Introduction
Glioblastoma multiforme (GBM) is a type of cancer
that affects the glial cells in the central nervous system (CNS).
It is the most common (1) and
aggressive type of primary brain tumour in adults and its outcome
is fatal (2).
In 2010, there were 22,020 new cases of primary
brain tumours diagnosed in the USA and approximately 13,140
mortalities (3). The incidence of
brain tumours has increased over the past 30 years, particularly in
the elderly (2). GBM is the most
common type of brain tumour in adults and accounts for 54% of all
gliomas (1). The highest incidence
is identified in adults over the age of 45 years (5). The prevalence is higher in males
compared with females, and in two studies conducted in France
(6) and the USA (7), the male to female ratio was 1.6:1 and
1.48:1, respectively.
The Hospital Universitario Central de Asturias
(HUCA) is the referral hospital in Asturias (Spain). Among the 1
million inhabitants of Asturias, 4,580 tumours were diagnosed in
2008, of which 3% were brain tumours. GBM had the highest incidence
and accounted for 19.3% of all brain tumours (8).
More than 90% of GBMs are primary tumours. They
generally have a clinical history of less than one year and are
more frequent in elderly patients. Despite a multitude of
scientific advances, the median survival time for these patients
receiving standard treatment [surgical resection, radiotherapy (RT)
and temozolomide (TMZ), followed by six maintenance cycles of TMZ]
remains low, at approximately 14 months (9,10).
The prognosis for patients with GBM appears to be
influenced by a number of factors. Certain factors that have been
suggested to predict a good prognosis include age (adults under 58
years have better rates of survival and progression-free disease)
(11), Karnofsky Performance Status
(KPS) ≥70–80% at diagnosis (12),
absence of anaplastic cells, existence of oligodendroglial
elements, tumour size and type of surgical resection (complete
resections have been identified to have better survival data)
(13), and preserved cognitive
status (10).
The use of bevacizumab and irinotecan (BVZ/CPT-11)
in GBM is based on the fact that these tumours are highly
vascularised, and preclinical data demonstrate that glioma growth
is dependent on tumour-associated neovascularisation (14,15).
In the 1970s, Folkman (16)
proposed the hypothesis of using angiogenesis as an anticancer
therapeutic target. Based on these theoretical premises, it appears
reasonable to use an antivascular endothelial growth factor agent
(anti-VEGF), including BVZ, for this type of tumour. However,
anti-VEGF is rarely used in GBM. BVZ studies are now at phase II
and it appears that in addition to being active in GBM, it is also
well tolerated (17,18). The antiglioma biological activity of
BVZ has also been demonstrated (19), with results superior to those
obtained in previous studies (20–35).
In May 2009, the US Food and Drug Administration
(FDA) approved the use of BVZ as a single agent for patients
diagnosed with GBM and disease progression who were previously
treated with the standard first-line treatment (36). However, BVZ is not yet administered
for any line of GBM treatment in Europe, with results pending from
phase III studies to confirm efficacy data.
Materials and methods
Study design
A retrospective cohort study with a control group
was conducted to compare the effectiveness of two chemotherapy
treatments in terms of overall survival (OS) in two patient
cohorts. The study was approved by the ethics committee of Central
University Hospital of Asturias, Asturias, Spain. Consent was
obtained for use of patient data.
Study population
Patients
Patients diagnosed with and treated for primary GBM
between 2002 and 2009 at HUCA were selected for this study. The
control cohort included all patients treated with TMZ between
January 2002 and December 2006 at the Hospital Pharmacy Service
(HUCA), and who met the inclusion criteria. The BVZ/CPT-11 cohort
included all patients diagnosed with any type of glioma and treated
with the study regimen as second-line treatment between January
2007 and December 2009, and who met the inclusion criteria.
Inclusion criteria. All patients were
histologically diagnosed with primary GBM between 2002 and 2009 at
HUCA. Patients were over the age of 18 and underwent surgical
procedures regardless of the type of resection, including biopsies.
The control cohort consisted of patients who received a first-line
treatment with TMZ concomitantly with RT and/or maintenance cycles
that were conducted until completion or progression. The control
patients did not receive second-line treatment or the second-line
treatment differed from that of the study cohort. The study cohort
consisted of patients who met the control cohort first-line
inclusion criteria and also received BVZ/CPT-11 regimen as
second-line treatment.
