Introduction
Amyloidosis is a clinical disorder caused by
extracellular deposition of insoluble abnormal fibrils in various
organs and is derived from the aggregation of misfolded, normally
soluble, proteins (1). Primary
pulmonary amyloidosis is a relatively rare pattern of amyloidosis
that is confined to the lungs and associated structures without any
other organ involvement. It occurs in 3 patterns: tracheobronchial,
diffuse interstitial and nodular parenchymal (2). Radiographically, the lesions of
primary nodular parenchymal pulmonary amyloidosis may be single or
multiple, and are able to calcify or cavitate. It is usually
considered in the differential diagnosis of pulmonary primary or
metastatic neoplasms. In the present study, we report a case of
primary nodular parenchymal pulmonary amyloidosis and review the
literature for related cases in Medline (1970-October 2011) and
Embase (1989-October 2011).
Patient and methods
Case report
A 44-year-old male was referred to our hospital for
further evaluation of multiple lobulated nodules of varying sizes
in both lungs that were detected on a chest computed tomography
(CT) scan conducted in a health examination 1 week earlier. The
patient’s medical history included an appendectomy that was
conducted 10 years previously. Additionally, the patient was a
non-smoker and did not suffer from pulmonary or systemic symptoms.
Physical examinations and laboratory findings, including analysis
of tumour markers, were all of no significance. As metastases was
suspected in the multiple lung nodules,
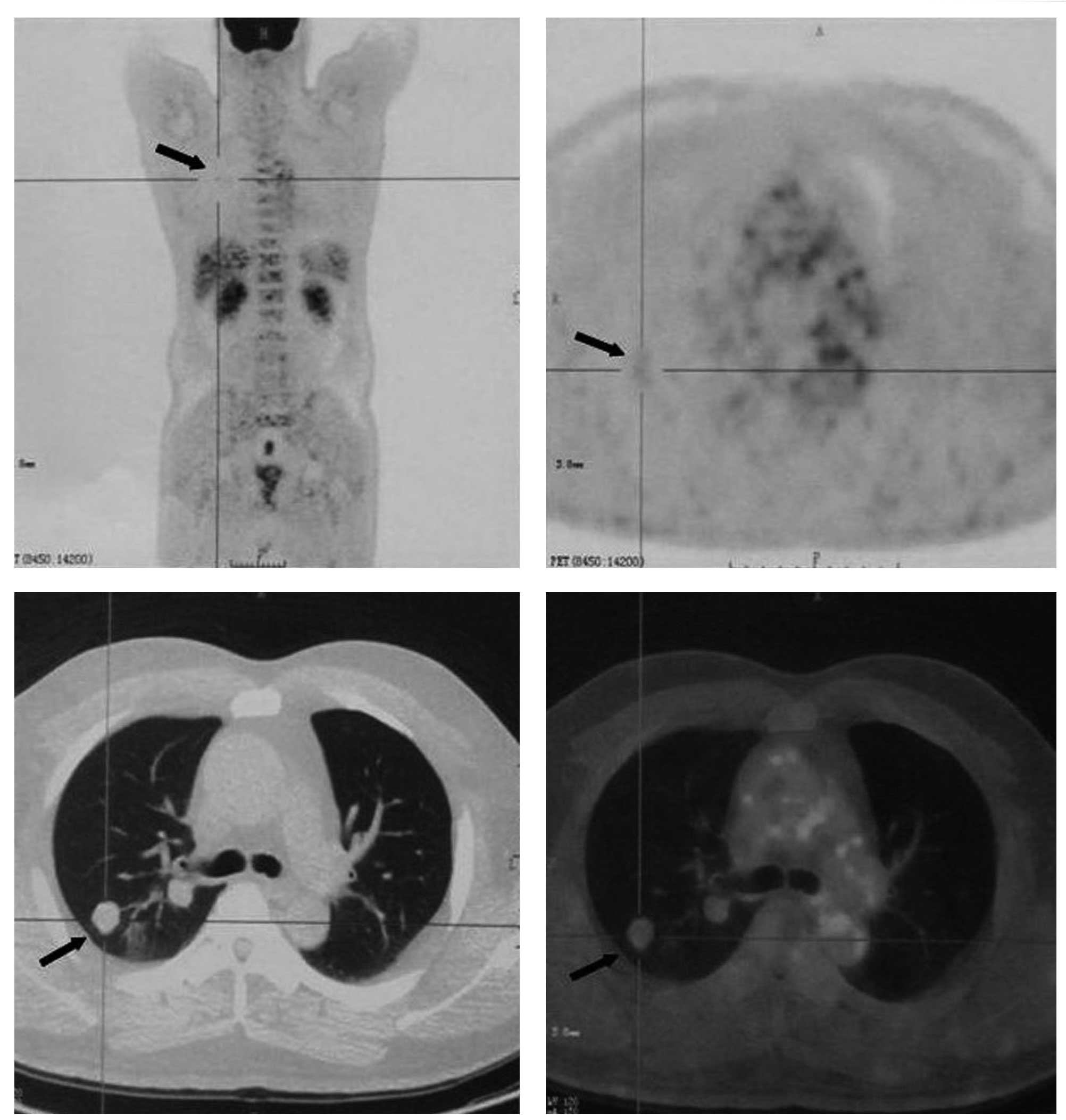
18F-fluorodeoxyglucose (FDG) positron emission
tomography (PET) /CT was conducted to characterize the nodules and
to detect a possible primary malignancy. The 18F-FDG
PET/CT revealed that the nodules had a mild uptake of
18F-FDG suggestive of malignancy, with a maximum
standardized uptake value (SUVmax) of 1.19 (Fig. 1). Other than these pulmonary
nodules, there was no evidence of a high-uptake lesion indicative
of a primary malignancy anywhere else in the body. A percutaneous
CT-guided fine-needle aspiration (FNA) biopsy was conducted in the
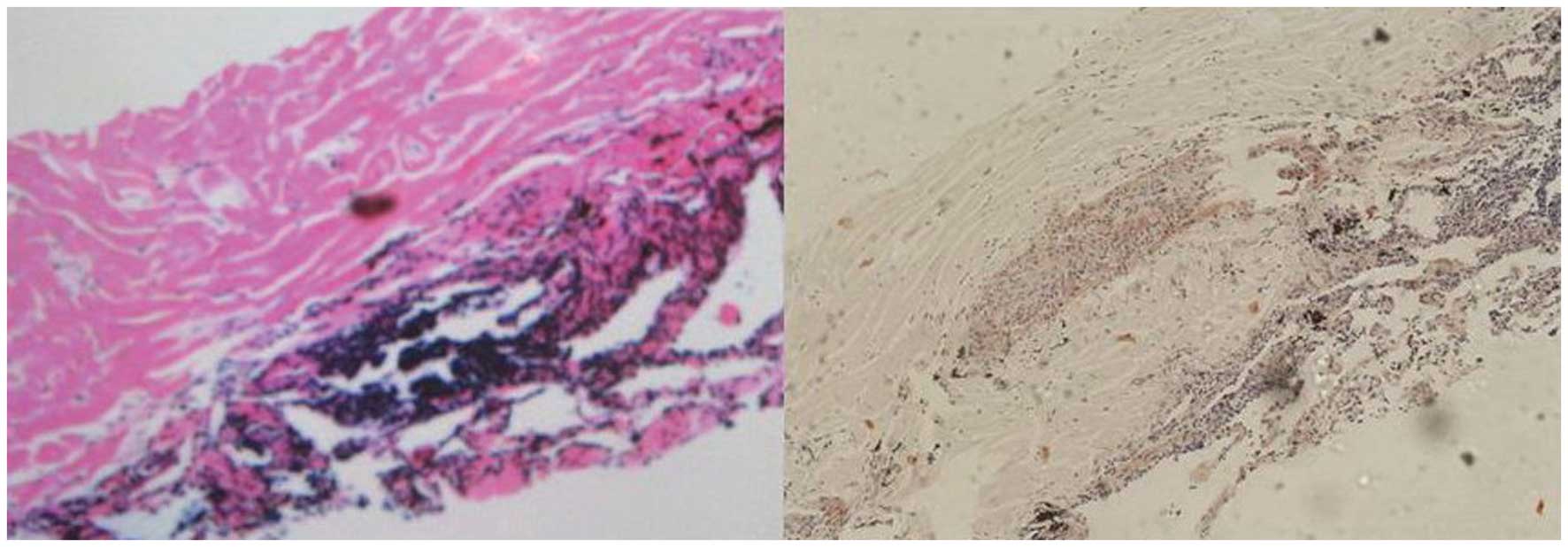
left lung nodule. Histologically, the specimens contained
amorphous, homogeneous material with a few lymphocytes. Congo red
staining was positive (Fig. 2),
which confirmed the deposition of amyloid within the specimen.
