransferase and alanine aminotransferase levels,
≤3-fold than the upper limit of the normal value; total bilirubin,
≤2.0 mg/dl; urea nitrogen level, ≤25 mg/dl; serum creatinine level,
≤1.5 mg/dl; and creatinine clearance, ≥50 ml/min.
Methods
The NDP/CPT regimen was administered as follows: on
day 1, 50 mg/m2 of CPT was administered intravenously
for 90 min, followed by 60 mg/m2 of NDP for 60 min. On
days 8 and 15, patients were administered CPT using the same
procedure. Patients were hydrated with 1,000 ml of electrolyte
fluids on day 1 only. This was considered as one course and was
repeated every four weeks. The CDDP/CPT regimen was administered as
follows: CPT at 60 mg/m2 was administered intravenously
for 90 min, followed by CDDP at 60 mg/m2 for 180 min. On
day 8 and day 15, CPT only was administered using the same
procedure. On days 0–4, patients were hydrated with 2,000 ml of
electrolyte fluids. This was considered as one course and was
repeated every four weeks.
Granulocyte-colony stimulating factor was
administered for patients with grade 4 neutropenia or grade 3
neutropenia with infection. Granisetron hydrochloride (3 mg), a
5-HT3 receptor antagonist, was administered
intravenously on days 1, 8 and 15 as a prophylactic antiemetic
treatment. Loperamide hydrochloride, an anti-diarrheal agent, was
orally administered at 1–2 mg/day when required. The subsequent
course of chemotherapy was administered when the following
conditions were met: white blood cell count, ≥3,000/μl;
neutrophil count, ≥1,500/μl; platelet count,
≥10x104/μl; and absence of diarrhea.
The response to treatment was evaluated by computed
tomography (CT) images every 2 cycles of chemotherapy in patients
with measurable disease. Tumor response was evaluated according to
World Health Organization criteria (1979). A complete response (CR)
was defined as the disappearance of all clinical and radiological
evidence of the tumor for at least four weeks. A partial response
(PR) was defined as a decrease of ≥50% in the sum of the products
of the longest perpendi cular diameters of all measurable lesions
for at least four weeks. Progressive disease (PD) was defined as an
increase of >25% in the sum of the products of the perpendicular
diameter of all measurable lesions or the appearance of new
lesions. Any other events were considered to indicate no change
(NC). Progression-free survival (PFS) was defined as the interval
from the date of the first chemotherapy administration until the
date of recurrence or tumor progression. Overall survival (OS) was
defined as the time from the date of chemotherapy until death or
the date of the last follow-up contact. Adverse events were graded
according to the National Cancer Institute's Common Terminology
Criteria for Adverse Events (NCI-CTAE) version 3.0.
Statistical analysis
The Kaplan-Meier method was used to calculate the
distribution of patient survival, and its significance in each
group was tested using the log-rank test. The χ2 test
was used for statistical analysis. P<0.05 was considered
statistically significant.
Results
Primary CCC
In the present study, 29 patients with primary CCC
were treated using NDP/CPT, and 20 patients were treated using
CDDP/CPT (Table I). The median age
was 54 years in the NDP/CPT group and 53.5 years in the CDDP/CPT
group. According to the International Federation of Gynecology and
Obstetrics staging criteria, there were 14 cases with stage
Ia/Ic(b) in the NDP/CPT group and only one such case in the
CDDP/CPT group. The reason for this observation may be the change
in the indication for postoperative adjuvant chemotherapy during
the period involved. During the period of CDDP/CPT use, patients
with stage Ic(b) did not undergo chemotherapy, whereas during the
period of NDP/CPT use, patients with stage Ic(b) underwent
chemotherapy. Patients with stage Ia do not usually receive
adjuvant therapy; however, if a residual tumor caused by adhesion
is suspected, adjuvant therapy is used. There was no significant
difference in the degree of completion of surgery.
 | Table ICharacteristics of patients with
primary CCC. |
Table I
Characteristics of patients with
primary CCC.
