Introduction
Overexpression of unmodified prolactin (U-PRL)
promotes prostate and breast cancer; however, phosphorylated PRL
has the opposite effect (1). A
recombinant PRL was constructed and used as a mimic of the
phosphorylated PRL (S179D PRL). In a previous study, we
demonstrated that S179D PRL inhibited breast and prostate cancer
cell growth (2). The mechanisms
involved were associated with short PRL signaling; however, it was
suggested that the short PRL receptor (PRLR) did not induce mammary
cell differentiation in response to U-PRL. It has been suggested
that the short PRLR lacks the structural Stat5 activation site.
Nevertheless, a short mouse PRLR was able to activate
mitogen-activated protein kinases (MAPKs), which led to the
proliferation of NIH/3T3 cells (3).
Binart et al revealed that a short form of mouse PRLR
rescues mammopoiesis in heterozygous PRLR mice (4). Notably, we demonstrated that S179D PRL
induced β-casein gene expression by regulating the ratio of short
to long PRLR in mouse mammary HC11 cells (5). Similarly, Meng et al and Qazi
et al identified that a lower ratio of short to long PRLR
contributed to breast cancer development, as the short PRLR form
inhibited signaling of the long PRLR form via heterodimerization
(6,7). Both the long and the short PRLR forms
have been identified in human prostate tissues (8). We previously demonstrated that S179D
PRL inhibited prostate cancer DU145 cell growth via the short human
PRLR, S1b (2), and inhibited the
growth of DU145-derived tumor cells in nude mice (9).
In previous studies, we also revealed that S179D PRL
activated short PRLR and ERK1/2 resulting in p21/waf1 expression in
prostate cancer cells (2,9). It is well known that p21/waf1 induces
cell cycle arrest, differentiation and apoptosis in a number of
cell lines. These results indicate that S179D PRL may induce
apoptosis via the short PRLR S1b and the upregulation of p21/waf1.
Short PRLR signaling may trigger the activation of JNK, c-jun and
c-fos, resulting in the activation of activating protein-1 (AP-1)
and the upregulation of p21/waf1 in human prostate cancer cells.
Previous studies have demonstrated that the accumulation of ERK1/2
resulted in the upregulation of p21 (10–12).
Structurally, the short PRLR has the potential to activate Jak 2
and further activate MAPKs. Studies have revealed that the short
PRLR activates AP-1 family members, including c-jun and c-fos
complexes, and U-PRL induces an increase in the c-jun content of
the AP-1 transcriptional complex via activating JNK (13,14).
AP-1 was considered to be a regulator of cell death, and p21/waf1
expression correlates with the expression of AP-1 family members
(c-jun and c-fos) in breast cancer cells (15). Additionally, it has been
demonstrated that JNK activation, as well as c-jun and c-fos
upregulation, contribute to p21/waf1 expression in selenite-induced
HepG2 cell apoptosis. In the present study, we investigated whether
S179D PRL activates JNK, c-jun and c-fos, leading to p21/waf1
upregulation and apoptosis in response to S179D PRL in the PC3
cells transfected with human short PRLR S1b. The JNK blocker,
SP600125, was used to determine whether JNK signaling contributes
to p21/waf1 upregulation. The aim of this study was to uncover the
molecular mechanisms associated with S179D PRL and prostate cancer
cells.
Materials and methods
Recombinant PRL
DNA cloning and mutagenesis techniques were used to
produce U-PRL and S179D PRL, as previously described (5). S179D PRL was generated by substituting
an aspartate for a normally phosphorylated serine. The proteins
were expressed and purified, and their activity was studied in an
Nb2 cell bioassay. U-PRL promotes Nb2 cell proliferation, while
S179D PRL antagonizes this effect.
Cell lines and constructs
The PC3 cell line was purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA). Cells were
maintained in RPMI-1640 medium containing 10% fetal bovine serum
(FBS; Invitrogen, Carlsbad, CA, USA) and antibiotics. The
constructs pEF4C-S1b and pEF6-LPRLR containing prolactin receptors
were gifts from Dr Barbara K. Vonderhaar (National Institute of
Health, National Cancer Institute, Bethesda, MD, USA) and the
β-galactosidase (β-gal) plasmid was provided by Dr Linda A. Schuler
(University of Wisconsin, Madison, WI, USA). AP-1 (7x) was
purchased from Stratagene Corp. (La Jolla, CA, USA). The constructs
pRSV-jun and pRSV-fos were gifts from Dr Robert Tjian (University
of California, Berkeley, CA, USA). The construct p21 Luc was
obtained from Dr Leonard P. Freedman (Memorial Sloan-Kettering
Cancer Center, New York, NY, USA).
