Introduction
S-1 is an oral anticancer preparation composed of a
mixture of tegafur [FT, a prodrug of 5-fluorouracil (5-FU)],
5-chloro-2,4-dihydroxypyridine (CDHP, a biochemical modulator that
inhibits 5-FU biodegradation) and potassium oxonate (Oxo, added to
reduce the gastrointestinal toxicity of 5-FU) (1–3). In
the two registration phase II studies in Japan, the rate of
response to treatment with S-1 alone exceeded 40% in patients with
advanced or recurrent gastric cancer (4,5). The
Japan Clinical Oncology Group (JCOG) conducted a randomized
prospective controlled study to evaluate the efficacy of
single-agent S-1 as adjuvant therapy for patients with stage II/III
(Japanese Classification of Gastric Carcinoma, JCGC) (6) gastric cancer following curative D2
dissection (7). When the final
analysis was performed in September 2006, 3-year overall survival
(OS) was 80.5% for S-1 treated patients and 70.1% for patients who
underwent surgery alone. The hazard ratio for death in S-1 treated
patients was 0.68 (P=0.0024). The results of this trial
demonstrated that adjuvant chemotherapy with S-1 for stage II/III
gastric cancer is effective and suggested that this therapy should
be adopted as the standard treatment following curative D2 gastric
dissection (8).
To investigate the tolerability of adjuvant
chemotherapy with S-1 for stage II/III gastric cancer following
curative D2 dissection, we reviewed treatment outcomes in patients
receiving this adjuvant therapy.
Materials and methods
Patients
Between August 2007 and July 2010, 283 patients
underwent gastrectomy for adenocarcinoma of the stomach with
curative intent at the National Defense Medical College Hospital
(Tokorozawa, Saitama, Japan). Of these, 64 patients (41–84 years
old) had pathological stage II/III disease according to the JCGC
(6). All patients were informed of
the efficacy of the adjuvant chemotherapy trial of S-1 for gastric
cancer (ACTS-GC) and provided their consent to the study (7).
Treatment regimen
S-1 was orally administered twice daily for 4 weeks,
followed by a 2-week rest. This schedule was repeated every 6 weeks
for 12 months until tumor recurrence, observation of unacceptable
toxicity levels or refusal by the patient to undergo further
treatment. Dosages were assigned according to the patient body
surface area: <1.25 m2, 80 mg/day; 1.25–1.5
m2, 100 mg/day; and ≥1.5 m2, 120 mg/day.
Dosage modification and treatment interruption were performed
according to the protocol in the registration trial (5,7). The
dose or treatment schedule was modified at the physician’s
discretion according to the toxicity profiles. In principle, if
patients had hematological toxic effects of grade 3 or 4 or
non-hematological toxic effects of ≥ grade 2, their daily dose was
reduced and/or their schedule was changed from a 4-week
administration followed by a 2-week rest, to a 2-week
administration followed by a 1-week rest.
Measures
If no gross residual disease was evident at the time
of surgery and the resection margins were tumor-free on
histological examination, surgery was considered curative.
Pathological findings in gastric cancer patients were described on
the basis of the JCGC (6). Adverse
reactions were evaluated according to the common toxicity criteria
of the National Cancer Institute, version 3.0 (http://ctep.cancer.gov).
OS was measured from the date of resection to the
date of mortality from any cause. Relapse-free survival was
measured from the date of resection to the date when relapse was
evident by computed tomography, gastrointestinal endoscopic
examination, abdominal ultrasonography, upper gastrointestinal
series and/or positron emission tomography. Data for the patients
who survived were censored in our survival analyses. The medication
completion rate was measured from the date of treatment
commencement to the date of treatment discontinuation. Data for
patients in whom S-1 treatment was discontinued due to tumor
recurrence or mortality were censored in this analysis. All
patients were observed at our hospital or outpatient clinic at 2-
to 4-week intervals up to 12 months after surgery, 3- to 4-month
intervals during the 2 years of the study and every 6 or 12 months
thereafter for 3 years.