| NDP/CPT (n=29) | CDDP/CPT
(n=20) |
|---|
| Age (years) | | |
| Median | 54 | 53.5 |
| Range | 31–69 | 37–67 |
| FIGO stage | | |
| Ia/Ic(b) | 14 | 1 |
|
Ic(1)/(2)/(a) | 5 | 7 |
| II | 4 | 3 |
| III | 4 | 6 |
| IV | 2 | 3 |
| Residual tumor size
(cm) | | |
| 0 | 27 | 16 |
| <1 | 2 | 2 |
| >1 | 0 | 2 |
Survival was assessed by excluding patients with
stage Ia/Ic(b). There was no significant difference in the 5-year
OS between the groups; OS was 58% in patients treated with NDP/CPT
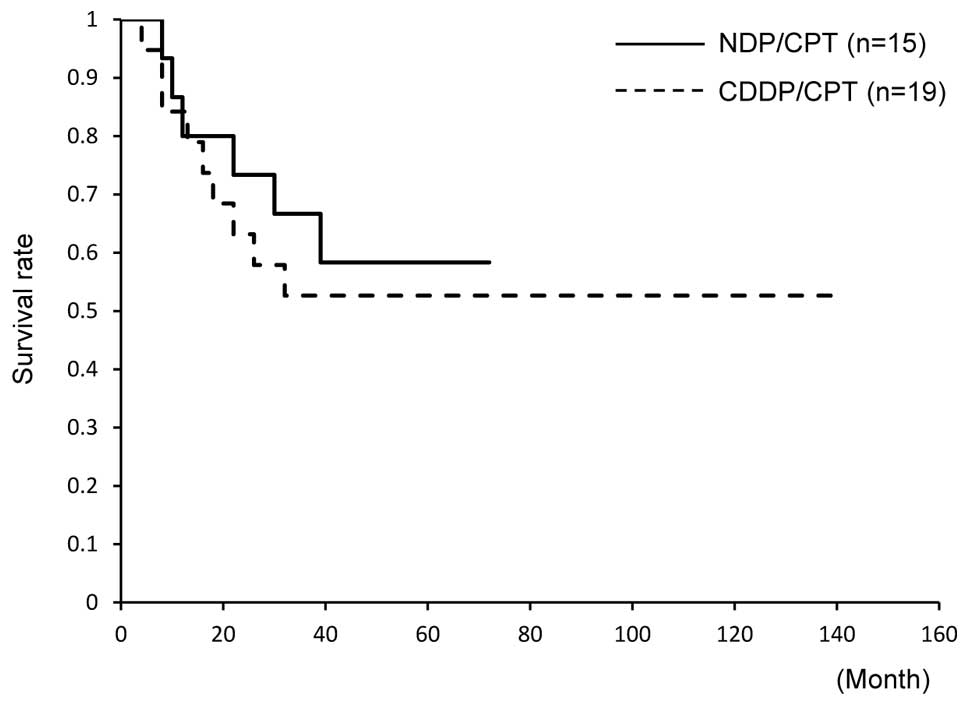
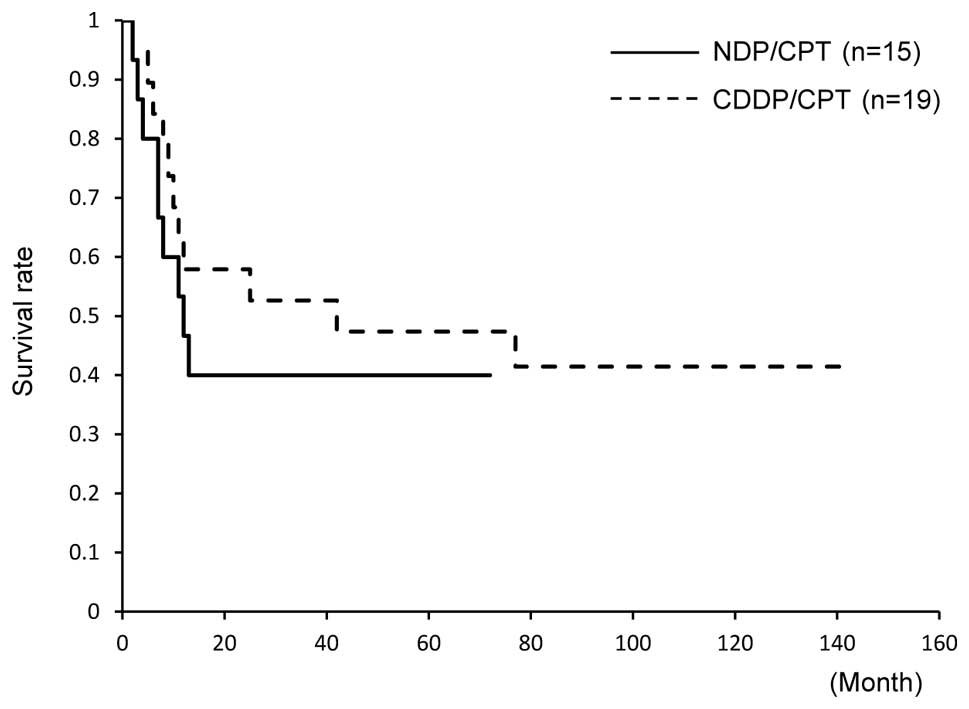
and 53% in those treated with CDDP/CPT (Fig. 1). Similarly, the 5-year PFS did not
significantly differ between the groups; PFS was 40% in patients
treated with NDP/CPT and 47% in those treated with CDDP/CPT
(Fig. 2).
Recurrent ovarian carcinoma
Sixty-six patients with recurrent ovarian carcinoma
were treated with either NDP/CPT or CDDP/CPT (Table II). The median age was 56 years in
the NDP/CPT group and 52 years in the CDDP/CPT group. In the two
groups, there was a large proportion of patients with stages
III–IV, and the most common histological type observed was serous
adenocarcinoma. In the two groups, previous treatments mostly
consisted of 1 or 2 regimens, and the groups did not show a
significant difference. The previous regimen most frequently used
in the NDP/CPT group was paclitaxel/carboplatin (TC), whereas it