Cotransfection and luciferase assay
PC3 cells were grown in RPMI-1640 medium
(Invitrogen) containing 10% charcoal-stripped horse serum (Cocalico
Biologicals, Reamstown, PA, USA), 100 U/ml penicillin and 100
μg/ml streptomycin (Invitrogen). Cells were grown in
six-well plates and transfection was conducted once the cells
reached 50–70% confluency. The β-gal plasmid (0.25 μg/5 ml)
was cotransfected in order to examine the transfection efficiency.
After cells were treated with PRL (U-PRL and S179D PRL; 1
μg/ml) for three days, the JNK blocker, SP600125 (25
μM; Calbiochem, San Diego, CA, USA), was used to inhibit
AP-1 Luc and p21 Luc expression in response to each PRL. In another
transfection study involving c-fos and c-jun transfection, c-fos
(0.5 μg/5 ml) only, c-jun (0.5 μg/5 ml) only and
c-fos (0.25 μg/5 ml) plus c-jun (0.25 μg/5 ml), AP-1
Luc (0.25 μg/5 ml) or p21 luciferase construct (0.25
μg/5 ml), short PRLR (0.25 μg/5 ml) and β-gal
plasmids were used to transfect the cells. In the RPMI-1640 medium
containing 5% charcoal-stripped horse serum, cells were treated
with U-PRL or S179D PRL for 24 h. A volume of 10 μl was
applied to test luciferase activity using a illuminometer,
following the Promega Luciferase assay manufacturer’s
instructions.
Preparation of whole cell and nuclear
extracts
Cells were rinsed with phosphate-buffered saline
(PBS) and scraped off in a buffer containing 20 mM Tris-HCl (pH
7.4), 140 mM NaCl, 0.05 mM EDTA, 10 μg/ml leupeptin, 10
μg/ml aprotinin, 25 μg/ml pepstatin, 1 mM PMSF, 1 mM
Na3VO4, 10 nM NaF, 1 mM EGTA and 1% NP-40.
The cell lysate was homogenized and centrifuged at 12,000 x g for 5
min, and the supernatant was considered as a whole cell extract.
The whole cell lysate was used to examine the phosphorylated c-jun
(p-c-jun), c-fos (p-c-fos) and JNK (p-JNK). If the cells were
scraped into a hypotonic buffer, consisting of 10 mM Tris (pH 7.4),
10 mM NaCl, 6 mM MgCl2, 1 mM dithiothreitol and 0.1 mM
Na3VO4, and disrupted with a Dounce
homogenizer, the supernatant was removed following centrifugation
at 12,000 x g for 5 min. The pellet from the centrifugation was
resuspended in hypertonic extraction buffer, consisting of 20%
glycerol, 20 mM HEPES (pH 7.9), 420 mM NaCl, 1.5 mM
MgCl2, 0.2 mM EDTA, 0.2 mM phenylmethylsufonyl fluoride,
1 mM dithiothreithol and 0.1 mM Na3VO4, and
incubated on ice for 30 min. The supernatant obtained following
centrifugation was a nuclear extract. This was used to examine the
p21/waf1 protein concentration, which was calculated using the
Bradford method.
Western blot analysis
A total of 20 μg of protein was loaded onto a
reducing SDS-PAGE gel. Following electrophoresis, the protein was
transferred to nitrocellulose membranes in 48 mM Tris, 39 mM
glycine, 0.1% SDS and 20% methanol (pH 8.3). Membranes were blocked
with 5% non-fat milk in washing buffer consisting of Dulbecco’s PBS
(DPBS; Invitrogen) and 0.1% Tween-20. Blotted and blocked membranes
were probed with primary rabbit polyclonal anti-p21/waf1 (1:500),
anti-c-fos (1:1000), anti-p-c-jun (1:1000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and anti-JNK (1:500;
Promega, Madison, WI, USA) in washing buffer for 3 h at room
temperature or overnight at 4°C. After washing three times for 15
min each, the blots were incubated in goat anti-rabbit conjugated
to horseradish peroxidase (Sigma, St. Louis, MO, USA) at a dilution
of 1:2000–1:10000, as appropriate, for 30–45 min at room
temperature. Following a further three washes, the membranes were
treated with ECL reagent (Amersham Biosciences, Piscataway, NJ,
USA) and an autoradiograph image was obtained.