Exclusion criteria. Patients who did not meet
all the inclusion criteria and those lacking any data required for
analysis were excluded from the study.
Definition of endpoints
Primary endpoints (quantitative
variables)
The primary efficacy endpoint of the study was OS,
which was calculated from the time of diagnosis to the date of the
last observation in the patient's medical record or mortality.
Interim survival (IS) following second-line treatment with
BVZ/CPT-11 was measured from the start of the second-line treatment
to the date of the last observation or mortality. Time to
progression (TTP) following first-line treatment was also
calculated, representing the time from the start of treatment until
the time of disease progression as defined by the oncologist.
Secondary endpoints (dichotomous and qualitative
variable). The secondary endpoint was the type of second-line
treatment received, identifying whether the treatment included the
BVZ/CPT-11 regimen.
Data analysis
An assessment of differences in effectiveness was
conducted by comparing the OS and TTP values between the cohorts
using SPSS version 18.0 (SPSS, Chicago, IL, USA). Firstly, the two
study samples were identified, the means and standard deviations
were calculated for the quantitative variables and absolute and
relative frequencies were calculated for the qualitative variables.
OS was identified using the Kaplan-Meier method, which was
conducted in each study group, and the curve was plotted using a
primary independent variable.
Subsequently, the difference in the survival rate of
each group was compared, taking into account each possible risk
factor, using the log rank hypothesis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients and treatment
The control cohort (without BVZ) consisted of 32
patients. Among the 128 patients initially receiving TMZ, the
following were excluded: 25 patients whose diagnosis was not brain
tumour, 55 with a diagnosis of low grade glioma, 11 with secondary
glioblastoma and six who were diagnosed prior to 2002.
The study cohort (with BVZ) consisted of 28
patients. Among the 36 patients who were initially diagnosed with
glioma and treated with BVZ/CPT-11, 8 patients were excluded when
their initial diagnosis did not correspond with primary GBM, which
was the subject of study.
The clinical and demographic characteristics of the
patients included in the two cohorts are summarised in Table I, and the different treatments that
were administered are identified in 1.
 | Table IPatient clinical and demographic
characteristics. |
Table I
Patient clinical and demographic
characteristics.
| Variable | With BVZ (%) | Without BVZ
(%) | P-value |
|---|
| No. of
patients | 46.7 (28) | 53.3 (32) | |
| Age (years) | | | 0.246 |
| Median | 52.6 | 56.1 | |
| Range | 23–69 | 27–73 | |
| Gender | | | 0.741 |
| Male | 60.7 (17) | 53.1 (17) | |
| Female | 39.3 (11) | 46.9 (15) | |
| KPS (%) | | | 0.729 |
| Median | 70 | 70 | |
| Range | 60–90 | 60–100 | |
| Type of
resection | | | 0.981 |
| Complete | 46.4 (13) | 43.7 (14) | |
| Partial | 42.9 (12) | 43.7 (14) | |
| Biopsy | 10.7 (2) | 9.3 (3) | |
| ND | | 3.1 (1) | |
Survival and progression
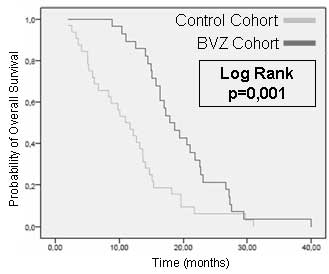
With regard to the primary endpoint, the median OS
was 17.94 months [95% confidence interval (CI), 14.91–20.96] in the
BVZ/CPT-11 treatment cohort and 10.97 months (95% CI, 7.65–14.30)
in the control cohort. A comparison of OS in the two groups was
plotted on a Kaplan-Meier curve (Fig.
1; P=0.001). The IS for patients receiving BVZ/CPT-11 was 8.8
months (95% CI, 5.3–12.3).