Statistical analysis
Statistical calculations were performed using
StatView version 5.0 (SAS Institute, Inc., Cary, NC, USA). Data are
expressed as the means ± SEM. Statistical analyses were performed
using the Mann-Whitney U test or Chi-square test with Fisher’s
exact test, as appropriate. Survival and medication completion
rates were calculated using the Kaplan-Meier method and the
significance of the difference was determined by a log-rank test.
P<0.05 was considered to indicate a statistically significant
result.
Results
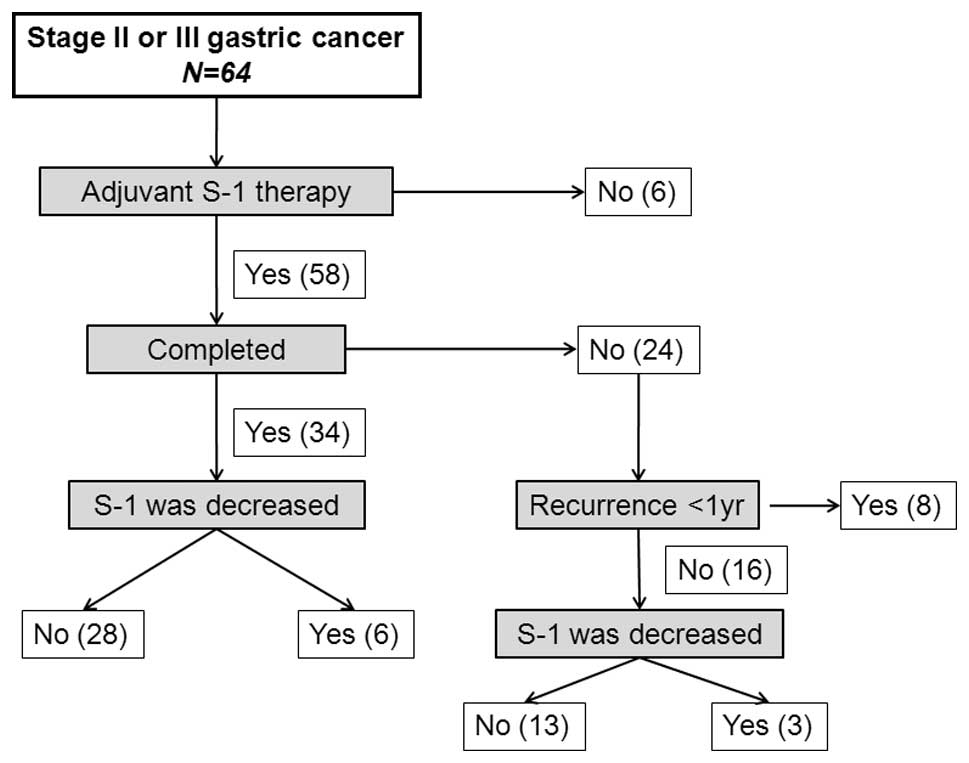
Of the 64 patients included in the study, 6 refused
adjuvant therapy with S-1 due to age (n=4) or financial concerns
(n=2). The remaining 58 patients received S-1 within 8 weeks of
surgery (Fig. 1). Twenty-four
patients (41.3%) discontinued treatment within 12 months as a
result of disease relapse (n=8) and intolerable adverse events
(n=16). The S-1 dose was decreased in 9 of the 58 patients (15.5%).
Of the 34 patients who underwent treatment for 12 months, the S-1
dose was decreased in 6 (17.6%), and of the 24 patients who
discontinued treatment, the S-1 dose was decreased in 3 (12.5%).
Among the 58 patients who received S-1 therapy, treatment was
continued for at least 3 months in 49 patients (84.5%), at least 6
months in 45 patients (77.6%), at least 9 months in 37 patients
(63.8%) and 12 months in 34 patients (58.6%).
