Introduction
High incidence of mortality from colorectal cancer
(CRC) in Western countries has prompted much research on CRC within
these countries. In Iran, there has been a significant increase in
CRC cases, particularly among the younger population (1). According to a previous study, there
are an estimated 3,641 new cases of CRC in Iran each year, of which
2,262 (62%) result in mortality (2). Therefore, it is necessary to draw
attention to CRC research in order to improve the diagnosis and
treatment of this type of cancer. Adjuvant chemotherapy following
curative resection is the most common approach to CRC treatment;
however, a decreased sensitivity to chemotherapeutic agents has
been observed in CRC patients who were subjected to previous
surgery (3).
Overexpression of ATP-binding cassette (ABC)
transporter proteins was established to confer drug resistance to
tumor cells (4). Multiple drug
resistance protein 2 (MRP2/ABCC2), a member of the ABC multidrug
transporters, is a polytopic membrane glycoprotein of 190–200 kDa,
which has two ATP-binding domains and 17 transmembrane regions
(5), and was previously named the
canalicular multispecific organic anion transporter (cMOAT). MRP2
is present in the apical membrane of polarized cells within the
liver, kidney and intestine (6) and
plays an important role in in vitro studies into drug
resistance (7,8). It exports a wide spectrum of
substrates using an ATP-dependent mechanism, including the
glucuronide, glutathione and sulfate conjugates of endogenous and
exogenous compounds (9,10).
Glutathione conjugation was identified as one of the
mechanisms for oxaliplatin resistance in CRC (11). The FOLFOX-4 regimen is the main
chemotherapeutic procedure used to treat CRC; it is a combination
of oxaliplatin (a third-generation platinum drug) and
5-fluorouracil/leucovorin (FL). Incorporation of oxalipatin into a
backbone of FL is able to improve the rate of response by 40–50% in
metastatic CRC cases (12). Several
mechanisms contribute to resistance against platinum compounds,
including enhanced DNA repair, decreased drug accumulation, drug
inactivation and enhanced tolerance to platinum-DNA adducts
(13,14). Glutathione conjugation is a
well-known mechanism involved in the detoxification and
inactivation of platinum compounds (15).
The role of the MRP2 gene has also been identified
in cisplatin resistance (16). The
functional inhibition of MRP2 appears to be an effective approach
in overcoming resistance to platinum-based drugs in human melanoma
cells (17). A recent in
vivo study demonstrated the involvement of MRP2 in drug
resistant phenotypes of CRC cell lines (18). However, the role of MRP2 in the
clinical outcome of CRC patients who received platinum-based
therapy remains to be clarified.
In this hospital-based study, we performed
immunohistochemical detection of MRP2 in paraffin-embedded samples
of 50 CRC patients. We investigated the putative association of
MRP2-positivity and early CRC relapse in patients who were treated
with FL and oxaliplatin.
Patients and methods
Study population and chemotherapy
A total of 50 CRC patients (30 males and 20 females;
age range, 17–77 years) who had undergone complete resection of
histologically verified stage II (T2 and T3, N0, M0) or stage III
(any T, N1 and 2, M0) CRC were selected for this study. The
clinical stage and pathological features of primary tumors were
defined according to the criteria of the American Joint Commission
on Cancer/International Union against Cancer (AJCC/UICC) (19). This study examined protein
expression in association with platinum-based drugs; therefore,
patients who had received prior chemotherapy or radiotherapy were
excluded, thus only the patient’s first response to chemotherapy
was analyzed. The clinicopathological features of the patients were
obtained from their medical records. This study was approved by the
Hazrate Rasoul Akram Hospital (Tehran University, Tehran, Iran) and
the Faculty of Medicine and Health Sciences (University Putra
Malaysia, Malaysia).
All patients were treated with 12 cycles of FOLFOX-4
chemotherapy for 6 months. The chemotherapeutic regimen consisted
of oxaliplatin (85 mg/m2) combined with leucovorin (200
mg/m2) and bolus fluorouracil (400 mg/m2) on
day 1, and continuous infusion of fluorouracil (600
mg/m2) on day 2. The cycle was then repeated after a
2-week rest period. Patients received premedication, including
dexamethasone or granisetron, 1 h prior to their treatment.