Therefore, we established a diagnosis of primary nodular
parenchymal pulmonary amyloidosis and discharged the patient
without chemotherapy. The patient enjoys good clinical condition 1
year later.
Methods
We searched for previous cases of primary nodular
parenchymal pulmonary amyloidosis in Medline (1970-October 2011)
and Embase (1989-October 2011), using a search strategy combining
medical subject headings and the key words ‘lung’ and
‘amyloidosis’.
Results
We identified 19 articles (3–21)
describing primary nodular parenchymal pulmonary amyloidosis in
Medline and Embase. Data on clinical presentation, radiographic
pattern, biopsy and survival of 58 patients (including our case)
are summarized in Table I. Ages
ranged from 44 to 89 years and, consistent with previous findings,
the average age of the patients was in the sixth decade (9,12,16).
There were 8 male and 12 female cases, while the gender was not
indicated in 38 cases. The patients were usually asymptomatic, and
the amyloidosis was discovered accidentally on routine chest
radiography. Few of these cases were associated with cough or
hemoptysis. Radiologically, the nodular parenchymal pattern
appeared as solitary or multinodular infiltrates in any lobe,
usually mimicking neoplastic growth. Nodules ranged in diameter
from 1 to 4 cm, with 15 cm being the largest nodule reported in the
literature (22). Biopsy data were
available for 22 patients. Of these 22 patients, nodule resection
was conducted in 9, lobectomy was conducted in 8, and percutaneous
FNA biopsy was conducted in 5. Patients with a nodular parenchymal
pattern were all in good condition during follow-up.
 | Table I.Cases of primary nodular parenchymal
pulmonary amyloidosis. |
Table I.
Cases of primary nodular parenchymal
pulmonary amyloidosis.
| Author/Year,
(Ref.) | Age (years)/
gender | Clinical
presentation | Radiographic
pattern | Biopsy
(pathology) | Survival |
|---|
| Chaudhuri and
Parker, 1970 (3) | 66/M | Asymptomatic | Smooth, round
shadow in the lateral basal segment of the right lower lung lobe
(CXR) | Right lower
lobectomy (a round nodule 2.5 cm in diameter, in the lateral basal
segment of the right lower lobe) | Patient was well
postoperatively |
| Moldow, et
al, 1972 (4) | 58/F | Six month history
of cough | Infiltrative
lesions involving both upperlung lobes (CXR) | Thoracotomy, a
wedge resection of an accessible nodule in the left lower lobe (the
left upper lobe was studded with numerous form to hard 1–3 cm
nodules) | Chest roentgenogram
remained unchanged 1 year later |
| Dyke, et al,
1974 (5) | 51/M | Chronic cough | Well-circumscribed
opacity in the periphery of the left upper lung lobe (CXR) | Thoracotomy, an
subpleural nodule (1.5 cm in diameter) of the anterior segment of
the left upper lobe was excised | Patient was well
until the age of 60 years |
| Brauner, et
al, (6) 1974 | 70/F | Chronic cough | 2.5 cm soft tissue
lesion in the upper lobe of the left lung (CXR) | A wedge resection
of the lesion in the left lung | ND |
| Bonfils-Roberts,
et al, 1975 (7) | 55/F | Asymptomatic | Lobulated left
parahilar mass (4 cm in diamter) (CXR) | Thoracotomy, a left
lower lobectomy | Postoperative
recovery was satisfactory |
| Makinen, et
al, 1977 (8) | 67/F | Asymptomatic | Tumour-like
infiltration in the lower lobe of the right lung (CXR) | Thoracotomy, the