Apoptosis assay
The treated PC3 cells were collected to examine the
DNA degradation. DNA was isolated using phenol/chloroform
extraction followed by ethanol precipitation procedures. A total of
10 μg of DNA was run on a 0.8% agarose gel. Apoptosis was
determined by DNA fragmentation.
Statistical analysis
Data were subjected to analysis of variance with
post-tests for comparison among specific groups using the INSTAT
program (Graph PAD Software, San Diego, CA, USA). Post-tests
comparing each potential pair of groups were conducted. Bonferroni
corrections for multiple comparisons against a single group were
used. All experiments were conducted a minimum of three times.
Following Bonferroni corrections, P<0.05 was considered to
indicate a statistically significant difference.
Results
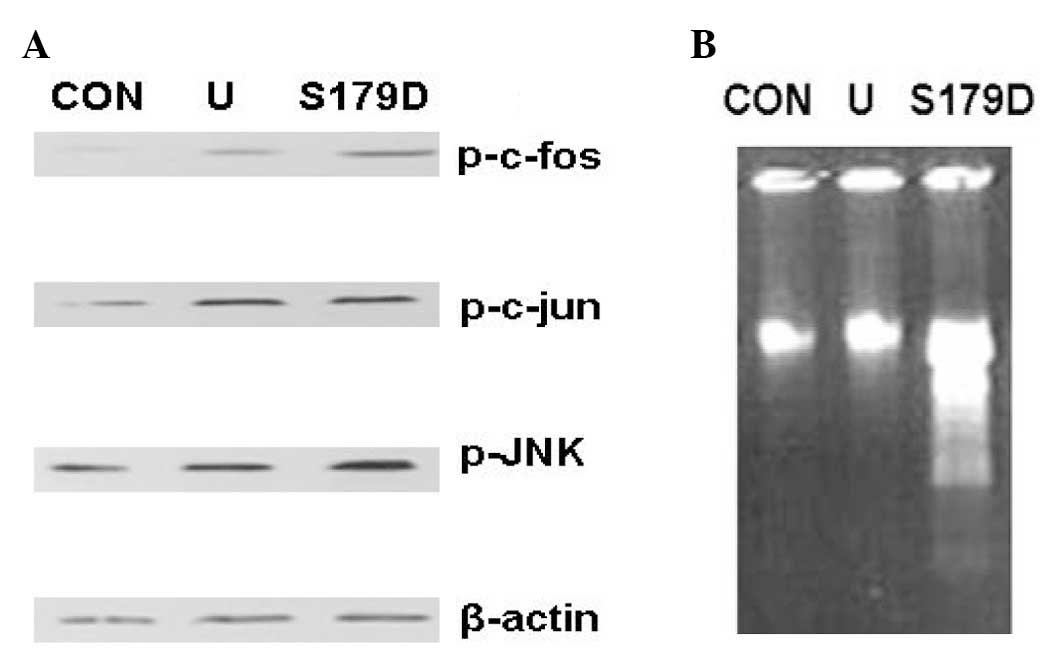
S179D PRL phosphorylates c-fos, c-jun and
JNK
PC3 cells were treated with U-PRL or S179D PRL for 4
days. Western blot analysis demonstrated that U-PRL and S179D PRL
phosphorylated c-fos, c-jun and JNK (Fig. 1A). Apoptosis assays revealed that
S179D PRL induced apoptosis in PC3 cells (Fig. 1B); however, U-PRL did not.
Previously, we identified that S179D PRL upregulated the short PRLR
S1b and ERK1/2 leading to p21 upregulation in PC3 cells (2). These results indicated that the short
PRLR S1b and c-jun/c-fos complex (AP-1) may contribute to S179D PRL
signaling and apoptosis.