Clinical response was assessed by measuring carcinoembryonic
antigen (CEA) levels at 3-month intervals for 2 years and at
6-month intervals thereafter. A colonoscopy and CT scan was usually
performed at 6-month intervals in the first 2 years and annually
thereafter; these tests were mandatory following an elevated CEA
level. Development of new recurrent or metastatic lesions following
surgery was considered as relapse and local relapse was
histopathologically/cytologically confirmed by specimen
examination.
Immunohistochemistry
The tumor and matched normal paraffin-embedded
tissues were cut into 4 μm sections, mounted onto slides and
incubated for 30 min at 60°C. Sections were routinely
deparaffinized in xylene and rehydrated in a graded ethanol series.
Endogenous peroxidase activity was blocked by incubation in 0.3%
H2O2 for 30 min. Antigen retrieval was
achieved by heating the tissue sections in an autoclave filled with
sodium citrate buffer for 10 min at 120°C. Following cooling at
room temperature, the sections were rinsed in phosphate-buffered
saline (PBS; pH 7.4), incubated with anti-MRP2 primary antibodies
(M2III-6, ab3373; Abcam, Cambridge, UK) diluted at 1:120 for 1.5 h,
and rinsed in PBS. The sections were subsequently treated with
EnVision+ Dual Link System-HRP secondary antibody (K4061; Dako,
Copenhagen, Denmark) for 45 min and rinsed in PBS. Immunoreactivity
was visualized with 3,3′-diaminobenzidine (Dako). The sections were
counterstained with hematoxylin and mounted. The protein expression
was evaluated by two pathologists, blinded to clinical outcomes.
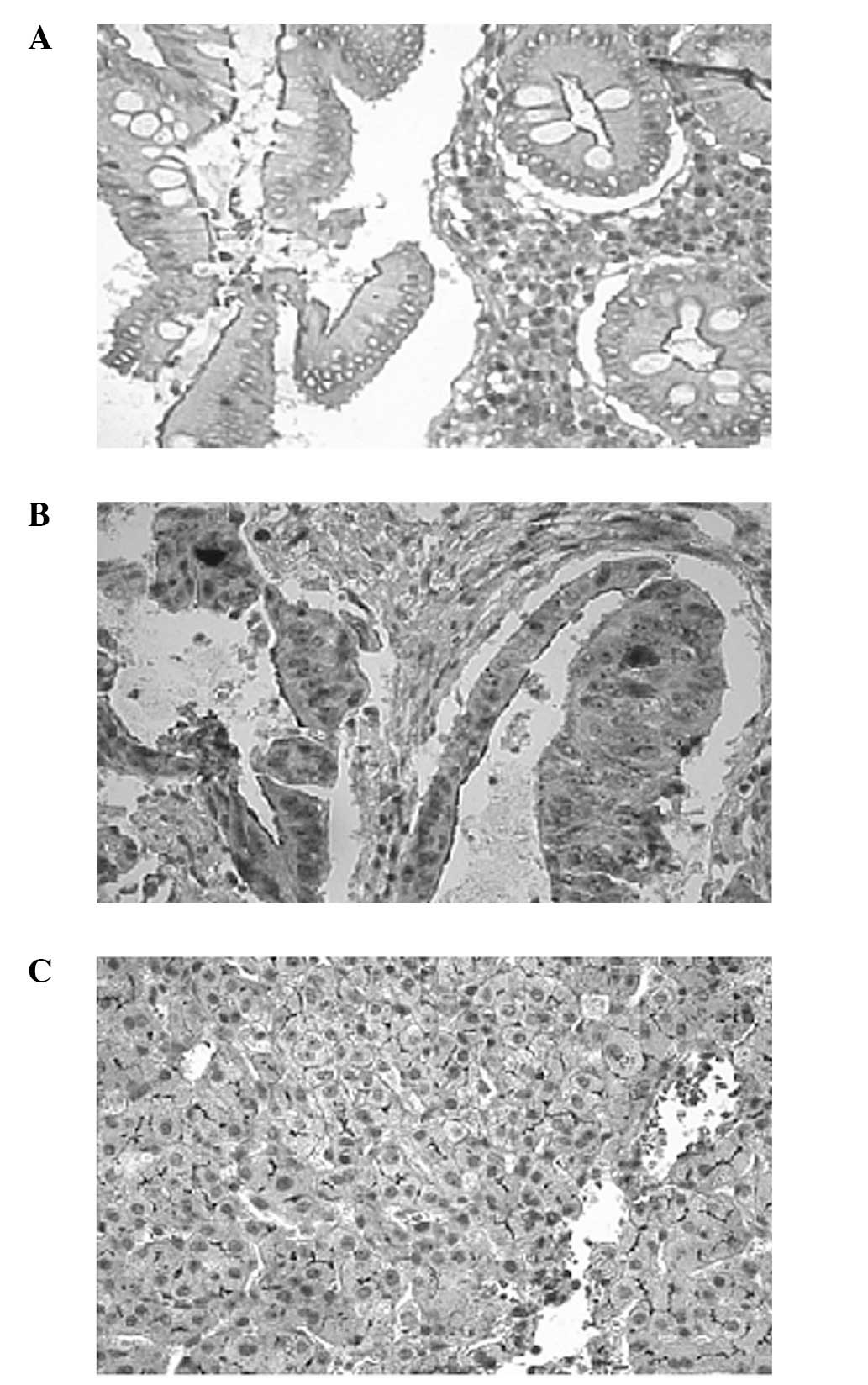
Liver tissues were used as a positive control for MRP2 (Fig. 1C) and the primary antibody was
replaced by PBS as a negative control. Positive and negative
controls were applied in each run of the procedure. Samples were
classified as positive for the expression when over 10% of tumor
cells demonstrated immunoreactivity, as in previous studies in
ovarian cancer by Arts et al (20) and in small cell lung cancer by
Ushijima et al (21).
Statistical analysis
The data were analyzed using the Statistical Package
for the Social Sciences software version 11.0 (SPSS, Inc., Chicago,
IL, USA). Correlations between positive expression,