was cisplatin and carboplatin (JP) in the CDDP/CPT group. The
NDP/CPT group tended to have a higher 6-month recurrence rate than
the CDDP/CPT group; however, this difference was not statistically
significant.
 | Table IICharacteristics of patients with
recurrent ovarian carcinoma. |
Table II
Characteristics of patients with
recurrent ovarian carcinoma.
| NDP/CPT (n=33) | CDDP/CPT
(n=33) |
|---|
| Age (years) | | |
| Median | 56 | 52 |
| Range | 20–72 | 34–72 |
| FIGO stage | | |
| I | 5 | 5 |
| II | 1 | 3 |
| III | 23 | 15 |
| IV | 4 | 10 |
| Histological
subtype | | |
| Serous | 19 | 18 |
| Clear | 4 | 5 |
| Endometrioid | 5 | 3 |
| Mucinous | 2 | 3 |
| Others | 3 | 4 |
| Number of prior
chemotherapy regimens | | |
| 0 | 3 | 2 |
| 1 | 20 | 19 |
| 2 | 9 | 10 |
| 3 | 1 | 2 |
| Prior chemotherapy
cycle (duplicated) | | |
| TC | 24 | 13 |
| JP | 1 | 16 |
| Weekly T | 8 | 5 |
| DC | 5 | 0 |
| Other | 3 | 8 |
| Time to
recurrence | | |
| <6 months | 24 | 18 |
| >6 months | 9 | 15 |
The NDP/CPT group had a better response rate than
the CDDP/CPT group; however, this difference was not statistically
significant (27 and 18%, respectively) (Table III). No significant difference was
found in the disease control rate between the two groups (62 and
68%, respectively).
 | Table IIITreatment outcome. |
Table III
Treatment outcome.
