Introduction
Several lines of evidence have suggested a strong
association between chronic inflammation and carcinogenesis
(1–4). It has been estimated that up to 20% of
all tumors arise from the sites of infection, chronic irritation
and inflammation (1). Persistent
stimulation of chronic inflammation may cause the infiltration of
inflammatory cells, the release of cytokines and the production of
reactive oxygen species, which results in DNA damage, angiogenesis,
tumor invasion and metastasis (1).
There are numerous types of inflammatory cells in the tumor
micro-environment, and tumor-associated macrophages (TAMs) are a
major component. While the role of TAMs in tumor development is
complex and multifaceted, in clinical studies, increased TAM
density has been found to be associated with poor prognosis
(5–7). TAMs contribute to tumor development
through several mechanisms, including releasing angiogenic factors,
promoting tumor cell proliferation, and facilitating tumor cell
invasion and metastasis (8).
Lung cancer is one of the models of
inflammation-driven carcinogenesis (9,10). It
has been shown that chronic exposure to tobacco smoke is the major
risk factor for development of lung cancer by inducing inflammation
(11,12). Takahashi et al (13) further demonstrated that tobacco
smoke promotes lung tumorigenesis by triggering IKKβ- and
JNK1-dependent inflammation. Moreover, regular use of nonsteroidal
anti-inflammatory drugs decreases the risk of lung cancer (14,15).
Therefore, inflammation plays an important role in lung
carcinogenesis.
Urotensin II (UII), a somatostatin-like cyclic
peptide, was originally isolated from the fish urophysis (16). Subsequently, the cDNAs encoding UII
in a human (17), monkey (18), mouse and rat (19) were cloned. It has been demonstrated
that UII is an endogenous ligand for the orphan G-protein-coupled
receptor (GRP14) (20), which is
now known as urotensin II receptor (UT-R) (21). Recent studies suggest that UII is
involved in the inflammatory reaction (22,23).
In cardiomyocytes, UII has been shown to stimulate interleukin-6
(IL-6) release through UT-R (22).
A study by Segain et al (23) demonstrates that UII is a new
chemotactic factor for UT-R-expressing monocytes. UII has also been
shown to induce cellular adhesion molecule expression in human
coronary endothelial cells (24).
On the other hand, inflammatory cells and cytokines also regulate
the release and expression of UII and UT-R. It has been
demonstrated that lymphocytes are by far the largest producers of
UII, while monocytes and macrophages are the largest producers of
UT-R (25). In the human
rhabdomyosarcoma cell line TE-671, the expression of UT-R has been
shown to be upregulated by interferon-gamma (26). In addition, stimulation of monocytes
with lipopolysaccharide (LPS), IL-1β and tumor necrosis factor-α
(TNF-α) upregulates UT-R expression (23).
Our previous study demonstrated that UII stimulated
the proliferation of lung adenocarcinoma A549 cells and promoted
lung adenocarcinoma growth in a nude mice xenograft model (27), suggesting that UII may contribute to
the pathogenesis of lung adenocarcinoma. However, further study is
needed to explore the underlying mechanism of UII in lung
adenocarcinoma. Since lung carcinogenesis is associated with
chronic inflammation, and UII is involved in certain inflammatory
reactions, we considered whether UII could promote lung
carcinogenesis through modulating the inflammatory
microenvironment. Therefore, in the present study, we observed the
effect of UII on the inflammatory microenvironment of lung
adenocarcinoma in tumor-bearing nude mice.
Materials and methods
Materials
Human UII was obtained from Sigma Chemical Co. (St.
Louis, MO, USA). Antibody for phosphorylated-nuclear factor-κB
(p-NF-κB) was obtained from Bioworld Technology (Minneapolis, MN,
USA). Antibodies for CD68 and NF-κB were purchased from
Biosynthesis Biotechnology Co., Ltd. (Beijing, China). Mouse IL-6
enzyme-linked immunosorbent assay (ELISA) kits, mouse TNF-α ELISA
kits, mouse matrix metalloproteinase-9 (MMP-9) ELISA kits and
horseradish peroxidase-coupled goat anti-rabbit IgG were purchased
from Boster Biological Technology, Ltd. (Wuhan, China). BCA Protein
Assay kit was obtained from Pierce Co. (Rockford, IL, USA). The
study was approved by the ethics committee of Xuzhou Medical
College, Xuzhou, China.
