Introduction
Hepatoid carcinomas are a rare group of tumors
resembling hepatocellular carcinoma; however, they arise outside
the liver and may be identified in the lung, stomach, kidneys,
ovary, pancreas, urinary bladder and renal pelvis (1). They demonstrate hepatic
differentiation and produce α-fetoprotein (AFP). Hepatoid carcinoma
of the ovary (HCO) was first reported in 1987 by Ishikura and
Scully (2). They described five
cases of ovarian carcinoma with hepatic differentiation, of which
three were confirmed to be primary and two were suspected to be
primary. Most patients are postmenopausal with non-specific signs
and symptoms.
Here, we report a case of HCO in a 55-year-old
female, and summarize the characteristics, therapies and prognosis
of cases that have been previously reported. The study was approved
by the Ethics Committee of Second Xiangya Hospital, Central South
University, Changsha, China. Informed consent was obtained from the
patient.
Case report
A 55-year-old postmenopausal female, G2P2, presented
with lower abdominal pain, abdominal distention and increasing
abdominal girth for approximately two months. The patient had no
familial history of inherited disease. On physical examination the
patient had abdominal distention, left upper and lower quadrant
pain, and no hepatosplenomegaly or lymphadenopathy. A left ovarian
tumor approximately 11 cm in diameter was palpable. Biochemical
examinations revealed normal renal and liver functions, an elevated
α-fetoprotein (AFP) level of 248.84 ng/ml (normal <20 ng/ml), an
elevated cancer antigen (CA)-125 level of 168.27 KU/l (normal
<35 KU/l), and normal carcinoembryonic antigen (CEA) and β-human
chorionic gonadotropin (β-HCG) levels. An X-ray examination of the
chest revealed a blunt bilateral costophrenic angle. A computed
tomography (CT) scan revealed massive ascites and omental and
peritoneal implants, and the liver, gallbladder, pancreas, spleen,
kidneys and adrenal glands were all normal in size and texture.
Upon primary diagnosis of stage IIIC poorly differentiated
adenocarcinoma, the patient underwent an exploratory laparotomy
with total hysterectomy, bilateral salpingo-oophorectomy,
omentectomy and tumor debulking. The ascites were approximately 3
liters in volume. Numerous tumor nodules were identified on the
pelvic peritoneum, omentum majus and mesentery, with the largest
nodule approximately 5x6 cm in diameter. No abnormalities were
observed in the liver, spleen, kidneys, stomach or diaphragm. The
left ovary had enlarged to 11x8x7 cm, and the cut surface was solid
and red-yellow in color with focal hemorrhaging and necrosis.
Nitrogen mustard (2%) was used to wash the abdominal cavity and
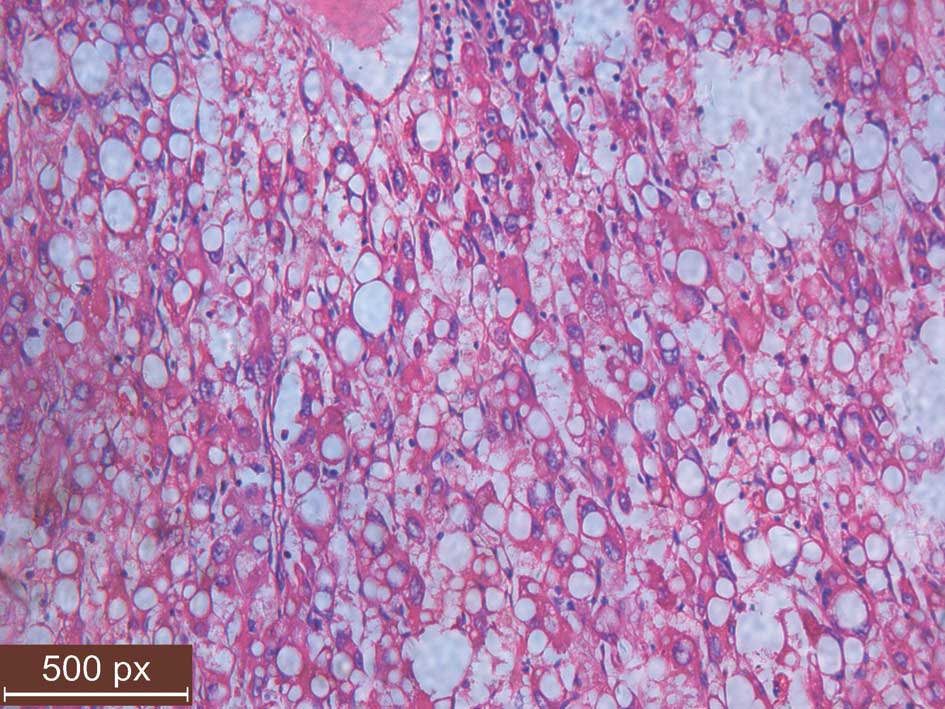
kill the residual cancer cells. Microscopically, the tumor cells,
which were uniform in size and shape, contained abundant amounts of
eosinophilic cytoplasm and had distinct cell borders resembling
hepatocellular carcinoma (Fig. 1).