clinicopathological features and early relapse were analyzed using
a Chi-square test. The Kaplan-Meier method was used for survival
analyses and the log-rank test was used for comparing survival
data. Disease-free survival (DFS) was defined as the number of
months from the date of surgery to the first event of documented
relapse or mortality. For those patients who did not experience
relapse, data were collected during the last follow-up. Overall
survival (OS) was considered as the duration between surgery and
mortality from any cause, and data on survivors were counted at the
last follow-up. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
In total, 50 patients were included in this study,
of which 30 were male and 20 were female (male to female ratio, 1.5
to 1). A total of 38 patients (76%) were >50 years and 12 (24%)
were <50 years (range, 17–77 years). A total of 22 cases (44%)
were stage II and 28 cases (56%) were stage III. The primary tumor
location for 35 (70%) patients was the colon and for 15 (30%) was
the rectum. Table I shows the
clinicopathological features of the patients and tumors. The median
follow-up time was 34 months (range, 24–50 months); in this time, a
total of 15 cases (30%) developed early relapse, either local
recurrence or distant metastasis, of which 5 were strongly
resistant to treatment and demonstrated recurrence at time of
chemotherapy.
 | Table IClinicopathological data of patients
and tumors. |
Table I
Clinicopathological data of patients
and tumors.
|
Characteristics | No. of cases | % |
|---|
| Gender | | |
| Male | 30 | 60 |
| Female | 20 | 40 |
| Age | | |
| >50 | 38 | 76 |
| <50 | 12 | 24 |
| Mean (years) | 57.2 | |
| Median
(years) | 57 | |
| Range
(years) | 17–77 | |
| Tumor size
(cm) | | |
| <5 | 28 | 56 |
| >5 | 22 | 44 |
| Location | | |
| Colon | 35 | 70 |
| Rectum | 15 | 30 |
| Depth of tumor
invasion | | |
| T4 | 3 | 6 |
| T3 | 47 | 94 |
| Lymph node
metastasis | | |
| Negative | 22 | 44 |
| Positive | 28 | 56 |
| Stage | | |
| II | 22 | 44 |
| III | 28 | 56 |
| Histology | | |
| WD + MD | 48 | 96 |
| PD | 2 | 4 |
Protein expression
Expression and subcellular localization of MRP2 was
determined by immunohistochemistry of 50 paraffin-embedded tumors
and matched normal tissues (Fig.