Cell culture and tumor-bearing nude mice
model
The cell culture process and establishment of the
tumor-bearing nude mice model were described in our previous study
(27).
Immunohistochemistry for CD68
expression
The tumor tissues were embedded in optimal cutting
temperature (OCT) compound and fresh-frozen with liquid nitrogen.
The tissues were sectioned at a thickness of 10 μm. For
immunohistochemistry, the frozen sections were immersed for 10 min
in 0.5% hydrogen peroxide to deplete endogenous peroxidase
activity. Following pre-incubation with 5% bovine serum albumin for
30 min to prevent nonspecific staining, the sections were incubated
with rabbit anti-mouse CD68 antibody (1:200) at 4°C overnight. The
sections were then incubated with horseradish peroxidase-coupled
goat anti-rabbit IgG antibody for 20 min, followed by incubation
with strep-avidin-biotin-peroxidase complex (SABC) for 20 min at
37°C. The peroxidase was visualized by incubation with 3,
3′-diaminobenzidine (DAB) in the dark for 3 min. The sections were
counterstained with hematoxylin, dehydrated, and observed under a
light microscope. Negative controls were established using PBS as a
substitute for CD68 antibody. Positive staining was indicated by
brown deposits.
ELISA analysis for the protein levels of
IL-6, TNF-α and MMP-9
The tumor tissues were homogenized in lysis buffer
(RIPA) containing phenylmethanesulfonyl fluoride (PMSF) at 4°C.
Homogenates were centrifuged at 12,000 x g for 15 min at 4°C, and
supernatant fractions were used for ELISA analysis. Next, 100
μl sample or standard was added to each well of 96-well
plates coated with primary antibody. The plates were incubated at
37°C for 90 min and then washed five times. Biotinylated specific
antibody was added into each well and incubated at 37°C for 60 min.
Plates were then washed, incubated with 100 μl diluted
streptavidin-HRP at 37°C for 30 min. Following washing, the color
was produced by addition of 100 μl substrate solution for 25
min. Finally, 100 μl stop solution was added to terminate
the reaction.
Western blot analysis for p-NF-κB
The lysis supernatants were used for western blot
analysis. Protein concentrations were determined by a BCA Protein
Assay kit. The protein sample was separated on a 12%
SDS-polyacrylamide gel and transferred onto a nitrocellulose
membrane by electrotransfer. Membranes were blocked with 4% non-fat
milk for 1 h at room temperature and then incubated with specific
antibodies at 4°C overnight. Membranes were subsequently washed
three times and incubated in horseradish peroxidase-coupled goat
anti-rabbit IgG antibody for 3 h at room temperature.
Immunoreactive bands were visualized by an enhanced
chemiluminescence assay. Band intensities were measured by an
MSF-300G Scanner (Microtek Laboratory).
Statistical analysis
Values are provided as the mean ± SD. Statistical
differences between mean values were determined using one-way
ANOVA. P<0.05 was considered to indicate a statistically
significant difference.