mass was excised (approximately 3 cm in diameter) | ND |
| Rubinow, et
al, 1978 (9) | 63/M | Asymptomatic | Mass lesion in the
left upper lung lobe (CXR) | Left upper
lobectomy | Lost in follow-up
and died in an automobile accident 3 years later |
| Desai, et
al, 1979 (10) | i) 69/M; | i) Chronic
cough | i) 2 nodular
opacities in the lower half of left lung | i) Transcutaneous
biopsy with a needle under uoroscopic guidance | i) ND |
| ii) 48/F | ii)
Aymptomatic | ii) Bilateral
pulmonary nodules (CXR) | ii) Open biopsy of
the lung (a nodule was resected) | ii) ND |
| Schoen, et
al, 1980 (11) | 64/M | A chronic cough
productive of white sputum | A chest
roentgenogram showed a peripheral 4x2-cm noncalcified pleural-based
mass with irregular borders in the left lateral mid-lung field
(CXR) | Wedge resection of
2 nodules in the left upper lobe (4.0×3.5×1.0 cm and 1.8×0.8×0.6
cm) | ND |
| Hui, et al,
1986 (12) | Mean, 64 (28
cases) | Asymptomatic | Nodular lesions
were circumscribed, showed no evidence of calcification, and ranged
in size from 1 to 4 cm (CXR) | ND | ND |
| Kamei, et
al, 1989 (13) | 77/M | Asymptomatic | Multiple nodular
shadows in both lungs (2 vessel like shadows connected to 1 nodular
lesion in the right lower lobe) (CXR) | Right lower
lobectomy | Patient was well
with no special treatment following surgery |
| Davis, et
al, 1991 (14) | 56/M | Pleuritic pain;
hemoptysis | Left hilar mass and
bilateral dense nodules in the pulmonary parenchyma (CT) | Thoracotomy; the
mass was resected and wedge resections were performed on 2 nodules
from the left upper lobe (those >1.5 cm in diameter) | Uneventful
recovery |
| Mollers, et
al, 1992 (15) | 88/F | A single episode of
hemoptysis | 3 partially
calcified nodules in both lower lung (CXR) | Transthoracic
coaxial fine needle | Good condition and
asymptomatic 20 months later |
| Utz, et al,
1996 (16) | Mean, 67 (7
cases) | NA | Single nodule (5
patients); multiple nodules (2 patients) (ND) | Biopsy (described
unclearly) | ND |
| Khoor, et
al, 2004 (17) | i) 62/F | i) Episode of
severe exacerbation of asthma | i) Multiple,
bilateral pulmonary nodules; | i) Thoracotomy
(right middle-lobe biopsy) and a video assisted thoracoscopic wedge
biopsy 9 months later (>5.0 cm) | i) Pulmonary
nodules were stable 1 year later |
| ii) 65/F | ii)
Asymptomatic | ii) Solitary
pulmonary nodule in the right upper lobe | ii) Right upper
lobectomy (2.4 cm) | ii) ND |
| iii) 69/F | iii)
Asymptomatic | iii) Solitary
pulmonary nodule in the left upper lobe (radiological method was
not mentioned) | iii) Left upper
lobectomy (>4.0 cm) | iii) ND |
| Biewend, et
al, 2006 (18) | i) 75 | NA | NA | NA | i) No disease |
| ii) 73 | NA | NA | NA | ii) Stable
disease |
| iii) 65 | NA | NA | NA | iii) ND |
| Adžić, et
al, 2008 (19) | 52/F | Hemoptysis for 1
year | Nodular, multiple,
bilateral soft tissue densities (HRCT) | Open lung biopsy
(the nodules measured >3 cm) | Good clinical
condition 3 years later |
| Yang, et al,
2009 (20) | 58/F | Asymptomatic | Multiple lung
nodules (CT) | CT guided
percutaneous FNA biopsy of 1 nodule | ND |
| Seo, et al,
2010 (21) | 54/F | Asymptomatic | Multiple nodules
(>2.5 cm) in both lungs (CT); mild FDG uptake in the pulmonary
nodules (SUVmax 1.8) (PET/CT) | Open lung wedge
resection of the right pulmonary nodules | ND |
| Present case | 44/M | Asymptomatic | Multiple lobulated