| NDP/CPT (n=33) | CDDP/CPT
(n=33) | p |
|---|
| CR | 3 | 3 | |
| PR | 4 | 1 | |
| NC | 9 | 11 | |
| PD | 10 | 7 | |
| NE | 7 | 11 | |
| CR+PR (%) | 27% (7/26) | 18% (4/22) | ns |
| CR+PR+NC (%) | 62% (16/26) | 68% (15/22) | ns |
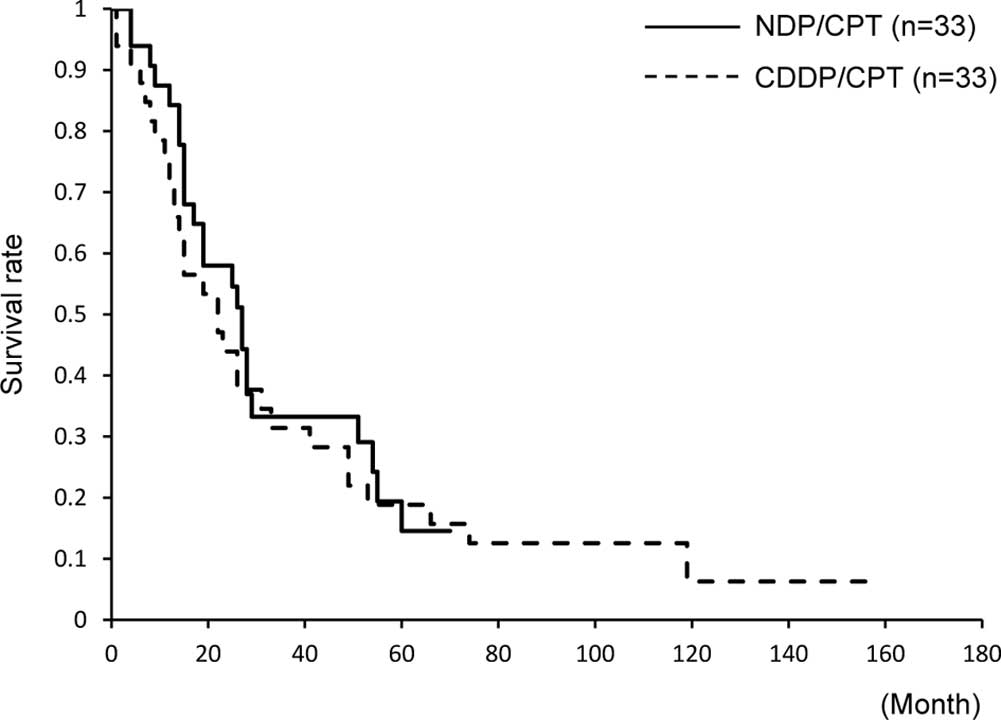
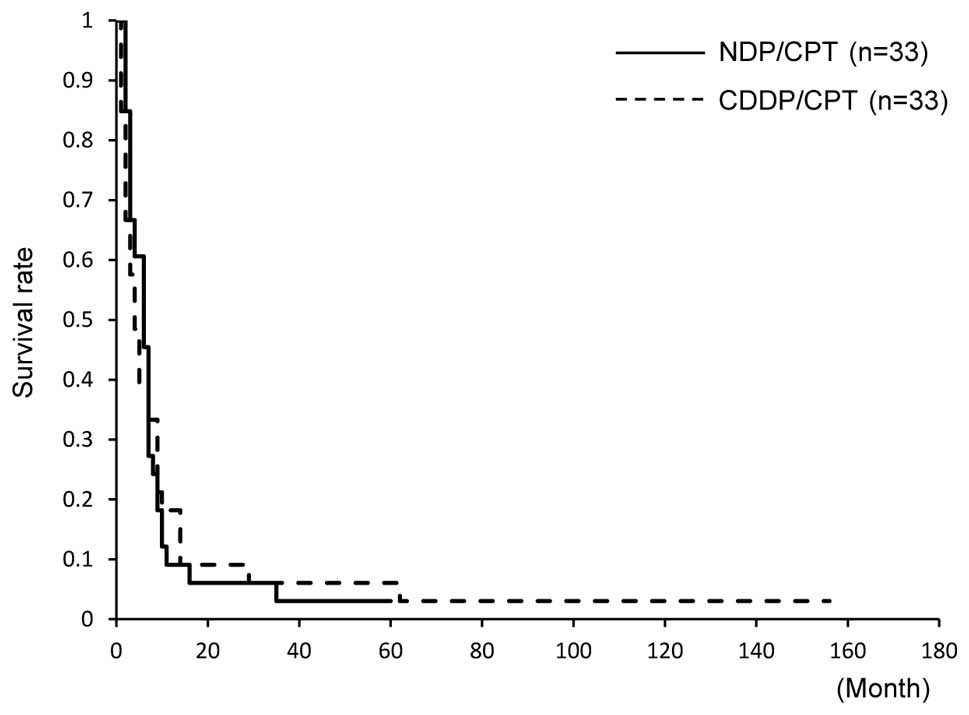
The 5-year OS was not significantly different (15
and 18%, respectively). The median OS was 19 months in the two
groups, and it was not significantly different (Fig. 3). The 5-year PFS was 3% in the
NDP/CPT group and 6% in the CDDP/CPT group; however, no significant
difference was found between the two groups. The median PFS also
did not show any difference (6 and 4 months, respectively, Fig. 4).
Toxicity
Toxicity was assessed among patients with primary
CCC and recurrent ovarian carcinoma. Toxicity was compared between
the 62 patients treated with NDP/CPT and the 53 patients treated
with CDDP/CPT (Table IV). For
hematotoxicity of grade 3 or above, neutropenia was observed in 23%
of patients treated with NDP/CPT, which was lower than that in
patients treated with CDDP/CPT (56%). Anemia and thrombocytopenia
were also lower in patients treated with NDP/CPT. With regards to
non-hematotoxicity of grade 2 or above, there was a lower rate of
nausea in patients treated with NDP/CPT compared to those treated
with CDDP/CPT (20 and 52%, respectively). There were also lower