Results
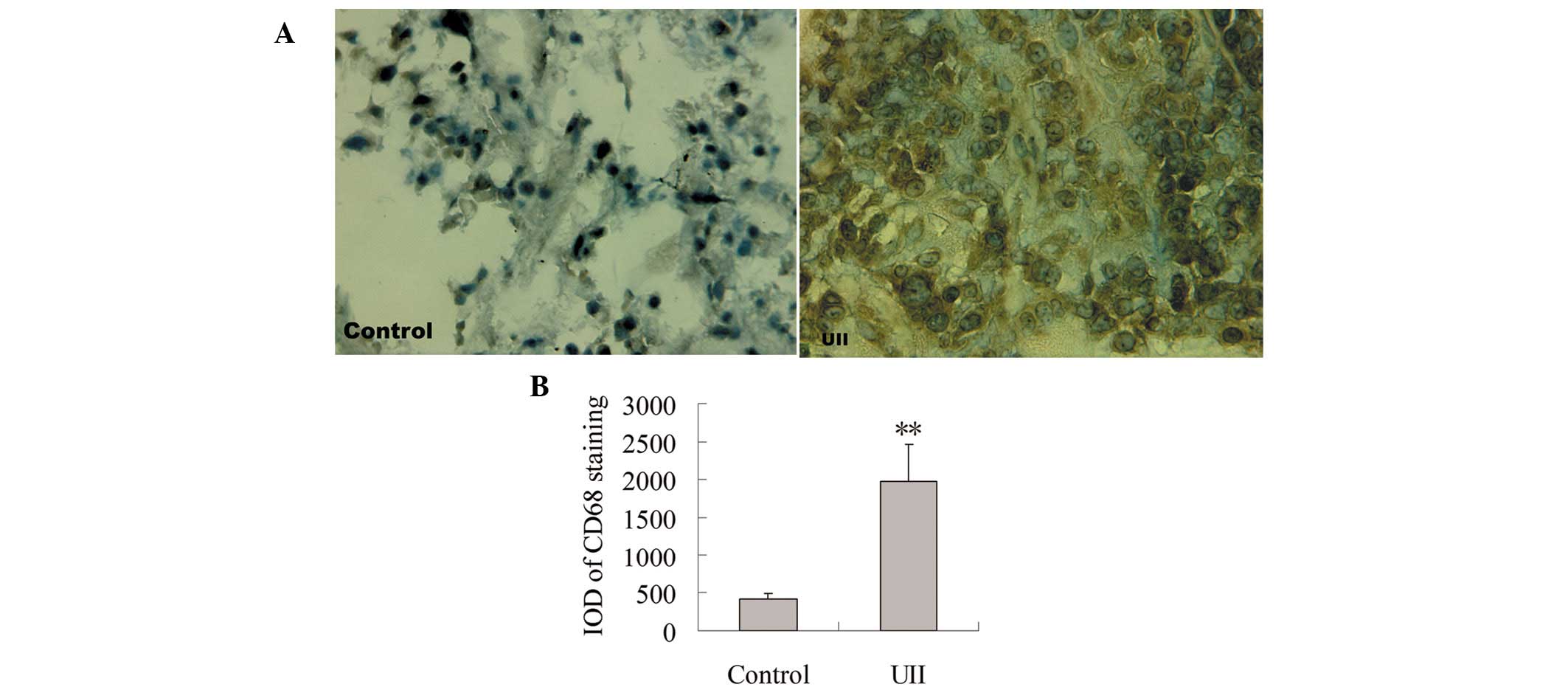
Effect of UII on TAM infiltration
To observe the effect of UII on TAM infiltration,
immunohistochemical staining for CD68 antibody was measured. As
shown in Fig. 1A, positive staining
for CD68 was localized mainly in the cytoplasm. Few
CD68+ TAMs were detected in the control group. A
significant increase of CD68+ TAMs was observed in the
UII group. Statistical analysis showed that compared with the
control group, the integral optical density (IOD) of
CD68+ immunostaining was significantly increased in the
UII group (P<0.01, Fig. 1B).
Effect of UII on the levels of TNF-α,
IL-6 and MMP-9
As presented in Fig.
2, the levels of IL-6, TNF-α and MMP-9 in the UII group
increased significantly compared with the control group
(P<0.05). This result shows that UII was capable of promoting
the production of IL-6, TNF-α and MMP-9 in the tumor tissues.