nodules of varying sizes in both lungs (CT); mild FDG uptake in the
pulmonary nodules (SUVmax 1.19) (PET/CT) | Percutaneous
CT-guided core biopsy was obtained from the left lung nodule | Patient enjoys good
clinical condition 1 year later |
Discussion
Amyloidosis is a disease caused by extracellular
amyloid deposits (23). Amyloid
fibres are formed by the folding of various fibril precursor
proteins into an alternative conformation rich in β-sheet
structures. This characteristic structure results in specific
staining with Congo red dye that yields an apple-green
birefringence under polarized light microscopy. The genetic and/or
environmental factors in individual susceptibility to amyloid
deposition have not been elucidated (22). Primary pulmonary amyloidosis is
characterized by amyloid deposition in the lungs and other
associated structures. Radiologically, the primary nodular
parenchymal pulmonary amyloidosis appear as single or multiple
nodules in any lobe, and should be considered in the differential
diagnosis of pulmonary primary or metastatic neoplasms. In our
study, there is no specific examination for the preoperative
diagnosis of primary nodular parenchymal pulmonary amyloidosis
without the presence of classic clinical findings, laboratory tests
and radiological results. The final diagnosis usually requires
histological confirmation.
The optimal technique for biopsy is uncertain. In
our study, open lung biopsy was the most commonly used method for
obtaining biopsy material, but it was more invasive than CT-guided
percutaneous FNA biopsy. It is worth noting that CT-guided
percutaneous FNA biopsy has also been used when a less invasive
approach is necessary and 5 cases of our study were diagnosed
solely on the basis of material obtained by percutaneous FNA
biopsy, avoiding unnecessary invasive surgical resection. Once the
diagnosis is clear, nodular parenchymal amyloidosis rarely requires
treatment, which may involve surgical resection if a large nodule
causes a space-occupying effect. Additionally, the majority of
patients with a nodular parenchymal pattern were in good condition
during follow-up.
18F-FDG PET/CT is most widely used for
cancer detection by revealing which tissues have a high metabolic
rate and take up greater amounts of glucose in comparison to the
surrounding tissues. To a certain extent, the high metabolic rate
usually correlates with more aggressive tumors and a greater number
of viable tumor cells (24). The
use of 18F-FDG PET/CT for the diagnostic workup of
pulmonary nodules to reduce inappropriate invasive diagnostic
investigation and subsequent complications is emerging.
Duhaylongsod et al (25)
reported that the SUV of 18F-FDG uptake in malignant
nodules (SUV≥2.5) was greater than benign pulmonary nodules; the
sensitivity, specificity and accuracy were 97, 82 and 92%,
respectively. However, 18F-FDG is known to have little
uptake in malignancies with low metabolic activity, including
bronchoalveolar cancer, carcinoid tumor and mucinous
adenocarcinoma. Furthermore, certain noncancerous conditions may
also demonstrate high metabolic rates (26). Increased 18F-FDG activity
has been demonstrated in cases of tuberculosis, sarcoidosis, fungal
disease, interstitial lung disease, osteoarthritis, vascular
thromboses, osteoporosis and rheumatoid nodules (27–29).