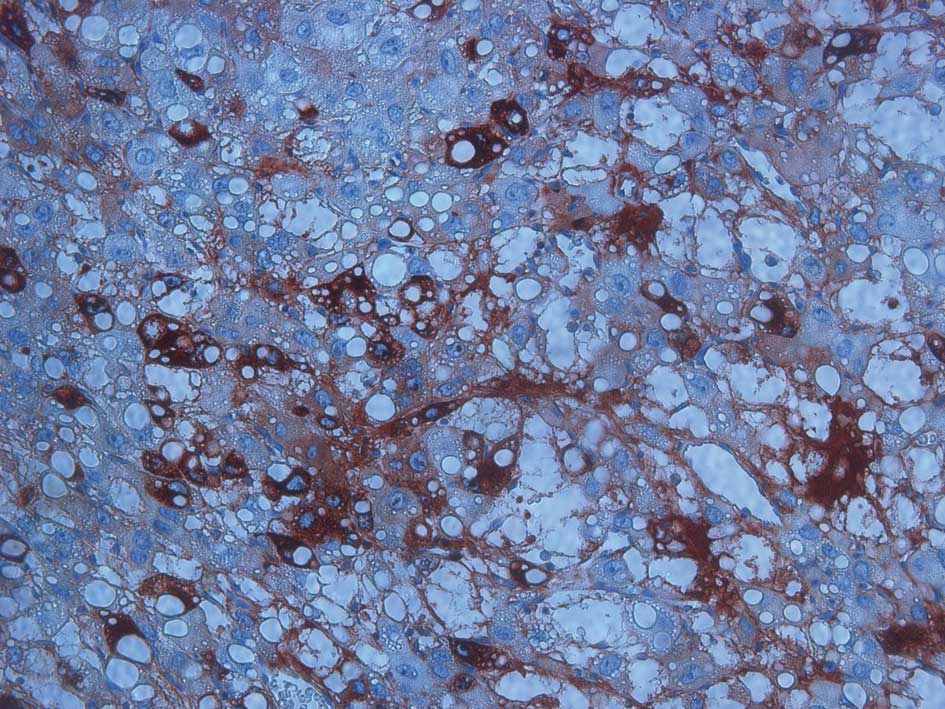
Immunohistochemical staining revealed that AFP (Fig. 2), cytokeratin (CK) and CK18 were
positive, while α-inhibin, CK5, CK6, CD30, PLAP, HPC, CA-125, PR,
ER and OCT3/4 were negative.
On postoperative day 6 an X-ray examination of the
chest was conducted and compared with the X-ray taken prior to the
surgery. An irregular shaped high-density mass approximately 4 cm
in length was identified under the left diaphragm. Upon diagnosis
of a metastatic tumor using needle puncture biopsy, the patient was
administered chemotherapy (docetaxel 120 mg and nedaplatin 80 mg)
on postoperative day 10. The patient received six cycles of the
same chemotherapy regimen for 10 months, and to date, no signs of
recurrence have been observed.