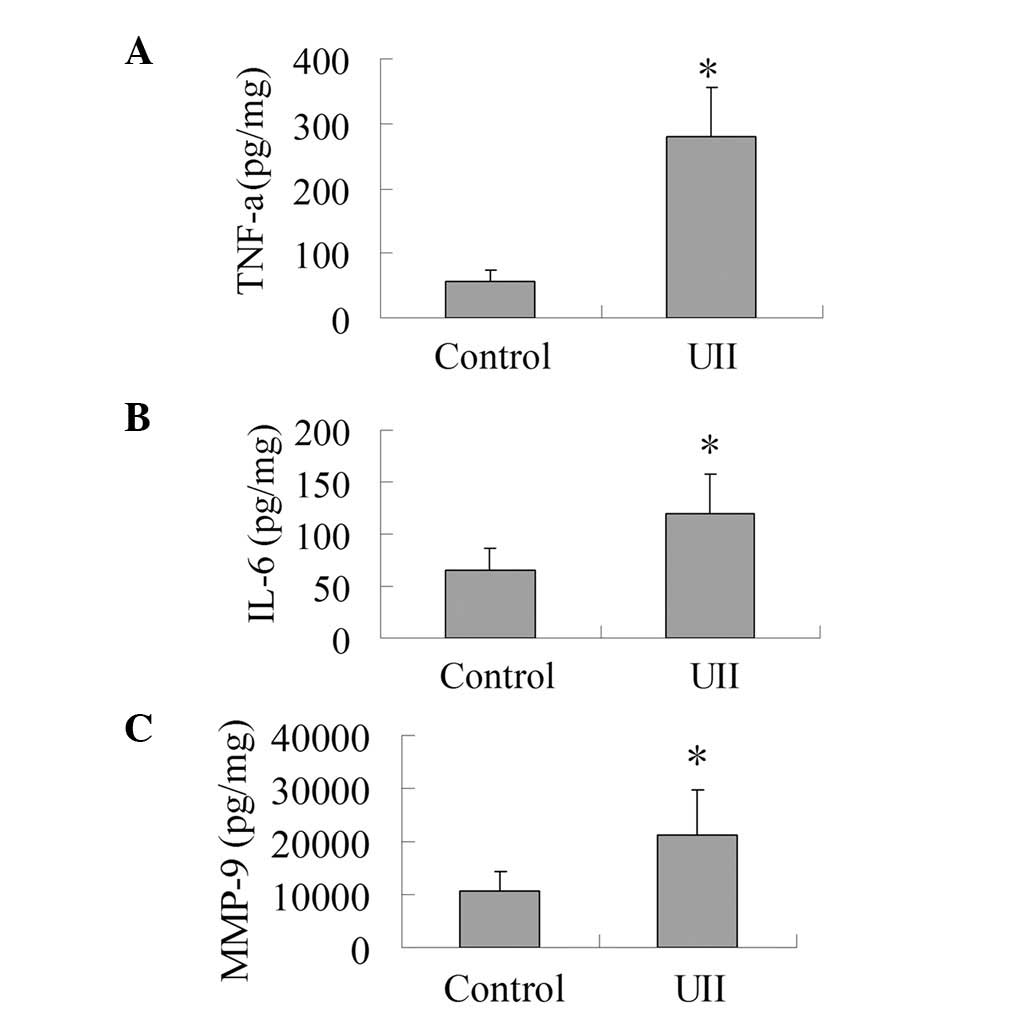 | Figure 2.Effect of UII on the levels of TNF-α,
IL-6 and MMP-9. The tumor tissues were homogenized in lysis buffer
(RIPA) containing PMSF at 4°C. Homogenates were centrifuged at
12,000 x g for 15 min at 4°C, and supernatant fractions were used
for ELISA analysis to determine the levels of TNF-α, IL-6 and
MMP-9. (A) Effect of UII on the level of TNF-α. (B) Effect of UII
on the level of IL-6. (C) Effect of UII on the level of MMP-9. The
results are expressed as mean ± SD (n=6). *P<0.05 vs.