1). MRP2-positive expression was observed in the tumor and
matched normal tissues of 24 (48%) and 7 (14%) cases, respectively.
Comparing MRP2 expression in the tumor tissues and matched normal
tissues indicated significant overexpression of MRP2 in cancerous
regions compared to normal mucosa (P=0.003).
Correlation between early relapse and
clinicopathological markers
The correlation between MRP2 expression and
clinicopathological parameters was also investigated. No
statistical associations were observed between early relapse and
gender, age, tumor size, tumor site, lymph node metastasis, cancer
stage, grade or histology (P>0.05). However, a higher frequency
of early relapse was observed among male patients (12/15
cases).
Correlation between expression of MRP2
and early relapse
When the patients were divided into groups according
to their MRP2 expression, the MRP2-positive group did not
demonstrate a significantly poorer response compared to the
MRP2-negative group in the tumor tissue or normal mucosa
(P>0.05). Therefore, no association between early relapse and
MRP2-positive expression was identified. All 5 cases who developed
recurrences at the time of chemotherapy (during 6 months) were
MRP2-positive (P=0.022).
Correlation between clinicopathological
factors and patient survival
Variables including clinical AJCC/UICC stage, lymph
node metastasis, histology, tumor size, tumor location, age, gender
and positive MRP2 expression in tumor and matched normal tissues
were analyzed using the Kaplan-Meier method. These parameters were
not associated with the OS or DFS rate of patients (P>0.05),
except in the cases of deep tumor invasion. Although a significant
correlation between deep tumor invasion and survival time was
observed, the results were not suitable for evaluation due to the
small number of cases (2 out of 50) in the T4 type group. In
addition, a similar trend was observed between MRP2-negative
patients and a higher OS.
Discussion
Development of drug resistance and recurrence that
frequently occurs in cancer therapy is a major limitation to
applying adequate high doses of drugs to eradicate tumor cells. The
detection of markers that predict drug resistance is of major
interest in selecting the most effective medication in first-line
treatment. At present, oxaliplatin is used in the treatment of CRC
(11) and combining oxaliplatin to
a backbone of FL was revealed to improve the adjuvant chemotherapy
of colon cancer (22). However,
certain patients remain unresponsive to this effective combination
chemotherapy.
MRP2 mediates the export of intracellular
glutathione conjugates of a number of clinically important drugs
(9,10). Glutathione-conjugate formation is a
well-known mechanism of resistance to oxaliplatin in CRC (11). Once oxaliplatin is passively
absorbed by cells, it is detoxified within the cytoplasm by forming
glutathione conjugates and consequently eliminated from the cancer
cells. However, in the nucleus it forms oxaliplatin-DNA adducts
that contribute to cytotoxicity. A
glutathione-conjugation-oxaliplatin complex was verified to be a
substrate of ABC transporters (11). Additionally, MRP2 mRNA upregulation
was identified to be associated with decreased formation of
platinum-DNA adducts and decreased G2-arrest in the
cisplatin-resistant cell line (17). Hinoshita et al (23) also observed the association between
MRP2 mRNA levels and cisplatin resistance in colorectal carcinoma
cells in an in vivo study.
Taking into consideration the involvement of MRP2 in
the resistance of platinating agents, we aimed to evaluate its
possible association with CRC recurrence in platinum-based
chemotherapy employing a homogenous population. Patients were
diagnosed at an age less than 78 years in stage II/III CRC and did
not receive preoperative treatment prior to their FOLFOX-4 regimen.
We anticipated that the overexpression of MRP2 may influence tumor
response and induce recurrence.
When the correlation of the MRP2-positive expression
to the clinicopathological markers was examined, the statistics did
not reveal any significant correlation. The MRP2-positive patients
did not demonstrate a significantly superior OS and DFS compared to
the MRP2-negative patients in the neoplastic tissue or normal
mucosa. However, a higher OS was observed in the MRP2-negative
patients. In addition, when the expression of MRP2 in the
colorectal carcinoma and matched normal mucosa of patients were
assessed by immunohistochemical staining, the statistical result
indicated significant overexpression in the tumor tissue.