rates of diarrhea and fever in the NDP/CPT group.
 | Table IVAdverse events. |
Table IV
Adverse events.
| A, Hematological
toxicity. |
|---|
|
|---|
| NDP/CPT | CDDP/CPT | |
|---|
|
|---|
| n=124 courses
| G3/4 (%) | n=106 courses
| G3/4 (%) | pa |
|---|
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 |
|---|
| Leukopenia | 29 | 51 | 5 | 0 | 4 | 21 | 52 | 24 | 7 | 29 | <0.01 |
| Neutropenia | 16 | 38 | 24 | 5 | 23 | 17 | 28 | 35 | 24 | 56 | <0.01 |
| Anemia | 26 | 14 | 1 | 0 | 1 | 40 | 37 | 17 | 4 | 20 | <0.01 |
|
Thrombocytopenia | 2 | 0 | 0 | 0 | 0 | 21 | 7 | 4 | 1 | 5 | <0.05 |
|
| B,
Non-hematological toxicity. |
|---|
|
|---|
| NDP/CPT | CDDP/CPT | |
|---|
|
|---|
| n=124 courses
| G2/3/4 (%) | n=106 courses
| G2/3/4 (%) | pa |
|---|
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 |
|---|
| Nausea | 54 | 24 | 1 | 0 | 20 | 35 | 43 | 12 | 0 | 52 | <0.01 |
| Diarrhea | 29 | 15 | 2 | 0 | 14 | 13 | 16 | 9 | 1 | 25 | <0.05 |
| Hepatotoxicity | 23 | 1 | 1 | 0 | 2 | 6 | 2 | 1 | 0 | 3 | ns |
| Nephrotoxicity | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ns |
| Fervescence | 2 | 2 | 0 | 0 | 2 | 1 | 12 | 0 | 0 | 11 | <0.01 |
Discussion
The results of this retrospective analysis
demonstrate that NDP/CPT treatment was equally effective yet less
toxic than CDDP/CPT in the treatment of primary CCC and recurrent
ovarian carcinoma.