Demographic and clinicopathological data of patients
are shown in Table I. Patients who
discontinued S-1 treatment within 12 months were older than those
who completed 12 months of adjuvant therapy. However, no
differences were observed in tumor stage and surgery type
(gastrectomy, reconstruction or resection of other organs) between
the two groups. Patients who completed 12 months of adjuvant
therapy with S-1 were more frequently treated by senior doctors
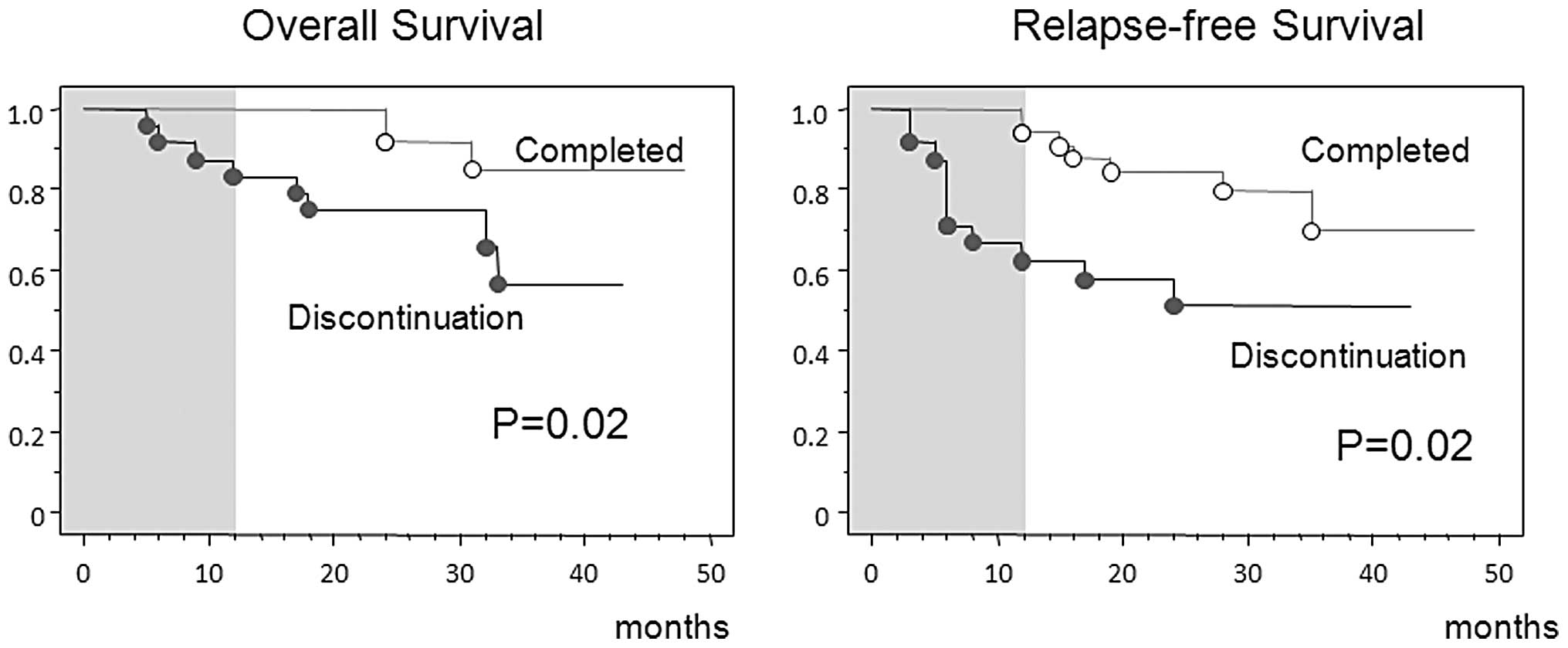
(>15 years of experience). More favorable outcomes in OS and
relapse-free survival were observed in these patients than in those
who discontinued treatment (Fig.