control group. UII, urotensin II; TNF-α, tumor necrosis factor-α;
IL-6, interleukin-6; MMP-9, matrix metalloproteinase-9; PMSF,
phenylmethanesulfonyl fluoride; ELISA, enzyme-linked immunosorbent
assay. |
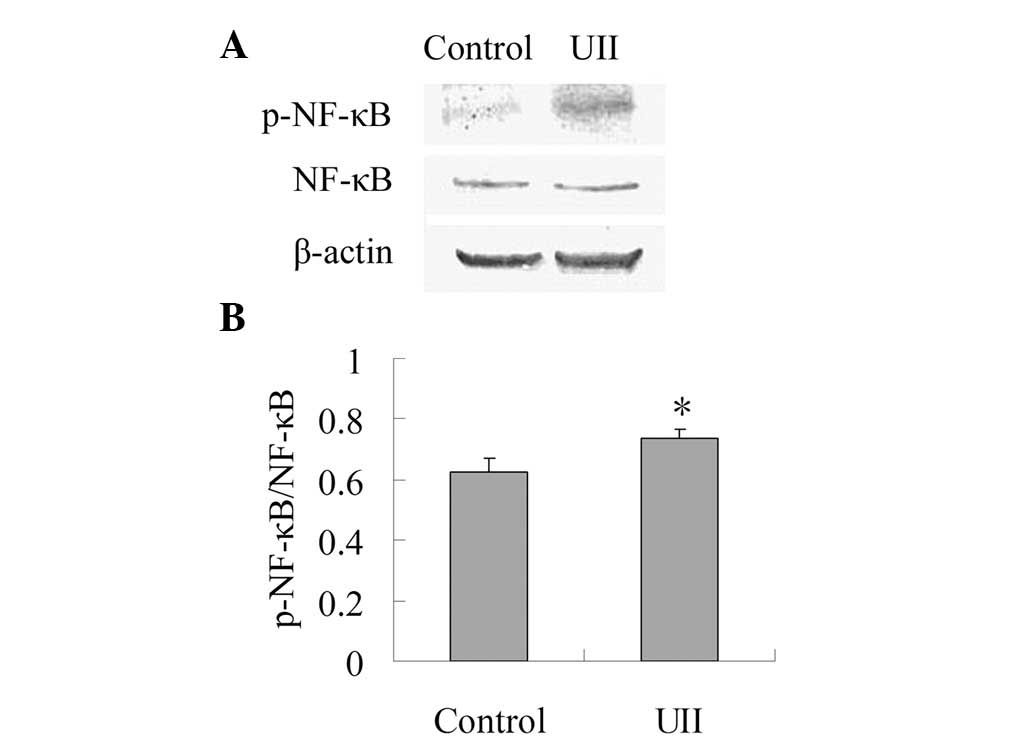
Effect of UII on the expression of
p-NF-κB
As shown in Fig. 3,
the expression of NF-κB between the control group and the UII group
was not statistically different. When compared with the control
group, UII increased the ratio of p-NF-κB/NF-κB significantly
(P<0.05), which suggests that UII was capable of promoting the
activation of NF-κB.
Discussion
The recent study in our laboratory showed that UII
stimulates the proliferation of lung adenocarcinoma A549 cells and
promotes lung adenocarcinoma growth in a nude mice xenograft model
(27). In this study, we
demonstrated that UII promotes the formation of lung adenocarcinoma
inflammatory microenvironment in tumor-bearing nude mice and
induces the activation of NF-κB. These findings implied that
modulation of lung adenocarcinoma inflammatory microenvironment by
the NF-κB pathway may be one of the mechanisms whereby UII promotes
lung adenocarcinoma growth.
Recent studies have shown that the tumor
inflammatory microenvironment plays an important role in cancer
progression. TAMs, in particular, have been found to be associated
with tumor growth and metastasis (5–7). A
large number of studies concerning numerous tumor types, including
colorectal cancer (5), supraglottic
laryngeal carcinoma (6), Ewing’s
sarcoma (7), intestinal type
gastric cancer (29), pancreatic
cancer (30), non-gynecologic
leiomyosarcoma (31) and thyroid
cancer (32), have shown that the
presence of TAMs in the tumor microenvironment is associated with a
worse prognosis. Therefore, TAMs play an important role in tumor
progression. Usually, the pan-macrophage/monocyte marker CD68 is
used as a marker for TAMs. In this study, we determined the number
of infiltrating TAMs in tumor tissues by immunohistochemical
staining of CD68. Our results showed that compared with the control
group, the number of TAMs in the UII group markedly increased,
suggesting that UII may contribute to the formation of the
inflammatory microenvironment in lung adenocarcinoma.