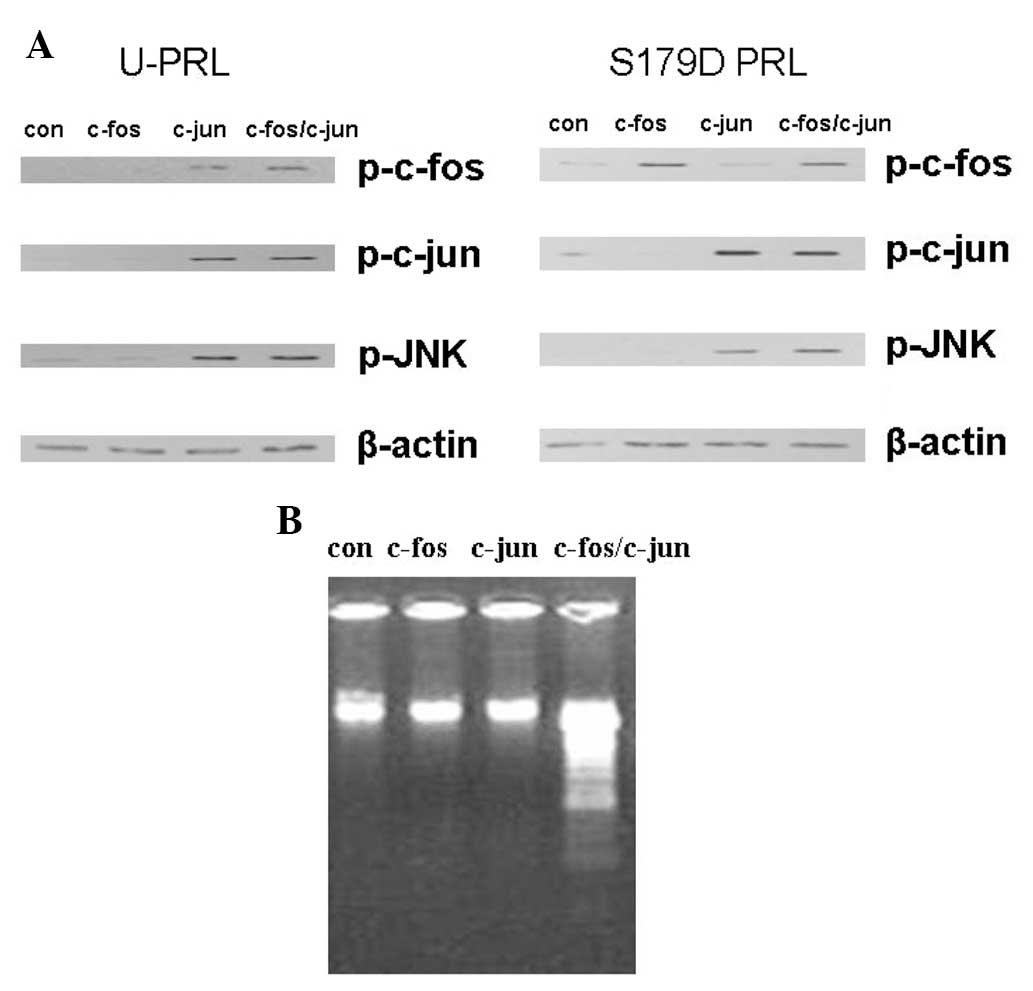
S179D PRL phosphorylates c-fos, c-jun and
JNK in the short PRLR S1b transfected cells
It was suggested that S1b activated the MAPK
pathway; however, in previous studies, S179D PRL did not upregulate
S1b following a 24 h incubation (2). In this study, we transfected PC3 cells
with S1b, and either c-fos, c-jun or c-fos/c-jun constructs, in
order to observe the activation of c-fos, c-jun or c-jun/c-fos.
Following transfection, cells were treated with U-PRL or S179D PRL.
The results demonstrated that U-PRL and S179D PRL activated c-fos,
c-jun and JNK in c-fos, c-jun and c-fos/c-jun transfected cells to
a certain extent (Fig. 2A). These
data reveal that S1b is involved in the activation of c-fos, c-jun
and JNK in S179D PRL treated PC3 cells. Additionally, apoptosis was
only observed in c-fos/c-jun-transfected cells, but not in the
c-fosor c-jun only-transfected cells (Fig. 2B), which suggests that AP-1
contributes to apoptosis.
S1b triggers p21/waf1 upregulation
In this study, we investigated whether S1b
contributed to p21/waf1 upregulation. Control PC3 cells were
transfected either with or without S1b and cells were treated
either with or without S179D PRL. After a 4-day incubation,
p21/waf1 was determined using western blot analysis. The results
revealed that p21/waf1 was upregulated in S179D PRL-treated cells
with S1b transfection (Fig. 3A). We
previously identified that S1b was expressed following a 4-day
treatment with S179D PRL (2);
therefore, S1b contributed to p21/waf1 upregulation in response to
S179D PRL.