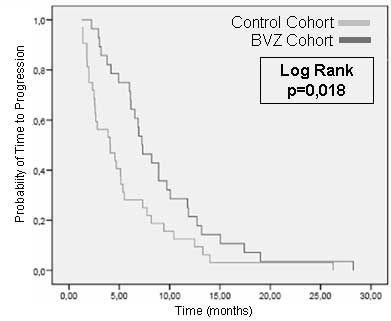
TTP in first-line treatment for the control cohort
was 4.07 months (95% CI, 1.66–6.49) and 7.26 months (95% CI,
5.51–9.01) in the BVZ cohort (P=0.018; Fig. 2). The difference in TTP was also
calculated following BVZ/CPT-11 administration as second-line
treatment. This was identified to be 5.75 months (95% CI,
4.58–6.92).
The duration of first-line treatment was also
compared between the two cohorts. The median duration of TMZ
treatment was 6.67 months (95% CI, 6-287–7.052) in the study cohort
and 4.14 months (95% CI, 1.686–6.594) in the control cohort (P=
0.004).
Survival and risk factors
Survival was analysed for these variables by
applying possible confounding variables. Table III reveals the median survival with
CIs for the two cohorts by gender and age divided into two groups
(<58 and ≥58 years). KPS score was also divided into two groups
(<80 and ≥80%). Finally, the type of surgical resection was
analysed.
 | Table IIIOS data by subgroup for each
variable. |
Table III
OS data by subgroup for each
variable.
| OS (95% CI)
| Log-rank (Mantel
Cox)
|
|---|
| Variable | Control | BVZ | BVZ | BVZ vs.
control |
|---|
| Age (years) | | | | |
| ≥58 | 6.70
(4.39–9.02) | 18.69
(16.49–20.90) | 0.602 | 0.022 |
| <58 | 14.06
(13.29–14.84) | 17.25
(12.95–21.55) | | |
| Gender | | | | |
| Male | 9.79
(5.60–13.98) | 18.69
(14.63–22.76) | 0.627 | 0.100 |
| Female | 14.06
(1.38–26.75) | 17.25
(11.43–23.06) | | |
| KPS (%) | | | | |
| ≥80 | 13.70
(2.50–24.90) | 22.57
(20.46–24.68) | 0.694 | 0.064 |
| <80 | 10.97
(9.08–12.87) | 17.25
(15.13–19.37) | | |
| Type of
resection | | | | |
| Complete | 14.06
(13.48–14.64) | 17.94
(12.38–23.49) | N/D | N/D |
| Incomplete | 9.79
(4.19–15.39) | 16.36
(11.23–21.49) | | |
| Biopsy | 6.70 | 22.57
(6.48–38.66) | | |
Discussion
As shown in Table I,
there are no differences between patients in the two cohorts with
regard to variables that may act as confounders. The variables were
similar to those identified in other studies. For example, the
median age, KPS score, gender ratio and the different types of
resection observed in the two cohorts, were similar to those
identified in previous studies (9,10,17–35).
When comparing OS data in patients treated with and
without BVZ, it is evident that the median OS from diagnosis
increased by almost 7 months in the BVZ treatment cohort (P=0.001).
In relative terms, this represents a 50–60% increase in OS.
However, it should be noted that patients did not receive the same
number of first-line treatment cycles in the two cohorts, as
discussed below.
Results for OS in the BVZ cohort were similar to
those reported in the few phase II trials that were available at
the time of writing. Vredenburgh et al (20) and Friedman et al (17) identified that the median OS from the
start of BVZ/CPT-11 treatment was 9.8 and 9.2 months, respectively,
compared with the 8.8 months demonstrated in our study.
Certain factors may have influenced these small
differences. In our study, 17.9% of patients in the BVZ cohort had
a KPS score of <70%, while other trials established a KPS score
of ≥70% as an inclusion criterion (19), and the percentage of these patients
was much lower than in our study (20). There were also other criteria, such
as <1.5 mg/ml bilirubin and a certain platelet count, which we
did not take into account. It should also be noted that we did not
have the date of mortality for 12 patients, which also may have
influenced our lower OS figures.