The role of TAMs in the tumor microenvironment and
tumor development is complex and multifaceted (33). TAMs release pro-angiogenic factors
and cytokines, including MMP-9, vascular-endothelial growth factor
(VEGF), platelet-derived growth factor (PDGF) and TNF-α. TAMs may
promote tumor growth by releasing epidermal growth factor (EGF),
IL-6 and PDGF. TAMs may also facilitate tumor cell invasion and
metastasis by releasing MMPs (MMP-2 and MMP-9), which degrade the
extracellular matrix and the basement membrane. These studies
suggest that TAMs contribute to tumor progression by modulating the
tumor inflammatory microenvironment. Our present study demonstrated
that UII promoted the release of IL-6, which may be one of the
causes of UII stimulation of lung adenocarcinoma growth. Consistent
with our results, it has been shown that UII stimulates the release
of IL-6 in cardiomyocytes (22). In
addition, our study showed that the levels of TNF-α and MMP-9 in
the UII group were higher than those of the control group, which
has not been demonstrated until now. Our study suggests that UII
may promote the release of inflammatory cytokines in the tumor
microenvironment, contributing to angiogenesis, tumor growth and
metastasis.
NF-κB is an important transcription factor for the
expression of numerous pro-inflammatory genes including IL-6, TNF-α
and MMP-9 (34). In the classic
pathway, activation of NF-κB, in particular the most abundant form
(p50/p65 heterodimer), depends on the phosphorylation of its
endogenous inhibitor (I-κB) primarily by I-κB kinases (IKKs). This
leads to ubiquitination and proteasomal degradation of I-κB. The
liberated NF-κB dimer then translocates to the nucleus, where it
activates specific target genes. Growing evidence indicates
post-translational modifications of NF-κB, particularly
phosphorylation and acetylation, also play significant roles in the
activation of the transcription factor. In response to certain
stimuli, the transactivation potential of NF-κB is regulated by
phosphorylation of its p65 subunit at specific serine residues
(35). Our present study showed
that compared with the control group, the expression of p-NF-κB in
the UII group was significantly higher. This was in accordance with
the levels of IL-6, TNF-α and MMP-9, suggesting that the
upregulated levels of IL-6, TNF-α and MMP-9 may have resulted from
enhanced activation of NF-κB.
In conclusion, our present study demonstrated that
UII increased the infiltration of CD68+ TAMs in lung
adenocarcinoma tissues. The enhanced levels of IL-6, TNF-α and
MMP-9 in the tumor microenvironment induced by UII (speculated to
be due to the increased activation of NF-κB) may be one of the
important mechanisms by which UII promotes lung adenocarcinoma
growth. These findings imply that antagonists of UII or UT-R have a
potential in the prevention and treatment of lung
adenocarcinoma.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (81001431, 81171013
and 81070889), the Key Subject of College and Universities Natural
Science Foundation of Jiangsu Province (10KJA320052), the Natural
Science Foundation of Jiangsu Higher Education Institutions of
China (11KJB310014), the Xuzhou Scientific and Technological
Project (xzzd1052) and the Priority Academic Program Development of
Jiangsu Higher Education Institutions.