2).
 | Table IDemographic and clinicopathological
data. |
Table I
Demographic and clinicopathological
data.
| Clinicopathological
data | Total | Completed | Discontinued | P-value |
|---|
| Number of
patients | 58 | 34 | 24 | |
| Age (years) | 63.4±8.0 | 61.4±83.4 | 66.3±6.5 | 0.02 |
| Gender | | | | |
| Male | 43 (74.1%) | 24 (70.6%) | 19 (79.2%) | 0.42 |
| Female | 15 (25.9%) | 10 (29.4%) | 5 (20.8%) | |
| Histological
classification | | | | |
| Intestinal | 23 (39.7%) | 12 (35.3%) | 11 (45.8%) | 0.52 |
| Diffuse | 32 (55.2%) | 19 (55.9%) | 13 (54.2%) | |
| Adenosquamous | 2 (3.4%) | 2 (5.9%) | 0 (0.0%) | |
| Tumor depth | | | | |
| T2 | 24 (41.4%) | 15 (44.1%) | 9 (37.5%) | 0.83 |
| T3 | 32 (55.2%) | 18 (52.9%) | 14 (58.3%) | |
| T4 | 2 (3.4%) | 1 (2.9%) | 1 (4.2%) | |
| Lymph node
metastasis | | | | |
| N0 | 10 (17.2%) | 6 (17.6%) | 4 (16.7%) | 0.85 |
| N1 | 27 (46.6%) | 15 (44.1%) | 12 (50.0%) | |
| N2 | 21 (36.2%) | 13 (38.2%) | 8 (33.3%) | |
| Stage | | | | |
| II | 25 (43.1%) | 15 (44.1%) | 10 (41.7%) | 0.18 |
| IIIA | 18 (31.0%) | 8 (23.5%) | 10 (41.7%) | |
| IIIB | 15 (25.9%) | 11 (32.4%) | 4 (16.7%) | |
| Type of
gastrectomy | | | | |
| Total | 30 (51.7%) | 20 (58.8%) | 10 (41.7%) | 0.24 |
| Distal | 28 (48.3%) | 14 (41.2%) | 14 (58.3%) | |
| Reconstruction | | | | |
| Billroth I | 22 (37.9%) | 11 (32.4%) | 11 (45.8%) | 0.48 |
| Billroth II | 3 (5.2%) | 2 (5.9%) | 1 (4.2%) | |