The OS value of the non-BVZ cohort (10.97 months)
was significantly different from the 14 and 15 month OS that was
demonstrated by Stupp et al (9) and Seiz et al (36), respectively. This discrepancy may be
attributed to the fact that 66.7% of the patients in our study were
diagnosed and treated before 2005, which is when the study by Stupp
et al (9) was published,
establishing the grounds for concomitant TMZ and RT and the six
maintenance cycles. Until then, fewer cycles of TMZ were
administered, usually entailing four 28-day cycles with one to five
days of treatment. Additionally, patients who underwent complete
resection did not receive cycles concomitantly with RT.
The characteristics of our patients also differed
from the study by Stupp et al (9). The latter required adequate renal and
liver function tests and blood values within a certain range as an
inclusion criterion, and we did not take these parameters into
consideration when enrolling patients in our study.
TTP data identified following first-line treatment
with TMZ and RT in the BVZ cohort was similar to data from previous
studies, which refers to progression-free survival (PFS) of 6.9
months (9). In the non-BVZ cohort,
TTP was 4.07 months. The difference between these figures can be
explained as previously stated.
It should be noted that TTP is not exactly
comparable with PFS. TTP does not take into account mortalities
without progression or mortalities from other causes, which are
included in PFS. Furthermore, we collected data from the time that
the oncologist reported clinical progression, and not when
progression was observed on nuclear magnetic resonance (NMR).
Therefore, there may be some time bias between the time when the
NMR was conducted and the time when the doctor in charge of
follow-up confirmed that the image represented disease
progression.
By comparing TTP data from the two cohorts, it is
apparent that there is a statistically significant difference
between the two (P=0.018). To discover the reason for this
difference, we measured the duration of TMZ treatment in both
cohorts and, as predicted, the median duration of TMZ treatment in
the BVZ cohort was longer than in the control cohort (P=0.004).
A multivariate analysis did not reveal any
statistically significant differences in OS among the variables we
considered as confounders. However, the clinically significant
differences we identified are noteworthy.
With regard to gender, no differences were
identified in either of the cohorts. In terms of age, OS data for
the BVZ cohort was almost equal in the two age groups (<58 and
≥58 years); however, there were differences in the OS data for the
non-BVZ cohort for both age groups. The <58-year-old group had
an OS of 14 months (95% CI, 13.27–14.84), while the ≥58-year-old
group had an OS of six months (95% CI, 4.69–7.16). We identified
similar data in a previous study (8). Younger patients (under the age of 58)
have been reported to have a better prognosis, with better rates of
survival and progression-free disease (11). Therefore, it cannot be said that BVZ
is more effective in one age group than another, because the data
are similar for this cohort.
With regard to KPS score, patients with a higher KPS
score demonstrated better survival data. This was clinically
significant in the BVZ cohort; however, it does not signify that
these will be the only patients who will benefit from BVZ
treatment.
Finally, analysis of the type of surgical resection
demonstrated that the OS data was better in patients who underwent
complete resection, with the exception of the BVZ cohort, where
patients who were biopsied had the longest median OS. However,
there were only two patients in this subgroup and the data should
not be considered.
In this study, based on the results obtained, we can
conclude that the BVZ/CPT-11 regimen is effective as a second-line
treatment of primary GBM. According to our analysis, we can predict
that patients with a higher KPS, lower age and complete resection
may have a longer life expectancy; although, we should not select
these patients alone as the sole beneficiaries of second-line
treatment with BVZ-CPT11. Cost-effectiveness studies should be
conducted to ascertain whether the cost of treatment is sustainable
when there are budget constraints.
References
|
1.
|
DN LouisH OhgakiOD WiestlerWK CaveneeThe
2007 WHO classification of tumours of the central nervous
systemActa
Neuropathol11497109200710.1007/s00401-007-0243-417618441
|
|
2.
|
JG GurneyN Kadan-LottickBrain and other
central nervous system tumors: rates, trends, and epidemiologyCurr
Opin Oncol13160166201010.1097/00001622-200105000-0000511307058
|
|
3.
|
SS BremPJ BiermanH BremCentral nervous
system cancersJ Natl Compr Canc Netw93524002011
|
|
4.
|
F Laigle-DonadeyJY DelattreGlioblastoma in
the elderlyGeriatr Psychol Neuropsychiatr Vieil91011062011(In
French)
|
|
5.