Discussion
HCO is a rare ovarian malignant tumor whose features
resemble hepatocellular carcinoma. The two basic diagnostic
criteria are abundant eosinophilic cytoplasm of the tumor cells
that resemble hepatocellular carcinoma, and positive
immunohistochemical AFP staining (2). HCO has been known for 25 years, but to
date, only 24 cases have been reported (1–19). The
clinical features are listed in Table
I and II.
 | Table IClinical features of HCO. |
Table I
Clinical features of HCO.
| Case No. | Age (years) | Signs and
symptoms | Site | Size (cm) | AFP (ng/ml) | CA-125 (U/ml) | FIGO stage | Outcome (follow-up
time) | Reference |
|---|
| 1 | 42 | Pelvic
peritonitis | B | L, 6.4; R, 5.4 | ND | ND | IIB | Died (5 years) | 2 |
| 2 | 71 | Abdominal
distension | L | 20 | ND | ND | III | Alive (2
years) | 2 |
| 3 | 57 | Abdominal
distension bloating, weight loss | R | 10.5x7.5x5.5 | ND | ND | III | Died (4
months) | 2 |
| 4 | 78 | Abdominal
distension, cramping | L | ND | 2420 | ND | III | Dies (8
months) | 2 |
| 5 | 68 | Abdominal pain and
a pelvic mass | R | 10x6x5 | ND | ND | III | Died (10
months) | 2 |
| 6 | 64 | Lower abdominal
mass | R | 18x17x16 | 23170 | 57.5 | IA | Alive (2
years) | 3 |
| 7 | 62 | Lower abdominal
pain | R | 8.2x7.8x6.4 | 2450 | ND | IA | Died (13
months) | 4 |
| 8 | 52 | ND | B | ND | 2500 | Elevated | III | Recurred (7
months) | 5 |
| 9 | 72 | Abdominal
distension, dyspnea, lethargy | B | L, 9.5; R, 5.4 | ND | 802 | III | Recurred (6
months) | 6 |
| 10 | 35 | ND | L | 35x30 | MS, 358; AF,
3250 | Normal | IIIA | Died (22
months) | 7 |
| 11 | 61 | Abdominal
distension | L | 12x9 | 73080 | 79.7 | III | Died (20
months) | 8 |
| 12 | 64 | Lower abdominal
pain, pelvic mass | R | 23x17x16 | 900 | 52.7 | IIIC | Recurred (18
months); Died (5 years) | 9 |
| 13 | 36 | Lower abdominal
pain | L | 10x8x8 | ND | 888 | IIIC | ND | 10 |
| 14 | 69 | Vaginal bleeding,
left lower quadrant mass | L | 12 | 589.5 | 11 | IA | ND | 11 |
| 15 | 53 | Ovarian mass | L | 10 | 257522 | Normal | IIB | Alive (13
months) | 11 |
| 16 | 76 | Ovarian mass | L | 16 | 24000 | ND | IIB | Alive (4
years) | 11 |
| 17 | 57 | Lower abdominal
pain | R | 13x9x8 | 24879 | ND | ND | Alive (3
years) | 12 |
| 18 | 63 | Vaginal bleeding,
lower abdominal pain | R | 16x12 | 454 | 84.59 | IA | Alive (10
months) | 13 |
| 19 | 40 | Lower abdominal
distension, amenorrhea | R | 11x9.5x3 | 32338 | 1297 | III | Alive (6
months) | 14 |
| 20 | 42 | Abdominal pain | R | 17x6 | 600 | ND | IA | Died (16
months) | 1 |
| 21 | 65 | Abdominal
distension, anorexia, progressive dyspnea | R | 12x10x6 | 329732.4 | 401.91 | III | ND | 15 |
| 22 | 42 | Abdominal pain | L | 11x7x7 | ND | 70 | ND | ND | 16 |
| 23 | 42 | Abdominal pain,
pelvic mass | L | 6x4x3 | ND | ND | I | ND | 17 |
| 24 | 46 | Weight loss,
abdominal pain, increasing abdominal girth | B | L, 4.5; R, 6.5 | <30,000 | 414 | III | ND | 18 |
| 25 | 55 | Lower abdominal
pain, abdominal distention, increasing abdominal girth | L | 11x8x7 | 248.84 | 168.27 | IIIC | Alive (now) | Present case |
 | Table IIPathological characteristics of
HCO. |
Table II
Pathological characteristics of
HCO.