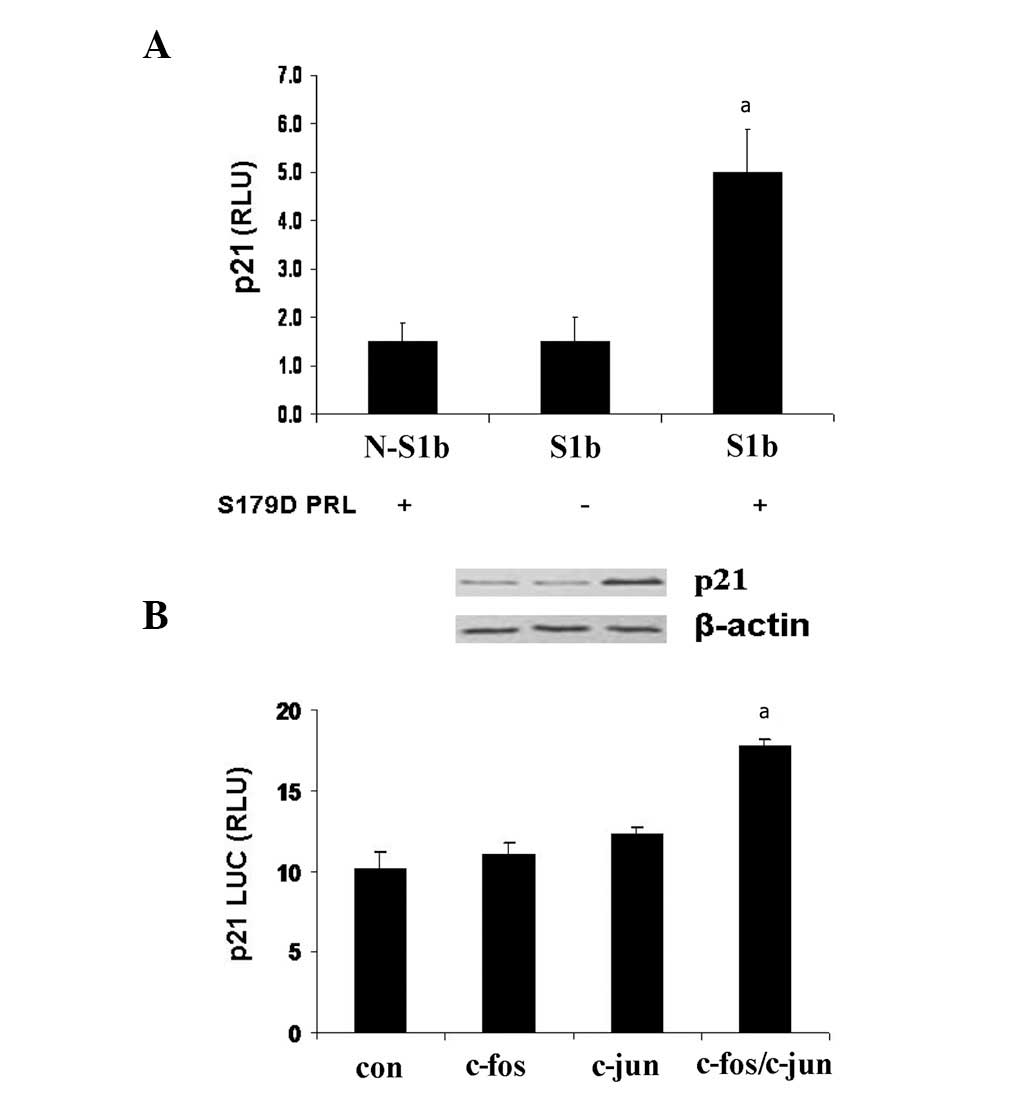 | Figure 3(A) Short PRLR S1b contributed to p21
upregulation. PC3 cells were transfected either with S1b (S1b) or
without S1b (N-S1b) for 4 days. Cells were treated with S179D PRL
(+) or without S179D PRL (−). After the 4-day incubation, p21
levels were determined by western blot analysis. The data revealed
that p21 was upregulated in the S179D PRL-treated cells transfected
with S1b, which indicated that short PRLR S1b contributed to
p21/waf1 upregulation. β-actin was used for normalization and equal
loading. (B) S179D PRL increases p21 luciferase activity. Cells
were transfected with S1b, c-fos, c-jun or fos/c-jun, and the p21
Luc constructs. After a 24 h incubation with S179D PRL, S179D PRL
increased p21 Luc in the c-fos/c-jun-transfected cells. All
experiments were conducted in triplicate. aP<0.05 vs.
the control cells. RLU, relative units; PRL, prolactin; con,
control. PRLR, PRL receptor. |
S179D PRL increases p21 luciferase
activity
Activated MAPK (ERK1/2 and JNK) was demonstrated to
activate AP-1 complexes (c-jun/c-jun or c-jun/c-fos dimers) in a
number of cell types (13–15). In this study, cells were transfected
with S1b, either c-fos, c-jun or c-fos/c-jun and p21 Luc
constructs, after 24-h incubation with S179D PRL. The results
revealed that S179D PRL increased p21 luciferase activity in
c-fos/c-jun transfected cells only (Fig. 3B).