|
I BaldiA HuchetL BauchetH
LoiseauEpidemiology of
glioblastomaNeurochirurgie56433440201010.1016/j.neuchi.2010.07.01120869733
|
|
6.
|
L BauchetH Mathieu-DaudéP
Fabbro-PeraySociété Française de Neurochirurgie (SFNC)Club de
Neuro-Oncologie of the Société Française de Neurochirurgie
(CNO-SFNC)Société Française de Neuropathologie (SFNP)Association
des Neuro-Oncologues d'Expression Française (ANOCEF)Oncological
patterns of care and outcome for 952 patients with newly diagnosed
glioblastoma in 2004Neuro
Oncol12725735201010.1093/neuonc/noq03020364023
|
|
7.
|
S DeorahCF LynchZA SibenallerTC
RykenTrends in brain cancer incidence and survival in the United
States: surveillance, epidemiology, and end results program, 1973
to 2001Neurosurg Focus20E1201010.3171/foc.2006.20.4.E116709014
|
|
8.
|
HUCA tumour register. 2008 Annual Report.
Hospital Universitario Central de Asturias and Instituto
Universitario Oncológico, Principality of Asturias, 2008. ISSN
1138–8501. http://10.15.65.11/huca/web/contenidos/servicios/rt/rt2008/rt2008.pdf.
Accesed January 21, 2012
|
|
9.
|
R StuppWP MasonMJ van den BentEuropean
Organisation for Research and Treatment of Cancer Brain Tumor and
Radiotherapy GroupsNational Cancer Institute of Canada Clinical
Trials Group: Radiotherapy plus concomitant and adjuvant
temozolamide for glioblastomaN Engl J
Med352987996200510.1056/NEJMoa04333015758009
|
|
10.
|
R StuppME HegiWP MasonEuropean
Organisation for Research and Treatment of Cancer Brain Tumour and
Radiation Oncology GroupsNational Cancer Institute of Canada
Clinical Trials Group: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trialLancet Oncol104594662009
|
|
11.
|
M LacroixD Abi-SaidDR FourneyA
multivariate analysis of 416 patients with glioblastoma multiforme:
prognosis, extent of resection, and survivalJ
Neurosurg95190198200110.3171/jns.2001.95.2.019011780887
|
|
12.
|
JF MineoA BordronM BaronciniPrognosis
factors of survival time in patients with glioblastoma multiforme:
a multivariate analysis of 340 patientsActa Neurochir
(Wien)149245253200710.1007/s00701-006-1092-y17273889
|
|
13.
|
AA BrandesA TosoniE FranceschiM ReniG
GattaC VechtGlioblastoma in adultsCrit Rev Oncol
Hematol67139152200810.1016/j.critrevonc.2008.02.005
|
|
14.
|
M MaxwellSP NaberHJ WolfeET Hedley-WhyteT
GalanopoulosJ Neville-GoldenHN AntoniadesExpression of angiogenic
growth factor genes in primary human astrocytomas may contribute to
their growth and progressionCancer Res511345135119911705174
|
|
15.
|
JA TakahasiM FukumotoK IgarashiY OdaH
KikuchiM HatanakaCorrelation of Basic fibroblast growth factor
expression levels with the degree of malignancy and vascularity in
human gliomasJ
Neurosurg76792798199210.3171/jns.1992.76.5.07921564542
|
|
16.
|
J FolkmanTumor angiogenesis: Therapeutic
implicationsN Engl J
Med28511821186197110.1056/NEJM1971111828521084938153
|
|
17.
|
HS FriedmanMD PradosPY WenBevacizumab
alone and in combination with irinotecan in recurrent glioblastomaJ
Clin Oncol2747334740200910.1200/JCO.2008.19.872119720927
|
|
18.
|
AD NordenGS YoungK SetayeshBevacizumab for
recurrent malignant gliomas, efficacy, toxicity and patterns of
recurrenceNeurology70779787200810.1212/01.wnl.0000304121.57857.3818316689
|
|
19.
|
TN KreislL KimK MoorePhase II trial of
single-agent bevacizumab followed by bevacizumab plus irinotecan at
tumor progression in recurrent glioblastomaJ Clin
Oncol27740745200910.1200/JCO.2008.16.305519114704
|
|
20.