|
Characteristics | Positive/total
cases |
|---|
| Hyaline
globule | 18/18 |
| AFP | 25/25 |
| Bile | 4/9 |
| α1-AT | 10/11 |
| α2-ACT | 7/7 |
| CEA | 12/17 |
| CA-125 | 5/10 |
| CK | 1/2 |
| CK7 | 3/6 |
| CK18 | 2/2 |
| CK19 | 2/8 |
| CK20 | 1/8 |
| Hep Par 1 | 4/4 |
| α-inhibin | 0/6 |
The origin of HCO is unknown. The majority of
patients are postmenopausal females; however, it may also affect
females of reproductive age and it has been identified in patients
between the ages of 35 and 78 years. The most common signs and
symptoms of HCO are abdominal distension, abdominal pain and pelvic
mass. All patients reported in this study presented with an
elevated serum AFP (16/16) level and the majority of patients had
an elevated CA-125 (12/15) level. HCO cases may occur alone or in
combination with other types of ovarian tumor, including serous
papillary carcinomas (6),
mucinous/serous/endometrioid denocarcinomas (11) or Sertoli-type sex cord-stromal
tumors (17). The tumor
differentiates poorly and progresses rapidly. When diag-nosed, the
FIGO stage is usually advanced. Of the reported cases, 14 cases
were stage III, three were stage II B, six were stage I and the
remaining two were of unknown stage. Patients usually succumb to
the disease within 2 years (1).
The tumor may appear solid, or solid with cystic
areas and multiple foci of hemorrhaging and necrosis.
Microscopically, HCO tumor cells are usually uniform in size and
shape with distinct borders. They also consist of a moderate to
abundant eosinophilic cytoplasm, centrally located nuclei arranged
in sheets, nests or trabeculae, and cytoplasm periodic acid-Schiff
(PAS)-positive hyaline globules, while bile canalicular structures
are rare. In previous studies, HCO revealed PAS-positive
diastase-resistant hyaline globules to varying degrees in each of
the 18 cases described. Among the nine patients that were examined
for bile canalicular structures, four revealed positive results;
therefore, we do not consider this to be a rare characteristic of
HCO. Electron microscopy identified abundant mitochondria that
possess lamella cristae with one or more electron-dense matrix
granule, distended smooth endoplasmic reticulum-formed vesicles, a
tight junction with few desmosomes and no microvilli, a rudimentary
canaliculi, occasional lipid droplets and an inconspicuous golgi
body and rough endoplasmic reticulum (14). During immunostaining, AFP, which is
the hallmark of the tumor, was positive in all cases. The existence
of CEA (1,2,8,10–13,15,16),
α1-AT (1,2,8,10,15–17)
and α1-ACT (2,8,13) was
demonstrated. CK18, which is positive in normal hepatocytes, bile
duct epithelium and hepatocellular carcinoma, is also focally
positive in HCO. CK19 and CK20, which are commonly positive in
normal gastrointestinal epithelial cells, bile duct cells and
certain adenocarcinomas, including ovarian surface epithelial
carcinomas, but negative in normal hepatocytes and hepatocellular
carcinomas, may occasionally be positive in HCO. CK7 was positive
in certain cases (8,12,14),
and a diffusely positive staining for CK7 is considered to be
highly consistent with HCO (14).