SP600125 inhibits AP-1 or p21 luciferase
activity
In this study, we examined the AP-1 (7x) and p21
luciferase activity after PC3 cells were transfected with S1b
c-fos/c-jun constructs in response to S179D PRL and the JNK
blocker, SP600125 (25 μM). After 24-h incubation, the
results demonstrated that SP600125 inhibited AP-1 and p21
luciferase activity induced by S179D PRL (Fig. 4A and B). Since the activation of
c-fos depends on activated ERK1/2 (15), both activated ERK1/2 and JNK
contributed to the activation of AP-1 and p21 upregulation.
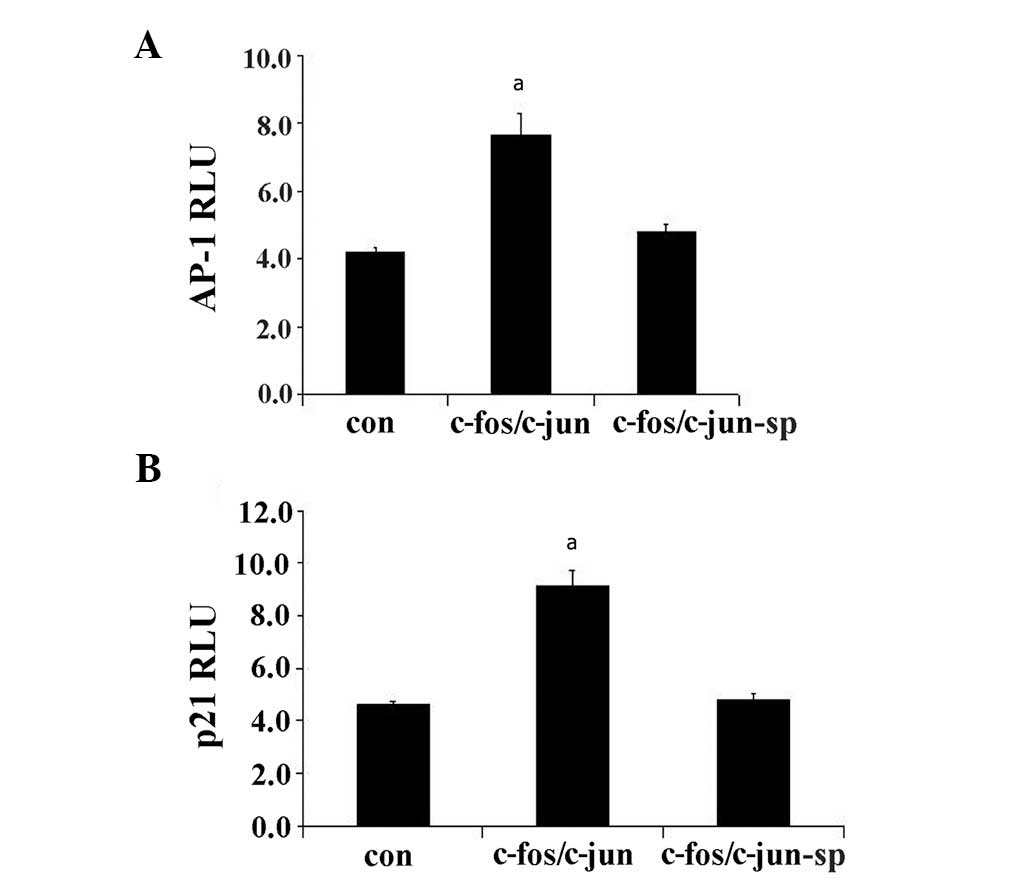 | Figure 4SP600125 inhibits AP-1 and p21
luciferase activities. PC3 cells were cotransfected with S1b,
c-fos/c-jun, AP-1 Luc and p21 Luc in response to S179D PRL and/or
the JNK blocker, SP600125 (25 μM). After a 24 h incubation,
SP600125 inhibited S179D PRL-induced (A) AP-1 and (B) p21/waf1
luciferase activity. This indicates that JNK contributes to the
activation of AP-1 and the upregulation of p21. All experiments
were conducted in triplicate. aP<0.05 vs. the control
cells. AP-1, activating protein-1; RLU, relative units; con,
control, sp, SP600125. PRL, prolactin; JNK, c-jun N-terminal
kinase. |
Discussion
In the previous studies, we demonstrated that S179D
PRL inhibited prostate cancer in vitro and in vivo
(1,9). In the present study, we identified
that S179D PRL induced apoptosis via activating AP-1 and
upregulating p21/waf1 in PC3 cells. Increased p21 and apoptosis
requires short human PRLR S1b and c-fos/c-jun heterodimerization.