| Roux en Y | 33 (56.9%) | 21 (61.8%) | 12 (50.0%) | |
|
Cholecystectomy | | | | |
| Yes | 24 (41.4%) | 14 (41.2%) | 10 (41.7%) | 0.95 |
| No | 34 (58.6%) | 19 (55.9%) | 14 (58.3%) | |
| Splenectomy | | | | |
| Yes | 20 (34.5%) | 12 (35.3%) | 8 (33.3%) | 0.81 |
| No | 38 (65.5%) | 22 (64.7%) | 16 (66.7%) | |
| Doctor in
charge | | | | |
| Junior (≤15
yrs) | 25 (43.1%) | 11 (32.4%) | 14 (58.3%) | 0.04 |
| Senior (>15
yrs) | 33 (56.9%) | 23 (67.6%) | 10 (41.7%) | |
| Total amount of S-1
(mg) | 16495.4±8851.9 | 23146.7±3335.6 | 7350.0±4954.9 | <0.0001 |
Table II summarizes
the data concerning the adverse reactions observed among the 58
patients in this study. No patient had ≥ grade 4 adverse events;
however, 3 patients had grade 3 leukopenia. In terms of
non-hematological adverse events, grade 3 anorexia was observed in
1 patient and grade 3 diarrhea was observed in 1 patient. The most
frequent cause of S-1 treatment discontinuation was tumor
recurrence. Non-hematological adverse events such as diarrhea and
nausea were also associated with treatment discontinuation
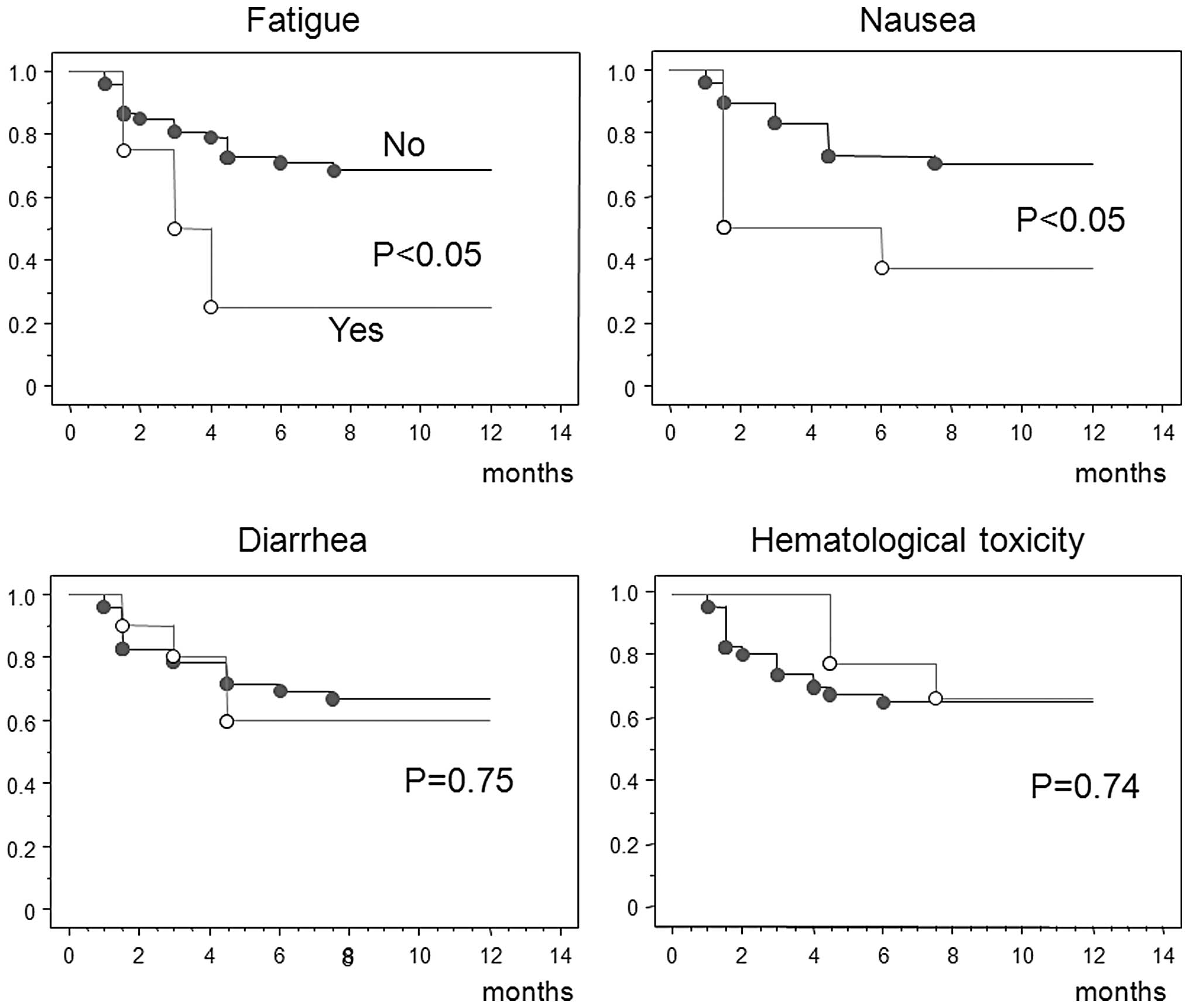
(Table III). Fig. 3 shows the medication completion
rates. S-1 treatment time was significantly shorter in patients who
experienced fatigue or nausea as an adverse event, whereas diarrhea
and hematological toxicity did not significantly affect the period
of treatment.