Therefore, the expression of MRP2 may play a role in the malignant
transformation of colorectal tissues in selected patients. In a
previous study by Hinoshita et al (23) using quantitative
reverse-transcription polymerase chain reaction (RT-PCR), it was
demonstrated that the expression of MRP2 was significantly
increased in CRC tissues compared to normal mucosal tissues.
Although overexpression of MRP2 was revealed to confer drug
resistance in tumor cells (24),
our observation did not identify any significant correlation
between early CRC relapse and MRP2-positive expression in the
neoplastic tissue or matched normal mucosa. Therefore, expression
of MRP2 did not appear to play a prognostic role in CRC patients
receiving adjuvant chemotherapy with FL and oxaliplatin.
We identified a subpopulation with MRP2-positive
expression among patients who had poor CRC prognosis and
demonstrated recurrence at time of chemotherapy. Although this
number of patients was small, they were the only patients who
demonstrated recurrence within 6 months. The findings indicated
that there are other existing mechanisms that affect the results of
the study. We suggest that further study of MRP2 expression with
proteins that simultaneously transport MRP2 substrates is required.
A study of the expression of organic anion transporting polypeptide
(OATP2) along with MRP2 in platinum-based chemotherapy may be
required.
The association between MRP2 expression and the
clinical outcome has been demonstrated in various types of tumors
(25–27), but its prognostic role in CRC
remains to be clarified. Further study is required to elucidate the
role of MRP2 expression in poor response patients who received
platinum-based therapy.
In conclusion, we demonstrated that MRP2 was
overexpressed in tumor tissues compared to normal tissues. To the
best of our knowledge, this is the first study to indicate the
protein expression of the ABC transporter MRP2 in tumor and normal
tissue. We identified that MRP2 expression is not a predictive
value of poor clinical outcome in patients with stage II/III CRC
treated with FL and oxaliplatin chemotherapy.
References
|
1.
|
R MalekzadehF BishehsariM MahdaviniaR
AnsariEpidemiology and molecular genetics of colorectal cancer in
Iran: a reviewArch Iran Med12161169200919249887
|
|
2.
|
A SadjadiM NouraieMA MohagheghiA
Mousavi-JarrahiR MalekzadehDM ParkinCancer occurrence in Iran in
2002: an international perspectiveAsian Pac J Cancer
Prev6359363200516236000
|
|
3.
|
V CatalanoAM BaldelliP GiordaniS
CascinuMolecular markers predictive of response to chemotherapy in
gastrointestinal tumorsCrit Rev Oncol
Hematol3893104200110.1016/S1040-8428(00)00114-111311657
|
|
4.
|
WT BeckCircumvention of multidrug
resistance with anti-P-glycoprotein antibodies: clinical potential
or experimental artifact?J Natl Cancer
Inst877375199510.1093/jnci/87.2.73
|
|
5.
|
M TakanoR YumotoT MurakamiExpression and
function of efflux drug transporters in the intestinePharmacol
Ther109137161200610.1016/j.pharmthera.2005.06.00516209890
|
|
6.
|
GE SanduskyKS MintzeSE PrattAH
DantzigExpression of multidrug resistance-associated protein 2
(MRP2) in normal human tissues and carcinomas using tissue
microarraysHistopathology416574200210.1046/j.1365-2559.2002.01403.x
|
|
7.
|
R KerbS HoffmeyerU BrinkmannABC drug
transporters: hereditary polymorphisms and pharmacological impact
in MDR1, MRP1 and
MRP2Pharmacogenomics25164200110.1517/14622416.2.1.5111258197
|
|
8.
|
R OhashiF TakahashiR CuiInteraction
between CD44 and hyaluronate induces chemoresistance in non-small
cell lung cancer cellCancer
Lett252225234200710.1016/j.canlet.2006.12.02517276588
|
|
9.
|
K TaniguchiM WadaK KohnoA human
canalicular multispecific organic anion transporter (cMOAT) gene is
overexpressed in cisplatin-resistant human cancer cell lines with
decreased drug accumulationCancer Res56412441291996
|
|
10.
|
E BakosR EversE SinkóA VáradiP BorstB
SarkadiInteractions of the human multidrug resistance proteins MRP1
and MRP2 with organic anionsMol Pharmacol57760768200010727523
|
|
11.