We previously demonstrated that S179D PRL primarily used the ERK1/2
pathways to activate downstream genes, including Stat5 and p21/waf1
(5). The short PRLR may play a role
in signaling. Structurally, the short PRLR has the potential to
activate Jak2, which is able to further activate the ERK1/2
pathway. In certain cell types, sustained ERK1/2 activation also
leads to p21/waf1 upregulation, cell cycle arrest, differentiation
and apoptosis (10–12). In the p21 gene promoter region,
there are 2 Stat5 activating sites; however, it is unclear whether
S179D PRL upregulates p21/waf1 via Stat5 activation. Serine
phosphorylation of Stat5 was revealed to have a more stable binding
affinity to the gene promoter (5);
therefore, the short PRLR S1b may contribute to Stat5 activation
leading to p21/waf1 upregulation and apoptosis. Thus, the short
PRLR has the potential to trigger various signaling pathways.
Additionally, S179D PRL may induce mRNA alternative splicing to
maintain a stable ratio of long and short PRLR (6,7).
Studies have demonstrated that activated AP-1 causes a p21/waf1
increase (16).
In the present study, we first identified that S179D
PRL upregulated p21, which resulted from c-fos/c-jun
heterodimerization. In the c-fos or c-jun only-transfected PC3
cells, p21/waf1 was not upregulated, suggesting that S179D PRL only
induced c-fos/c-jun heterodimerization and not c-fos or c-jun
homodimerization. The JNK blocker, SP600125, inhibited S179D
PRL-induced AP-1 activation and p21/waf1 upregulation by blocking
the c-fos/c-jun dimerization. We found that S179D PRL also
phosphorylated JNK. Whether the short PRLR is necessary for this
activation requires further investigation; however, p21/waf1
upregulation requires the short PRLR S1b. In a previous study, we
revealed that the p21/waf1 increase was induced by upregulating the
short PRLR S1b and activating ERK1/2. S179D PRL may activate
p21/waf1 via activating ERK1/2 and JNK. It is unknown whether MAPK
translocates into the nucleus; however, activated c-fos/c-jun
(AP-1) was found to induce apoptosis in numerous cell lines, and a
particular conformation of the c-fos/c-jun complex is necessary for
AP-1 to bind to p21/waf1. Consequently, S179D PRL may inhibit
prostate cancer via apoptosis. In these studies, U-PRL also
phosphorylated JNK and c-jun; however, no apoptotic effect was
identified. JNK is a c-jun N-terminal kinase; therefore, activated
JNK may not activate c-fos since c-fos was activated by ERK1/2.
U-PRL triggers the Jak2-Stat5 pathway via activating the long PRLR
rather than the short PRLR. This effect has been revealed to
contribute to cancer cell proliferation (13,17).
The short PRLR resulted from the translation of alternatively
spliced mRNA. These PRLRs may constitute a regulatory system to
regulate the long PRLR signaling. Additionally, S179D PRL, a U-PRLR
antagonist, may also block the U-PRL signals by competing with the
long PRLR. Nevertheless, S179D PRL primarily uses the short PRLR to
activate ERK1/2, JNK and transcription factors, leading to p21/waf1
upregulation and apoptosis.
In conclusion, S179S PRL activated c-fos, c-jun and
JNK leading to p21/waf1 upregulation and apoptosis, suggesting that
S179D PRL may be used as a potential drug to repress prostate
cancer.
Acknowledgements
We are grateful to Dr Ameae Walker,
(University of California, Riverside, CA, USA) for her support in
the experiments. This study was supported by The Science and
Development Plan of Beijing Education Committee (Grant No.
KM200910025008) and The National Natural Science Foundation of
China (Grant No. 81041067). We would also like to thank Dr Barbara
K. Vonderhaar (NCI NIH, Bethesda, MD, USA), Dr Linda A. Schuler
(University of Wisconsin, Madison, WI, USA), Dr Leopard Freeman
(Memorial Sloan-Kettering Cancer Center, New York, NY, USA) and Dr
Robert Tijan (University of California, Berkeley, CA, USA) for
their generosity in providing constructs.