 | Table IIAdverse reactions to adjuvant therapy
with S-1 among the 58 patients included in this study. |
Table II
Adverse reactions to adjuvant therapy
with S-1 among the 58 patients included in this study.
| No. of patients
| Percentage (%)
|
|---|
| Adverse
reaction | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 or 2 | Grade 3 or 4 |
|---|
| Leukopenia | 5 | 3 | 3 | - | 14.0 | 5.3 |
| Anemia | 26 | 3 | - | - | 50.9 | 0 |
| Elevated t-bil
level | 4 | 2 | - | - | 10.5 | 0 |
| Stomatitis | 5 | 2 | - | - | 12.3 | 0 |
| Anorexia | 20 | 1 | 1 | - | 36.8 | 1.8 |
| Nausea | 6 | 2 | - | - | 14.0 | 0 |
| Diarrhea | 8 | 6 | 1 | - | 24.1 | 1.8 |
| Skin lesions | 8 | 1 | - | - | 15.5 | 0 |
| Fatigue | 8 | 3 | - | - | 19.3 | 0 |
| Watering or dry
eye | 7 | 6 | - | - | 22.8 | 0 |
 | Table IIIChief causes of S-1 treatment
discontinuation. |
Table III
Chief causes of S-1 treatment
discontinuation.
| Adverse
reaction | No. of
patients | Percentage (%) |
|---|
| Recurrence | 8 | 33.3 |
| Diarrhea | 3 | 12.5 |
| Nausea | 3 | 12.5 |
| Elevated t-bil
level | 2 | 8.3 |
| Intestinal
obstruction | 2 | 8.3 |
| Fatigue | 2 | 8.3 |
| Neutropenia | 1 | 4.2 |
| Ascites | 1 | 4.2 |
| Stroke | 1 | 4.2 |
| Death of another
cause | 1 | 4.2 |
| Total | 24 | 100.0 |
Discussion
This study demonstrated that patients who completed
12 months of adjuvant therapy with S-1 were younger and more
frequently treated by doctors with >15 years of experience than
those who did not. Non-hematological adverse events such as nausea
and fatigue were frequent causes of S-1 treatment discontinuation.
Adverse events of ≥ grade 3 were significant causes of treatment
discontinuation in a small number of patients.
In a postmarketing survey of S-1 (9), including 3,294 patients with advanced
or recurrent gastric cancer, the incidence of adverse reactions
following administration of the drug at the usual dose level
according to the patient body surface area was 74.1%, which was
approximately equal to that obtained in premarketing trials. The
major reasons for drug discontinuation during the first and second
course of therapy were exacerbation of symptoms (43%) and adverse
drug reactions (33%). Therefore, to facilitate S-1 administration
for prolonged time periods, the incidence of adverse reactions
should be reduced. To accomplish this goal, several regimens have
been established (10). Kimura
et al developed a new S-1 dosing regimen in which S-1 is
administered for a 2-week period separated by 1-week drug-free
intervals, as adverse reactions due to S-1 therapy begin to appear
2–3 weeks after initial dosing (11). Sakuma et al also proposed
alternate-day treatment with S-1 as a strategy for reducing
toxicity, although the total dose of this regimen was 75% that of
standard treatment (12). Both
regimens decreased the incidence of adverse reactions and improved
treatment compliance when compared with the conventional 4-week
administration followed by a 2-week rest regimen.
In the ACTS-GC trial, 143 of 517 (27.7%) patients
discontinued S-1 treatment due to adverse events, which was
consistent with our results (27.6%). Only 5% patients in the
ACTS-GC trial had metastasis or relapse of gastric cancer. Our
study, which includes potentially more cases of advanced stage
disease than the ACTS-GC trial, involved relatively shorter time
periods of the treatment than the ACTS-GC trial.
Patients who experienced fatigue or nausea as
adverse events continued S-1 treatment for significantly shorter
time periods. However, diarrhea and hematological toxicity did not
significantly affect the treatment period. Following gastrectomy,
fatigue and gastrointestinal symptoms such as nausea and appetite
loss, even of ≤ grade 2, appeared to have a major impact on
treatment compliance.