NDP is an analog of cisplatin that has the same
amine carrier ligands as cisplatin but has a different leaving
group, a 5-membered ring structure in which glycolate is bound to
the platinum ion as a bidentate ligand. This product is
approximately 10 times more water-soluble than cisplatin and,
unlike cisplatin, shows limited binding to plasma proteins. As
such, it causes less renal, gastrointestinal, and neural toxicity,
and there is no need for hydration during its administration
(17).
In an in vitro study of chemosensitivity, an
MTT assay of the ovarian cancer cell strain 65 showed that the
inhibition rate of the NDP tumor was 62% and that of CDDP was 55%.
For clear cell adenocarcinoma, the inhibition rate of the NDP tumor
was 50% and that of CDDP was 37% (15). In a phase II clinical study,
patients with ovarian carcinoma who were administered a dose of 100
mg/m2 of NDP every 4 weeks showed a response rate of
37.7% (23/61). The response rate was 35.7% (15/42) in patients with
a previous history of adjuvant therapy, whereas the response rate
was 33% in patients with clear cell adenocarcinoma. This study
concluded that NDP was equally effective and less toxic than CDDP
for the treatment of ovarian carcinoma (13).
CPT is a derivate of camptothecin, and it inhibits
topoisomerase I. It may be used either as a single agent or in
combination therapy for gastric, colon, lung and cervical
carcinomas (18). As a single
agent, CPT had a response rate of 20–25% in cases of recurrent or
refractory ovarian carcinoma (10).
NDP/CPT has been used as chemotherapy for lung,
cervical, and testicular carcinomas (12,19–21).
In a phase I study, the Japanese Gynecologic Oncology Group (JGOG)
established a recommended dose for patients with cervical carcinoma
patients. A phase II study investigating its use as postoperative
adjuvant chemotherapy for cervical carcinoma is currently being
performed (19). Few available
reports regarding the use of NDP/CPT for ovarian carcinoma are
available; however, one study showed CR to NDP/CPT in patients with
CCC metastasis to the common iliac lymph node (16). It was also reported that
platinum/CPT therapy, including CDDP/CPT and NDP/CPT, was effective
in 31 patients with ovarian carcinoma (14).
The doses of NDP and CPT are determined by body
surface area; however, this method may be improved in the future.
For instance, Ishibashi et al developed Ishibashi's formula
to calculate the dose of NDP on the basis of renal function as with
carboplatin (CBDCA) (22). The
correlation between the predicted area under the curve (AUC) and
observed AUC values suggested by Ishibashi's formula was confirmed
from a study in which we participated (23). In the future, Ishibashi's formula
may be used to determine the appropriate dose of NDP. SN-38, an
active metabolite of CPT, is detoxified by glucuronidation with
uridine diphosphate gluconosyltransferase (UGT)1A1. The homozygotes
and double heterozygotes of UGT1A1*6 and *28
(*6/:6, *28/*28,
*6/*28) were significantly associated with
severe neutropenia (24). However,
the necessity of the dose adjustment of CPT on the basis of the
UGT1A1 polymorphism has yet to be determined (25).
Sugiyama et al observed that CDDP/CPT
treatment had a response rate of 40% in patients with recurrent or
refractory ovarian carcinoma (11).
Gershenson et al reported that platinum-sensitive and
non-sensitive ovarian carcinomas had response rates of 75 and 33%,
respectively (10). Recently,
single-agent chemotherapy has been recommended for cases of
platinum-resistant ovarian carcinoma. Platinum-based combination
chemotherapy is recommended for platinum-sensitive recurrent
ovarian carcinoma (Ovarian Cancer Guideline, http://www.nccn.org/professionals/physician_gls/f_guidelines.asp).
Paclitaxel/carboplatin (TC) is often used for platinum-sensitive
recurrent ovarian carcinoma. Consequently, the applicability of
platinum/CPT is limited for recurrent cases. For patients with
platinum-sensitive recurrent ovarian carcinoma that cannot tolerate
paclitaxel due to numbness and allergy, NDP/CPT is an alternative
chemotherapeutic agent.