The reason that noncancerous conditions uptake 18F-FDG
may be due to lesions with a high concentration of inflammatory
cells, including neutrophils and activated macrophages, which
increase glucose uptake (30,31).
Our case and the case reported by Seo et al (21) exhibited multiple lung nodules of
pulmonary amyloidosis with moderate 18F-FDG uptake, and
an SUVmax of 1.19 and 1.8, respectively. Our results suggest that
positive results of 18F-FDG PET/CT on pulmonary nodules
should be interpreted with caution in differentiating pulmonary
nodular amyloidosis from malignant lesions.
In conclusion, primary nodular parenchymal pulmonary
amyloidosis is a relatively rare condition without classic clinical
findings, laboratory tests and radiological results. Despite its
rarity, primary nodular parenchymal pulmonary amyloidosis with a
pattern of multiple nodules should be cautioned with the
differential diagnosis of pulmonary metastases with high
18F-FDG uptake on PET/CT.
References
|
1.
|
HY KimJG ImKS SongKS LeeSJ KimJS
KimLocalized amyloidosis of the respiratory system: CT featuresJ
Comput Assist
Tomogr23627631199910.1097/00004728-199907000-0002610433298
|
|
2.
|
JF CordierR LoireJ BruneAmyloidosis of the
lower respiratory tract. Clinical and pathologic features in a
series of 21
patientsChest90827831198610.1378/chest.90.6.8273780328
|
|
3.
|
MR ChaudhuriDJ ParkerA solitary amyloid
nodule in the lungThorax25382386197010.1136/thx.25.3.3825452296
|
|
4.
|
RE MoldowS BearmanMH EdelmanPulmonary
amyloidosis simulating tuberculosisAm Rev Respir
Dis10511411719725007606
|
|
5.
|
PC DykeMJ DemarayJW DelavanRA
RasmussenPulmonary amyloidomaAm J Clin
Pathol6130130519744855808
|
|
6.
|
GJ BraunerF al-BazzazMC Mihm JrAcquired
bullous disease of the skin and solitary amyloidoma of the lungAm J
Med57978986197410.1016/0002-9343(74)90178-84432876
|
|
7.
|
E Bonfils-RobertsAJ MarxTF NealonPrimary
amyloidosis of the respiratory tractAnn Thorac
Surg19313318197510.1016/S0003-4975(10)64023-4
|
|
8.
|
J MakinenJ NickelsPE HalttunenAmyloid
tumour of the lung. Report of a case and a short review of the
literatureActa Pathol Microbiol Scand A859079101977602776
|
|
9.
|
A RubinowBR CelliAS CohenBG RigdenJS
BrodyLocalized amyloidosis of the lower respiratory tractAm Rev
Respir Dis1186036111978707881
|
|
10.
|
RA DesaiVK MahajanS BenjaminHS Van
OrdstrandEM CordascoPulmonary amyloidoma and hilar adenopathy. Rare
manifestations of primary
amyloidosisChest76170173197910.1378/chest.76.2.170456056
|
|
11.
|
FJ SchoenRW AlexanderCI HoodLJ DunnNodular
pulmonary amyloidosis. Description of a case with
ultrastructureArch Pathol Lab Med104666919806892551
|
|
12.
|
AN HuiMN KossL HochholzerWD
WehuntAmyloidosis presenting in the lower respiratory tract.