In conclusion, the completion rate of S-1 treatment
did not depend on the type of surgical procedures, i.e.,
gastrectomy, reconstruction or resection of other organs. Fatigue
and gastrointestinal symptoms affected the period of treatment
continuation. In addition, patients who completed 12 months of
adjuvant therapy with S-1 were more frequently treated by doctors
with ≥15 years of experience. Thus, to facilitate the continuation
of adjuvant therapy with S-1, patients and doctors must be made
completely aware of the issues of toxicity, compliance and efficacy
associated with this therapy.
References
|
1.
|
T ShirasakaK NakanoT TakechiH SatakeJ
UchidaA FujiokaAntitumor activity of 1 M tegafur-0.4 M
5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against
human colon carcinoma orthotopically implanted into nude ratsCancer
Res56260226061996
|
|
2.
|
K TatsumiM FukushimaT ShirasakaS
FujiiInhibitory effects of pyrimidine, barbituric acid and pyridine
derivatives on 5-fluorouracil degradation in rat liver extractsJpn
J Cancer Res7874875519873114201
|
|
3.
|
T ShirasakaY ShimamotoM
FukushimaInhibition by oxonic acid of gastrointestinal toxicity of
5-fluorouracil without loss of its antitumor activity in ratsCancer
Res534004400919937689420
|
|
4.
|
Y SakataA OhtsuN HorikoshiK SugimachiY
MitachiT TaguchiLate phase II study of novel oral fluoropyrimidine
anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat
potassium) in advanced gastric cancer patientsEur J
Cancer3417151720199810.1016/S0959-8049(98)00211-19893658
|
|
5.
|
W KoizumiM KuriharaS NakanoK HasegawaPhase
II study of S-1, a novel oral derivative of 5-fluorouracil, in
advanced gastric cancer. For the S-1 Cooperative Gastric Cancer
Study GroupOncology58191197200010.1159/00001209910765119
|
|
6.
|
Japanese Gastric Cancer
AssociationJapanese Classification of Gastric Carcinoma - 2nd
English EditionGastric
Cancer11024199810.1007/PL0001168111957040
|
|
7.
|
S SakuramotoM SasakoT YamaguchiT
KinoshitaM FujiiA NashimotoAdjuvant chemotherapy for gastric cancer
with S-1, an oral fluoropyrimidineN Engl J
Med35718101820200710.1056/NEJMoa07225217978289
|
|
8.
|
Japanese Gastric Cancer
AssociationJapanese gastric cancer treatment guidelines 2010 (ver.
3)Gastric Cancer14113123201110.1007/s10120-011-0042-421573742
|
|
9.
|
H KawaiA OhtsuN BokuY HamamotoF NagashimaM
MutoEfficacy and safety profile of S-1 in patients with metastatic
gastric cancer in clinical practice: results from a post-marketing
surveyGastric Cancer61923200310.1007/s10120-003-0216-912775015
|
|
10.
|
S IwasaY YamadaT FukagawaT Eguchi
NakajimaK KatoT HamaguchiManagement of adjuvant S-1 therapy after
curative resection of gastric cancer: dose reduction and treatment
schedule modificationGastric
Cancer142834201110.1007/s10120-011-0003-y21327440
|
|
11.
|
Y KimuraN KikkawaS IijimaT KatoY NaoiT
HayashiA new regimen for S-1 therapy aiming at adverse reaction
mitigation and prolonged medication by introducing a 1-week
drug-free interval after each 2-week dosing session: efficacy and
feasibility in clinical practiceGastric
Cancer63439200310.1007/s10120-003-0230-y
|
|
12.
|
K SakumaY HosoyaW AraiH HarutaT UiK
KurashinaAlternate-day treatment with S-1 in patients with gastric
cancer: a retrospective study of strategies for reducing
toxicityInt J Clin
Oncol15166171201010.1007/s10147-010-0036-y20195683
|