The expression profiles of CK reflect the origin of HCO (11), and the degree of staining of
hepatocyte paraffin (Hep Par) 1 may be related to the
differentiation of the tumor.
The most effective way to manage HCO remains
unknown. In almost all cases in this report, the patients received
a hysterectomy, bilateral salpingo-oophorectomy, with or without
lymph node dissection, and/or omentectomy. The majority of patients
received postoperative chemotherapy with or without irradiation,
but the prognosis is poor. A total of seven (1,2,4,7,8)
out of nine patients were followed up until mortality; 7 succumbed
within two years, while the other two patients (2,9)
succumbed within five years. Pandey et al (18) reported that sorfenib, a first line
drug for hepatocellular carcinoma, administered postoperatively,
had no effect on HCO therapy.
Firstly, HCO must be distinguished from hepatoid
yolk sac tumors (HYST), a tumor with hepatoid differentiation and
regarded as a germ cell tumor of yolk sac type. HYST (16) occurs mainly in the younger
population (range, 7–54 years; average, 22 years) and is associated
with gonadal dysgenesis and dysgerminoma. HCO usually occurs in the
older population (range, 35–78 years; average, 56 years) and is
associated with normal gonad development. Immunostaining revealed
that HYST is negative for Hep Par 1 and focally positive for
polyclonal CEA (pCEA), while HCO is positive for Hep Par 1 and
diffusely positive for pCEA. HCO also needs to be distinguished
from other ovarian tumors, including undifferentiated carcinomas,
steroid cell tumors, clear cell carcinomas and endometrioid
carcinomas. Although they may possess features resembling HCO, none
of these tumors demonstrate AFP staining except in one case of
endometrioid carcinoma where transformation of an endometrioid
carcinoma to a yolk sac tumor occurred (19). Ovarian metastatic hepatocellular
carcinoma should also be excluded, although to date there is no
means of distinguishing between the two tumors (14).
Our patient presented with HCO in middle age. The
lack of lesions in the liver made the diagnosis of ovarian
metastatic hepatocellular carcinoma unlikely. Positivity for AFP
excluded the diagnosis of undifferentiated carcinomas, steroid cell
tumors, clear cell carcinomas and endometrioid carcinomas. The
elevated serum CA-125 levels, common epithelial origin of hepatoid
carcinomas, lack of gonadal dysgenesis or dysgerminoma, and
positive Hep Par 1 ruled out a diagnosis of HYST. A short time
after surgery, the tumor metastasized to the left diaphragm. The
reasons for this are unknown as this has not been previously
observed. We decided to administer chemotherapy on postoperative
day 10, by which time the condition of the patient had improved
from the surgery. An early start of therapy was beneficial to
treatment, and at present, the patient lives a relatively healthy
life.
In conclusion, HCO is a highly malignant tumor that
mainly affects elderly females and progresses rapidly. The signs
and symptoms are non-specific, and the treatments, which include
surgery, chemotherapy and radiotherapy, are similar to those of
other ovarian carcinomas. With these therapies, patients may live a
longer life, but most of them succumb within 2 years. Therefore, a
new therapeutic method should be identified. The use of nitrogen
mustard during the surgery and the early start of postoperative
therapy may prolong survival and improve quality of life.
References
|
1.
|
J LazaroD RubioM RepollesL CapoteHepatoid
carcinoma of the ovary and managementActa Obstet Gynecol
Scand86498499200710.1080/0001634060059311717486476
|
|
2.
|
H IshikuraRE ScullyHepatoid carcinoma of
the ovary. A newly described
tumorCancer6027752784198710.1002/1097-0142(19871201)60:11%3C2775::AID-CNCR2820601130%3E3.0.CO;2-S2445465
|
|
3.
|
M MatsutaH IshikuraK MurakamiT KagabuI
NishiyaHepatoid carcinoma of the ovary: a case reportInt J Gynecol
Pathol10302310199110.1097/00004347-199107000-000091717389
|
|
4.