|
E CasadoJ De CastroC
Belda-IniestaMolecular markers in colorectal cancer: genetic bases
for a customised treatmentClin Transl
Oncol9549554200710.1007/s12094-007-0102-817921101
|
|
12.
|
S GiacchettiB PerpointR ZidaniPhase III
multicenter randomized trial of oxaliplatin added to
chronomodulated fluorouracil-leucovorin as first-line treatment of
metastatic colorectal cancerJ Clin Oncol181361472000
|
|
13.
|
WL AllenVM CoylePG JohnstonPredicting the
outcome of chemotherapy for colorectal cancerCurr Opin
Pharmacol6332336200610.1016/j.coph.2006.02.00516750422
|
|
14.
|
CA RabikME DolanMolecular mechanisms of
resistance and toxicity associated with platinating agentsCancer
Treat Rev33923200710.1016/j.ctrv.2006.09.00617084534
|
|
15.
|
K ZhangP MackKP WongGlutathione-related
mechanisms in cellular resistance to anticancer drugsInt J
Oncol1287188219989499449
|
|
16.
|
Y ItohM TamaiK YokogawaInvolvement of
multidrug resistance-associated protein 2 in in vivo cisplatin
resistance of rat hepatoma AH66 cellsAnticancer
Res2216491653200212168849
|
|
17.
|
B LiedertV MaternaD SchadendorfJ ThomaleH
LageOverexpression of cMOAT (MRP2/ABCC2) is associated with
decreased formation of platinum-DNA adducts and decreased G2-arrest
in melanoma cells resistant to cisplatinJ Invest
Dermatol121172176200310.1046/j.1523-1747.2003.12313.x12839578
|
|
18.
|
K ShenD CuiL SunM HanJ LiuInhibition of
IGF-IR increases chemosensitivity in human colorectal cancer cells
through MRP-2 promoter suppressionJ Cell BiochemJan242012(Epub
ahead of print)
|
|
19.
|
LH SobinC WittekindInternational Union
Against CancerTNM Classification of Malignant Tumors6th editionJohn
Wiley & SonsNew Jersey2002
|
|
20.
|
HJ ArtsD KatsarosEG de VriesDrug
resistance associated markers P-glycoprotein, multidrug resistance
associated protein 1, multidrug resistance-associated protein 2,
and lung resistance protein as prognostic factors in ovarian
carcinomaClin Cancer Res5279828051999
|
|
21.
|
R UshijimaK TakayamaUM
IzumiImmunohistochemical expression of MRP2 and clinical resistance
to platinum-based chemotherapy in small cell lung cancerAnticancer
Res2743514358200718214043
|
|
22.
|
T AndréC BoniL
Mounedji-BoudiafOxaliplatin, fluorouracil, and leucovorin as
adjuvant treatment for colon cancerN Engl J Med350234323512004
|
|
23.
|
E HinoshitaT UchiumiK TaguchiIncreased
expression of an ATP-binding cassette superfamily transporter,
multidrug resistance protein 2, in human colorectal carcinomasClin
Cancer Res6240124072000
|
|
24.
|
P BorstR EversM KoolJ WijnholdsA family of
drug transporters: the multidrug resistance-associated proteinsJ
Natl Cancer Inst9212951302200010.1093/jnci/92.16.129510944550
|
|
25.
|
M YamasakiT MakinoT MasuzawaRole of
multidrug resistance protein 2 (MRP2) in chemoresistance and
clinical outcome in oesophageal squamous cell carcinomaBr J
Cancer104707713201110.1038/sj.bjc.660607121206495
|
|
26.
|
GB Van den BroekM WildemanCR
RaschMolecular markers predict outcome in squamous cell carcinoma
of the head and neck after concomitant cisplatin-based
chemoradiationInt J Cancer12426432650200919253368
|
|
27.
|
A MaciejczykE JagodaT WysockaR MatkowskiB
GyörffyH LageP SurowiakABCC2 (MRP2, cMOAT) localized in the nuclear
envelope of breast carcinoma cells correlates with poor clinical
outcomePathol Oncol
Res18331342201210.1007/s12253-011-9449-921986666
|