|
JJ VredenburghA DesjardinsJE
HerndonBevacizumab plus irinotecan in recurrent glioblastoma
multiformeJ Clin
Oncol2547224729200710.1200/JCO.2007.12.244017947719
|
|
21.
|
RM ZunigaR TorcuatorR JainEfficacy, safety
and patterns of response and recurrence in patients with recurrent
high-grade gliomas treated with bevacizumab plus irinotecanJ
Neurooncol91329336200910.1007/s11060-008-9718-y18953493
|
|
22.
|
V Stark-VanceBevacizumab and CPT-11 in the
treatment of relapsed malignant gliomaNeuro Oncol7Suppl369abstr
3422005
|
|
23.
|
S RavalS HwangL DorsettBevacizumab and
irinotecan in patients with recurrent glioblastoma multiformeJ Clin
Oncol25Supplabstr 20782007
|
|
24.
|
JJ VredenburghA DesjardinsJE Herndon
IIPhase II trial of bevacizumab and irinotecan in recurrent
malignant gliomaClin Cancer
Res1312531259200710.1158/1078-0432.CCR-06-230917317837
|
|
25.
|
SA AliWM McHaylehA AhmadBevacizumab and
irinotecan therapy in glioblastoma multiforme: a series of 13
casesJ
Neurosurg109268272200810.3171/JNS/2008/109/8/026818671639
|
|
26.
|
F BoksteinS ShpigelDT BlumenthalTreatment
with bevacizumab and irinotecan for recurrent high-grade glial
tumorsCancer11222672273200810.1002/cncr.2340118327820
|
|
27.
|
TF CloughesyMD PradosPY WenA phase II,
randomized, non-comparative clinical trial of the effect of
bevacizumab (BV) alone or in combination with irinotecan (CPT) on
6-month progression free survival (PFS6) in recurrent, treatment
refractory glioblastoma (GBM)J Clin Oncol26Supplabstr 2010b2008
|
|
28.
|
A DesjardinsDA ReardonJE Herndon
IIBevacizumab plus irinotecan in recurrent WHO grade 3 malignant
gliomasClin Cancer
Res1470687073200810.1158/1078-0432.CCR-08-026018981004
|
|
29.
|
S GuiuS TaillibertO
ChinotBevacizumab/irinotecan. An active treatment for recurrent
high grade gliomas: preliminary results of an ANOCEF Multicenter
StudyRev Neurol (Paris)1645885942008(In French)
|
|
30.
|
MR GilbertM WangK AldapeRTOG 0625: A phase
II study of bevacizumab with irinotecan in recurrent glioblastoma
(GBM)J Clin Oncol27Supplabstr 20112009
|
|
31.
|
PL NghiemphuW LiuY LeeBevacizumab and
chemotherapy for recurrent glioblastoma: a single-institution
experienceNeurology7212171222200910.1212/01.wnl.0000345668.03039.9019349600
|
|
32.
|
HS PoulsenK GrunnetM SorensenBevacizumab
plus irinotecan in the treatment patients with progressive
recurrent malignant brain tumoursActa
Oncol485258200910.1080/0284186080253792419031176
|
|
33.
|
T CloughesyJJ VredenburghB DayUpdated
safety and survival of patients with relapsed glioblastoma treated
with bevacizumab in the BRAIN studyJ Clin Oncol28Supplabstr
20082010
|
|
34.
|
A DesjardinsJJ VredenburghDA
ReardonLongterm survival from the initial trial of bevacizumab and
irinotecanJ Clin Oncol28Supplabstr 20452010
|
|
35.
|
MH CohenYL ShenP KeeganR PazdurFDA drug
approval summary: bevacizumab (Avastin) as treatment of recurrent
glioblastoma
multiformeOncologist1411311138200910.1634/theoncologist.2009-012119897538
|
|
36.
|
M SeizU KraVtCF FreyschlagLong-term
adjuvant administration of temozolomide in patients with
glioblastoma multiforme: experience of a single institutionJ Cancer
Res Clin Oncol13616911695201010.1007/s00432-010-0827-620177703
|