Clinicopathologic, radiologic, immunohistochemical, and
histochemical studies on 48 casesArch Pathol Lab
Med1102122181986
|
|
13.
|
K KameiK KusumotoT SuzukiPulmonary
amyloidosis with pulmonary arteriovenous
fistulaChest9614351436198910.1378/chest.96.6.14352582859
|
|
14.
|
CJ DavisEG ButchartAR GibbsNodular
pulmonary amyloidosis occurring in association with pulmonary
lymphomaThorax46217218199110.1136/thx.46.3.2172028438
|
|
15.
|
MJ MollersJP van SchaikSC van der
PuttePulmonary amyloidoma. Histologic proof yielded by
transthoracic coaxial fine needle
biopsyChest10215971598199210.1378/chest.102.5.15971424899
|
|
16.
|
JP UtzSJ SwensenMA GertzPulmonary
amyloidosis. The Mayo Clinic experience from 1980 to 1993Ann Intern
Med124407413199610.7326/0003-4819-124-4-199602150-000048554249
|
|
17.
|
A KhoorJL MyersHD TazelaarPJ
KurtinAmyloid-like pulmonary nodules, including localized
light-chain deposition: clinicopathologic analysis of three casesAm
J Clin Pathol121200204200410.1309/3GECPW2402F6V8EK
|
|
18.
|
ML BiewendDM MenkeKT CalamiaThe spectrum
of localized amyloidosis: a case series of 20 patients and review
of the
literatureAmyloid13135142200610.1080/1350612060087677317062379
|
|
19.
|
TN AdžićJM StojšićGD Radosavljević-AšićD
BourosMultinodular pulmonary amyloidosis in primary Sjögren’s
syndromeEur J Intern Med19e97e982008
|
|
20.
|
MC YangA BlutreichK DasNodular pulmonary
amyloidosis with an unusual protein composition diagnosed by
fine-needle aspiration biopsy: a case reportDiagn
Cytopathol37286289200910.1002/dc.2102319217042
|
|
21.
|
JH SeoSW LeeBC AhnJ LeePulmonary
amyloidosis mimicking multiple metastatic lesions on F-18 FDG
PET/CTLung
Cancer67376379201010.1016/j.lungcan.2009.11.01420022134
|
|
22.
|
JD GillmorePN HawkinsAmyloidosis and the
respiratory tractThorax54444451199910.1136/thx.54.5.44410212113
|
|
23.
|
MB PepysAmyloidosisAnnu Rev
Med57223241200610.1146/annurev.med.57.121304.13124316409147
|
|
24.
|
RL WahlTargeting glucose transporters for
tumor imaging: ‘sweet’ idea, ‘sour’ resultJ Nucl
Med37103810411996
|
|
25.
|
FG DuhaylongsodVJ LoweEF Patz JrAL
VaughnRE ColemanWG WolfeDetection of primary and recurrent lung
cancer by means of F-18 fluorodeoxyglucose positron emission
tomography (FDG PET)J Thorac Cardiovasc
Surg110130139199510.1016/S0022-5223(05)80018-27609536
|
|
26.
|
MM AbouziedES CrawfordHA Nabi18F-FDG
imaging: pitfalls and artifactsJ Nucl Med
Technol33145155200516145222
|
|
27.
|
G OllenbergerS KnightA TauroFalse-positive
FDG positron emission tomography in pulmonary amyloidosisClin Nucl
Med29657658200410.1097/00003072-200410000-0001815365447
|
|
28.
|
L ShinD KatzE YungHypermetabolism on F-18
FDG PET of multiple pulmonary nodules res ulting from bronchiolitis
obliterans organizing pneumoniaClin Nucl
Med29654656200410.1097/00003072-200410000-0001715365446
|
|
29.
|
M HashefiR CurielFuture and upcoming
non-neoplastic applications of PET/CT imagingAnn NY Acad
Sci1228167174201110.1111/j.1749-6632.2011.06082.x21718331
|
|
30.
|
RS BrownJY LeungS FisherIntratumoral
distribution of tritiated-FDG in breast carcinoma: correlation
between glut-1 expression and FDG uptakeJ Nucl
Med371042104719968683298
|
|
31.
|
R KubotaK KubotaS YamadaM TadaT IdoN
TamahashiMicroautoradiographic study for the differentiation of
intratumoral macrophages, granulation tissues and cancer cells by
the dynamics of fluorine-18-fluorodeoxyglucose uptakeJ Nucl
Med351041121994
|