References
|
1.
|
W WuE GinsburgBK VonderhaarAM WalkerS179D
prolactin increases vitamin D receptor and p21 through
up-regulation of short 1b prolactin receptor in human prostate
cancer cellsCancer
Res6575097515200510.1158/0008-5472.CAN-04-335016103106
|
|
2.
|
R DasBK VonderhaarTransduction of
prolactins (PRL) growth signal through both long and short forms of
the PRL receptorMol Endocrinol91750175919958614411
|
|
3.
|
N BinartP Imbert-BolloreN BaranC
VigliettaPA KellyA short form of the prolactin (PRL) receptor is
able to rescue mammopoiesis in heterozygous PRL receptor miceMol
Endocrinol1710661074200310.1210/me.2002-018112624115
|
|
4.
|
W WuD CossMY LorensonCB KuoXL XuAM
WalkerDifferent biological effects of unmodified prolactin and a
molecular mimic of phosphorylated prolactin involve different
signaling
pathwaysBiochemistry4275617570200310.1021/bi034217s12809512
|
|
5.
|
JP MengCH Tsai-MorrisML DufauHuman
prolactin receptor variants in breast cancer: Low ratio of short
forms to the long-form human prolactin receptor associated with
mammary carcinomaCancer
Res6456775682200410.1158/0008-5472.CAN-04-101915313907
|
|
6.
|
AM QaziCH Tsai-MorrisML
DufauLigand-independent homo- and heterodimerization of human
prolactin receptor variants: inhibitory action of the short forms
by heterodimerizationMol
Endocrinol2019121923200610.1210/me.2005-029116556730
|
|
7.
|
MT NevalainenEM ValvePM IngletonM NurmiPM
MartikainenPL HarkonenProlactin and prolactin receptors are
expressed and functioning in human prostateJ Clin
Invest99618627199710.1172/JCI1192049045863
|
|
8.
|
X XuE KreyeCB KuoAM WalkerA molecular
mimic of phosphorylated prolactin markedly reduced tumor incidence
and size when DU145 human prostate cancer cells were grown in nude
miceCancer Res61609861042001
|
|
9.
|
W WuL ZanelloAM WalkerS179D prolactin
sensitizes human prostate cancer cells such that physiological
concentrations of 1,25 dihydroxy vitamin D3 result in growth
inhibition and cell
deathProstate6714981506200710.1002/pros.20598
|
|
10.
|
T ChenYS WongSelenocystine induces S-phase
arrest and apoptosis in human breast adenocarcinoma MCF-7 cells by
modulating ERK and Akt phosphorylationJ Agric Food
Chem561057410581200810.1021/jf802125t18959417
|
|
11.
|
EJ LeeGS MoonWS ChoiWJ KimSK
MoonNaringin-induced p21WAF1-mediated G(1)-phase cell cycle arrest
via activation of the Ras/Raf/ERK signaling pathway in vascular
smooth muscle cellsFood Chem
Toxicol4638003807200810.1016/j.fct.2008.10.00218951945
|
|
12.
|
SJ LeeHM KimYH ChoK ParkEJ KimKH
jungAqueous extract of Magnolia officinalis mediates
proliferative capacity, p21WAF1 expression and TNF-alpha-induced
NF-kappaB activity in human urinary bladder cancer 5637 cells;
involvement of p38 MAP kinaseOncol Rep187297362007
|
|
13.
|
I OlazabalJ MunozS OguetaE ObregonJP
Garcia-RuizProlactin (PRL)-PRL receptor system increases cell
proliferation involving JNK (c-jun amino terminal kinase) and AP-1
activation: inhibition by glucocorticoidsMol
Endocrinol14564575200010.1210/mend.14.4.044210770493
|
|
14.
|
KL SchwertfegerS HunterLE HeasleyV
LevresseRP LeonJ DeGregoriSM AndersonProlactin stimulates
activation of c-jun N-terminal kinase (JNK)Mol
Endocrinol1415921602200010.1210/mend.14.10.053611043575
|
|
15.
|
JM GonzalezA Navarro-PucheB CasarP CrespoV
AndresFast regulation of AP-1 activity through interaction of lamin
A/C, ERK1/2, and c-fos at the nuclear envelopeJ Cell
Biol183653666200810.1083/jcb.20080504919015316
|
|
16.
|
M RoyuelaG Rodriguez-BerrigueteB FraileR
PaniaguaTNF-alpha/IL-1/NF-kappaB transduction pathway in human
cancer prostateHistol Histopathol2312791290200818712680
|
|
17.
|
W WuYH ChenE UedaD TanP BartoliniAM
WalkerDifferent forms of prolactin have opposing effects on the
expression of cell cycle regulatory proteins in differentiated
mammary epithelial cellsOncol Res167584200616898268
|