Findings of recent studies have suggested that
CDDP/CPT has an efficacy similar to or better than T/platinum in
patients with primary CCC. In a retrospective study that compared
46 cases of CDDP/CPT and 126 cases of T/platinum with optimal
debulking in stages II–IV, the 2-year PFS for CDDP/CPT was 86%,
which was higher than that of T/platinum (44%) (7). In another retrospective study of 82
patients treated with TC and 35 patients treated with CDDP/CPT,
equal efficacy was observed between the groups (8). In the JGOG's randomized phase II
trial, a comparison between 48 cases treated with CDDP/CPT and 50
cases treated with TC, the treatments were equally tolerated, and
there was no significant difference in PFS. Since there were
numerous patients in the CDDP/CPT group with large residual tumor
cells, a sub-analysis was performed in those with 2-cm residual
tumors. In this sub-analysis, the PFS tended to be longer in the
CDDP/CPT group, although the difference was not statistically
significant (p=0.056) (9).
At present, the JGOG and Gynecologic Cancer
Intergroup (GCIG) are participating in an international cooperative
randomized phase III trial (GCIG/JGOG3017 ovarian trial) to compare
CDDP/CPT and TC as initial first-line chemotherapy for the
treatment of CCC (9). If results
are obtained that show CDDP/CPT is more suitable than TC for
treating patients with CCC, a prospective study should be performed
to establish whether a regimen with less toxicity, such as NDP/CPT,
is suitable for the treatment of CCC.
References
|
1.
|
SF SerovRE ScullyLH SobinInternational
Histologic Classification of Tumors, No 9 Histological Typing of
Ovarian TumorsWorld Health OrganizationGeneva1973
|
|
2.
|
AP HeintzF OdicinoP MaisonneuveCarcinoma
of the ovary. FIGO 6th Annual Report on Results of Treatment in
Gynecological CancerInt J Gynaecol Obstet95S161S192200617161157
|
|
3.
|
T SugiyamaT KamuraJ KigawaClinical
characteristics of clear cell carcinoma of the ovary: a distinct
histologic type with poor prognosis and resistance to
platinum-based
chemotherapyCancer8825842589200010.1002/1097-0142(20000601)88:11%3C2584::AID-CNCR22%3E3.0.CO;2-510861437
|
|
4.
|
A Du BoisJ HerrstedtAC Hardy-BessardPhase
III trial of carboplatin plus paclitaxel with or without
gemcitabine in first-line treatment of epithelial ovarian cancerJ
Clin Oncol28416241692010
|
|
5.
|
H UtsunomiyaJ AkahiraS
TannoPaclitaxel-platinum combination chemotherapy for advanced or
recurrent ovarian clear cell adenocarcinoma: a multicenter trialInt
J Gynecol
Cancer165256200610.1111/j.1525-1438.2006.00289.x16445610
|
|
6.
|
T EnomotoC KuragakiM YamasakiIs clear cell
carcinoma and mucinous carcinoma of the ovary sensitive to
combination chemotherapy with paclitaxel and carboplatin?Proc Am
Soc Clin Oncol22abs. 17972003
|
|
7.
|
M TakanoY KikuchiN YaegashiAdjuvant
chemotherapy with irinotecan hydrochloride and cisplatin for clear
cell carcinoma of the ovaryOncol Rep1613011306200617089053
|
|
8.
|
M TakanoT SugiyamaN
YaegashiProgression-free survival and overall survival of patients
with clear cell carcinoma of the ovary treated with
paclitaxel-carboplatin or irinotecancisplatin: retrospective
analysisInt J Clin
Oncol12256260200710.1007/s10147-007-0670-117701003
|
|
9.
|
S TakakuraM TakanoF TakahashiRandomized
phase II trial of paclitaxel plus carboplatin therapy versus
irinotecan plus cisplatin therapy as first-line chemotherapy for
clear cell adenocarcinoma of the ovary: a JGOG studyInt J Gynecol
Cancer20240247201010.1111/IGC.0b013e3181cafb4720169667
|
|
10.