References
|
1.
|
F BalkwillA MantovaniInflammation and
cancer: back to
Virchow?Lancet357539545200110.1016/S0140-6736(00)04046-011229684
|
|
2.
|
TA GondaS TuTC WangChronic inflammation,
the tumor microenvironment and carcinogenesisCell
Cycle820052013200910.4161/cc.8.13.898519550141
|
|
3.
|
J MarxCancer research. Inflammation and
cancer: the link grows
strongerScience306966968200410.1126/science.306.5698.96615528423
|
|
4.
|
LM CoussensZ WerbInflammation and
cancerNature420860867200210.1038/nature0132212490959
|
|
5.
|
JC KangJS ChenCH LeeJJ ChangYS
ShiehIntratumoral macrophage counts correlate with tumor
progression in colorectal cancerJ Surg
Oncol102242248201010.1002/jso.2161720740582
|
|
6.
|
JY LinXY LiN TadashiP DongClinical
significance of tumor-associated macrophage infiltration in
supraglottic laryngeal carcinomaChin J
Cancer30280286201110.5732/cjc.010.1033621439250
|
|
7.
|
T FujiwaraJ FukushiS YamamotoMacrophage
infiltration predicts a poor prognosis for human ewing sarcomaAm J
Pathol17911571170201110.1016/j.ajpath.2011.05.03421771572
|
|
8.
|
H LuW OuyangC HuangInflammation, a key
event in cancer developmentMol Cancer
Res4221233200610.1158/1541-7786.MCR-05-026116603636
|
|
9.
|
WC ChoCK KwanS YauPP SoPC PoonJS AuThe
role of inflammation in the pathogenesis of lung cancerExpert Opin
Ther Targets1511271137201110.1517/14728222.2011.59980121751938
|
|
10.
|
DS O’CallaghanD O’DonnellF O’ConnellKJ
O’ByrneThe role of inflammation in the pathogenesis of non-small
cell lung cancerJ Thorac Oncol520242036201021155185
|
|
11.
|
G LeeTC WalserSM DubinettChronic
inflammation, chronic obstructive pulmonary disease, and lung
cancerCurr Opin Pulm
Med15303307200910.1097/MCP.0b013e32832c975a19417670
|
|
12.
|
T WalserX CuiJ YanagawaSmoking and lung
cancer: the role of inflammationProc Am Thorac
Soc5811815200810.1513/pats.200809-100TH19017734
|
|
13.
|
H TakahashiH OgataR NishigakiDH BroideM
KarinTobacco smoke promotes lung tumorigenesis by triggering
IKKbetaand JNK1-dependent inflammationCancer
Cell178997201010.1016/j.ccr.2009.12.00820129250
|
|
14.
|
CG SlatoreDH AuAJ LittmanJA SatiaE
WhiteAssociation of nonsteroidal anti-inflammatory drugs with lung
cancer: results from a large cohort studyCancer Epidemiol
Biomarkers
Prev1812031207200910.1158/1055-9965.EPI-08-111019293309
|
|
15.
|
A AkhmedkhanovP TonioloA
Zeleniuch-JacquotteKL KoenigRE ShoreAspirin and lung cancer in
womenBr J Cancer874953200210.1038/sj.bjc.660037012085255
|
|
16.
|
D PearsonJE ShivelyBR ClarkUrotensin II: a
somatostatin-like peptide in the caudal neurosecretory system of
fishesProc Natl Acad Sci
USA7750215024198010.1073/pnas.77.8.50216107911
|
|
17.
|
Y CoulouarnI LihrmannS JegouCloning of the
cDNA encoding the urotensin II precursor in frog and human reveals
intense expression of the urotensin II gene in motoneurons of the
spinal cordProc Natl Acad Sci
USA951580315808199810.1073/pnas.95.26.158039861051
|
|
18.
|
NA ElshourbagySA DouglasU ShabonMolecular
and pharmacological characterization of genes encoding urotensin-II
peptides and their cognate G-protein-coupled receptors from the
mouse and monkeyBr J Pharmacol136922200210.1038/sj.bjp.0704671
|
|
19.
|
Y CoulouarnS JégouH TostivintH VaudryI
LihrmannCloning, sequence analysis and tissue distribution of the
mouse and rat urotensin II precursorsFEBS
Lett4572832199910486557
|
|
20.
|
RS AmesHM SarauJK ChambersHuman
urotensin-II is a potent vasoconstrictor and agonist for the orphan
receptor GPR14Nature401282286199910.1038/4580910499587
|
|
21.