|
K TamakoshiJ HorioT OkamotoK SakakibaraS
HattoriA case report of hepatoid carcinoma of the ovaryNihon Sanka
Fujinka Gakkai Zasshi454794811993(In Japanese)
|
|
5.
|
J BadreddineY RabouilleJF HeronAM
MandardOvarian tumor with hepatoid differentiation. Discussion and
review of the literature. Report of a caseAnn Pathol1337391993(In
French)
|
|
6.
|
JP ScurryRW BrownT JoblingCombined ovarian
serous papillary and hepatoid carcinomaGynecol
Oncol63138142199610.1006/gyno.1996.02938898184
|
|
7.
|
E MaymonB PiuraM MazorA BashiriT
SilbersteinI Yanai-InbarPrimary hepatoid carcinoma of ovary in
pregnancyAm J Obstet
Gynecol179820822199810.1016/S0002-9378(98)70092-49757999
|
|
8.
|
H SenzakiY KiyozukaH MizuokaAn autopsy
case of hepatoid carcinoma of the ovary with PIVKA-II production:
immunohistochemical study and literature reviewPathol
Int49164169199910.1046/j.1440-1827.1999.00840.x10355972
|
|
9.
|
CH LeeKG HuangSH UengH SweiHY ChuehCH LaiA
hepatoid carcinoma of the ovaryActa Obstet Gynecol
Scand8110801082200210.1034/j.1600-0412.2002.811115.x12421179
|
|
10.
|
Y WatanabeM UmemotoH UedaH NakaiH HoshiaiK
NodaCytopathologic and clinicopathologic features of ovarian
hepatoid carcinoma. A case reportActa
Cytol477882200310.1159/00032647912585035
|
|
11.
|
N TochigiT KishimotoY SupriatnaY NagaiT
NikaidoH IshikuraHepatoid carcinoma of the ovary: a report of three
cases admixed with a common surface epithelial carcinomaInt J
Gynecol
Pathol22266271200310.1097/01.PGP.0000055173.04957.6612819394
|
|
12.
|
JS TsungPS YangHepatoid carcinoma of the
ovary: characteristics of its immunoreactivity. A case reportEur J
Gynaecol Oncol25745748200415597858
|
|
13.
|
S YigitMA UyarogluZ KusN EkinciO
OztekinHepatoid carcinoma of the ovary: immunohistochemical finding
of one case and literature reviewInt J Gynecol
Cancer1614391441200610.1111/j.1525-1438.2006.00564.x16803543
|
|
14.
|
JE KwonSH KimNH ChoNo ancillary finding is
valid to distinguish a primary ovarian hepatoid carcinoma from
metastatic hepatocellular carcinomaInt J Gynecol
Cancer1616911694200610.1111/j.1525-1438.2006.00646.x16884387
|
|
15.
|
E Tejerina GonzalezM ArguellesJA
Jimenez-HeffernanP DhimesB VicandiF PinedoCytologic features of
hepatoid carcinoma of the ovary: a case report with immunocytologic
evaluation of HepPar1Acta Cytol52490494200818702372
|
|
16.
|
A Zizi-SermpetzoglouN PetrakopoulouME
NikolaidouN TepelenisV SavvaidouT VasilakakiHepatoid carcinoma of
the ovary. A case report and review of the literatureEur J Gynaecol
Oncol303413432009
|
|
17.
|
A D’AntonioG De DominicisM AddessoA CaleoA
BoscainoHepatoid carcinoma of the ovary with sex cord stromal
tumor: a previously unrecognized associationArch Gynecol
Obstet281765768201019856182
|
|
18.
|
M PandeyC TruicaHepatoid carcinoma of the
ovaryJ Clin Oncol29e446e448201110.1200/JCO.2010.33.632121422440
|
|
19.
|
JL RutgersRH YoungRE ScullyOvarian yolk
sac tumor arising from an endometrioid carcinomaHum
Pathol1812961299198710.1016/S0046-8177(87)80418-53679203
|