|
DM GershensonIrinotecan in epithelial
ovarian cancerOncology (Williston Park)162931200212109803
|
|
11.
|
T SugiyamaM YakushijiT NishidaK UshijimaN
OkuraJ KigawaN TerakawaIrinotecan (CPT-11) combined with cisplatin
in patients with refractory or recurrent ovarian cancerCancer
Lett128211218199810.1016/S0304-3835(98)00065-29683285
|
|
12.
|
S MachidaM OhwadaH FujiwaraPhase I study
of combination chemotherapy using irinotecan hydrochloride and
nedaplatin for advanced or recurrent cervical
cancerOncology65102107200310.1159/00007233312931014
|
|
13.
|
T KatoH NishimuraM YakushijiPhase II study
of 254-S (cis-diammine glycolato platinum) for gynecological
cancerGan To Kagaku Ryoho1969570119921580643
|
|
14.
|
T OtaN TakeshimaK TakizawaSecond-line
chemotherapy for carboplatin/paclitaxel-refractory ovarian cancer:
are multi-agent chemotherapies of little value truly?Eur J Gynaecol
Oncol32471475201122053655
|
|
15.
|
M KoshiyamaM KinezakiT UchidaM
SumitomoChemosensitivity testing of a novel platinum analog,
nedaplatin (254-S), in human gynecological carcinomas: a comparison
with cisplatinAnticancer Res2544994502200516334133
|
|
16.
|
M NishidaH TsunodaY IchikawaH
YoshikawaComplete response to irinotecan hydrochloride and
nedaplatin in a patient with advanced ovarian clear cell
carcinomaInt J Clin
Oncol9403405200410.1007/s10147-004-0413-515549593
|
|
17.
|
Y SasakiT AmanoM MoritaPhase I study and
pharmacological analysis of cis-diammine (glycolato) platinum
(254-S; NSC 375101D) administered by 5-day continuous intravenous
infusionCancer Res511472147719911997185
|
|
18.
|
LS RosenIrinotecan in lymphoma, leukemia,
and breast, pancreatic, ovarian, and small-cell lung
cancersOncology (Williston Park)1210310919989726101
|
|
19.
|
K YamamotoK KokawaN UmesakiPhase I study
of combination chemotherapy with irinotecan hydrochloride and
nedaplatin for cervical squamous cell carcinoma: Japanese
gynecologic oncology group studyOncol
Rep2110051009200910.3892/or_00000316
|
|
20.
|
F OshitaM OheT HondaPhase II study of
nedaplatin and irinotecan with concurrent thoracic radiotherapy in
patients with locally advanced non-small-cell lung cancerBr J
Cancer10313251323201010.1038/sj.bjc.660587520940720
|
|
21.
|
T MikiT NomotoS NakagawaThe salvage
chemotherapy for refractory testicular cancer with novel anticancer
agentsHinyokika Kiyo45811814199910637749
|
|
22.
|
T IshibashiY YanoT OgumaA formula for
predicting optimal dose of nedaplatin based on renal function in
adult cancer patientsCancer Chemother
Pharmacol50230236200210.1007/s00280-002-0488-512203105
|
|
23.
|
S SatoH FujiwaraT OishiEvaluation of a
formula for individual dosage of nedaplatin based on renal
functionCancer Chemother
Pharmacol69599603201210.1007/s00280-011-1739-021918903
|
|
24.
|
H MinamiK SaiM SaekiIrinotecan
pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms
in Japanese: roles of UGT1A1*6 and
*28Pharmacogenet
Genomics17497504200710.1097/FPC.0b013e328014341f17558305
|
|
25.
|
G ToffoliE CecchinG CoronaThe role of
UGT1A1*28 polymorphism in the pharmacodynamics and
pharmacokinetics of irinotecan in patients with metastatic
colorectal cancerJ Clin Oncol2430613068200616809730
|