|
AP DavenportJJ MaguireUrotensin II: fish
neuropeptide catches orphan receptorTrends Pharmacol
Sci218082200010.1016/S0165-6147(00)01449-810689357
|
|
22.
|
DG JohnsZ AoD
NaselskyUrotensin-II-mediated cardiomyocyte hypertrophy: effect of
receptor antagonism and role of inflammatory mediatorsNaunyn
Schmiedebergs Arch
Pharmacol370238250200410.1007/s00210-004-0980-z15549273
|
|
23.
|
JP SegainM Rolli-DerkinderenN GervoisD
Raingeard de la BlétièreG LoirandP PacaudUrotensin II is a new
chemotactic factor for UT receptor-expressing monocytesJ
Immunol179901909200710.4049/jimmunol.179.2.90117617581
|
|
24.
|
P CirilloS De RosaM PacileoHuman urotensin
II induces tissue factor and cellular adhesion molecules expression
in human coronary endothelial cells: an emerging role for urotensin
II in cardiovascular diseaseJ Thromb
Haemost6726736200810.1111/j.1538-7836.2008.02923.x
|
|
25.
|
N BousetteL PatelSA DouglasEH OhlsteinA
GiaidIncreased expression of urotensin II and its cognate receptor
GPR14 in atherosclerotic lesions of the human
aortaAtherosclerosis176117123200410.1016/j.atherosclerosis.2004.03.02315306183
|
|
26.
|
M Birker-RobaczewskaC BoukhadraR StuderC
MuellerC BinkertO NaylerThe expression of urotensin II receptor
(U2R) is up-regulated by interferon-gammaJ Recept Signal Transduct
Res23289305200310.1081/RRS-12002697214753294
|
|
27.
|
YQ WuZ SongCH ZhouSH XingDS PeiJN
ZhengExpression of urotensin II and its receptor in human lung
adenocarcinoma A549 cells and the effect of urotensin II on lung
adenocarcinoma growth in vitro and in vivoOncol
Rep2411791184201020878108
|
|
28.
|
J ShiD ZhengY LiuMH ShamP TamF FarzanehR
XuOverexpression of soluble TRAIL induces apoptosis in human lung
adenocarcinoma and inhibits growth of tumor xenografts in nude
miceCancer
Res6516871692200510.1158/0008-5472.CAN-04-274915753363
|
|
29.
|
A KawaharaS HattoriJ AkibaInfiltration of
thymidine phosphorylase-positive macrophages is closely associated
with tumor angiogenesis and survival in intestinal type gastric
cancerOncol Rep24405415201010.3892/or_0000087320596627
|
|
30.
|
H KuraharaH ShinchiY MatakiSignificance of
M2-polarized tumor-associated macrophage in pancreatic cancerJ Surg
Res167e211219200910.1016/j.jss.2009.05.02619765725
|
|
31.
|
CH LeeI EspinosaS VrijaldenhovenPrognostic
significance of macrophage infiltration in leiomyosarcomasClin
Cancer Res1414231430200810.1158/1078-0432.CCR-07-171218316565
|
|
32.
|
M RyderRA GhosseinJC Ricarte-FilhoJA
KnaufJA FaginIncreased density of tumor-associated macrophages is
associated with decreased survival in advanced thyroid cancerEndocr
Relat Cancer1510691074200810.1677/ERC-08-003618719091
|
|
33.
|
A SicaRole of tumour-associated
macrophages in cancer-related inflammationExp
Oncol32153158201021403610
|
|
34.
|
BB AggarwalS ShishodiaSK SandurMK PandeyG
SethiInflammation and cancer: how hot is the link?Biochem
Pharmacol7216051621200610.1016/j.bcp.2006.06.02916889756
|
|
35.
|
J SunRD RamnathL ZhiR TamizhselviM
BhatiaSubstance P enhances NF-kappaB transactivation and chemokine
response in murine macrophages via ERK1/2 and p38 MAPK signaling
pathwaysAm J Physiol Cell
Physiol294C15861596200810.1152/ajpcell.00129.200818434625
|