Introduction
Hepatocellular carcinoma (HCC), one of the most
prevalent malignancies worldwide, is recently rapidly increasing in
the United States, and is particularly prevalent in Taiwan and
other Asian counties (1–5). For individuals with unresectable HCC,
the effect of either chemotherapy or the present target therapy is
limited. The need to find new therapies is urgent.
Previous studies have suggested that abnormal
activation of the sonic hedgehog (Shh) signaling pathway may
be essential for carcinogenesis in certain cancer types, including
HCC (6–18). Cyclopamine, a well-known antagonist
of Smoothened (Smoh), may inhibit the Shh pathway.
The effect of cyclopamine on hepatocarcinogenesis has been
described in a previous study (19). However, the in vivo effect
remains unknown. We conducted this study to investigate the
treatment effect of cyclopamine upon HCC in an in vivo model
of mice.
Materials and methods
Treatment groups
Eighty C57BL/6 mice (6–8 weeks old, 19–24 g) were
purchased and divided into 4 groups (A, B, C and D) with 20 mice in
each. Group A formed the control group. Under isoflurane general
anesthesia, we injected mouse hepatoma cells, i.e., Mistheton
Lectin-1 (ML-1) cells (5×106 cells/20 μl), into
the left liver of mice in groups B, C and D. In the second week, we
analyzed each mouse to assess the tumor growth status. Following
the initial tumor injection, group B did not receive any additional
drug injections. Group C received cyclopamine 10 mg/kg/day i.p and
group D received cyclopamine 30 mg/kg/day i.p. The injections were
administered every day for 10 days. After an interval of 4 weeks,
exploration and harvesting of the liver was performed for each
group. The tumor size was measured for groups B, C and D. Real-time
PCR analysis of Shh pathway factors [Shh, patched
homolog-1 (Ptch-1), glioma-associated oncogene homolog-1
(Gli-1) and Smoh) of the livers in group A and of the
tumors in groups B, C and D were undertaken. The experiment was
conducted under the Guidelines for the Care and Use of Laboratory
Animals of the Far Eastern Memorial Hospital, Taiwan. The
institutional licensing committee had approved the experiments
undertaken.
Definition of effective reduction of
tumor size following treatment
The maximal diameter of the tumor size of each mouse
(groups B, C and D) was measured at the final evaluation. Effective
reduction of the tumor size following treatment was defined if
there was a statistically significant reduction in the tumor size
as measured at the end of the study compared to the tumor size
prior to treatment (measured in the second week after ML-1 cell
injection).
Detection of mouse mRNA of Shh, Ptch-1,
Gli-1 and Smoh
The examination included extraction of RNA and
reverse transcription, and amplification of cDNA of Shh,
Ptch-1, Gli-1, Smoh and housekeeping gene
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by
real-time PCR.
Extraction of RNA and reverse
transcription PCR
We homogenized each resected cancer and liver tissue
completely in 1 ml TRIzol® reagent (Invitrogen,
Carlsbad, CA, USA), and added 0.2 ml chloroform and agitated it
vigorously by hand for 15–30 sec, then incubated them on ice for 20
min. The samples were then centrifuged at 13,000 rpm for 20 min at
4°C. Following centrifugation, the mixture was separated into a
lower red, phenol-chloroform phase, an interphase and a colorless
upper aqueous phase. RNA remained exclusively in the aqueous phase.
We transferred the aqueous phase to a fresh tube, and precipitated
the RNA from the aqueous phase by mixing in 0.5 ml of isopropanol.
The samples were incubated on ice for 20 min and were centrifuged
at 13,000 rpm for 20 min at 4°C. The RNA precipitation, often
invisible before centrifugation, formed a gel-like pellet on the
side and bottom of the tube. We removed the supernatant and washed
the RNA pellet once with 75% ethanol, adding at least 1 ml 75%
ethanol. We centrifuged it at 13,000 rpm for 5 min at 4°C. The RNA
pellet was dried and RNA was dissolved in RNase-free water. Then it
was incubated for 10 min at 60°C and was stored at −80°C.
cDNA was synthesized from 2 mg mRNA. The reverse
transcription reaction solution consisted of 2.0 ml 10X RT buffer,
0.8 ml 100 mM dNTP mixed with 2.0 ml 10X RT random primers and 1.0
ml MultiScribe™ Reverse Transcriptase (Applied Biosystems, Foster
City, CA, USA). The RNA solution was mixed with the reverse
transcription reaction solution (total volume 20 ml) and incubated
at 25°C for 10 min, 37°C for 120 min and 85°C for 5 sec. It was
stored at −20°C.
Amplification of cDNA of Shh, Ptch-1,
Gli-1, Smoh and GAPDH by real-time PCR
Total RNA was extracted from each liver tissue and
HCC tissue using TRIzol reagent. RT-PCR was performed using
high-capacity cDNA reverse transcription kits (Applied Biosystems).
In brief, 2–5 μg total RNA was used in a 20-μl
reverse transcription assay. Subsequently, the cDNA was diluted at
1:4 for real-time PCR assays which were carried out in a 96-well
plate in the LightCycler 480 (Roche Diagnostics, Mannheim, Germany)
using SYBR-Green I Master dye (Roche Diagnostics). Each real-time
PCR assay (10 μl) contained 3 μl water, 0.5 μl
forward and reverse primers, respectively, 5 μl 2X
SYBR-Green I Master and 1 μl diluted cDNA. All primer
sequences used for real-time analysis are listed in Table I. Real-time PCR parameters were
cycled as follows: hot start at 95°C for 1 min, followed by 45
cycles of denaturing at 95°C for 10 sec, annealing at 58°C for 5
sec and extension at 72°C for 20 sec. PCR products were detected
using 2% agarose gel to confirm the expected sizes. To normalize
the total amount of cDNA in each reaction, GAPDH was
coamplified as the internal control. Each sample was analyzed 3
times and quantified with the analysis software for LightCycler
(Roche Diagnostics).
 | Table I.Sequences of primer pairs. |
Table I.
Sequences of primer pairs.
| Gene | Direction | Primers
(5′-3′) |
|---|
| GAPDH | sense |
5′-CACCACCAACTGCTTAG-3′ |
| antisense |
5′-CTTCACCACCTTCTTGATG-3′ |
| Shh | sense |
5′-AAAGCTGACCCCTTTAGCCTA-3′ |
| antisense |
5′-TTCGGAGTTTCTTGTGATCTTCC-3′ |
| Ptch-1 | sense |
5′-CCGTTCAGCTCCGCACAGA-3′ |
| antisense |
5′-CTCACTCGGGTGGTCCCATAAA-3′ |
| Gli-1 | sense |
5′-TGTGGCGAATAGACAGAGGT-3′ |
| antisense |
5′-TGCCAGATATGCTTCAGCA-3′ |
| Smoh | sense |
5′-GAGCGTAGCTTCCGGGACTA-3′ |
| antisense |
5′-CTGGGCCGATTCTTGATCTCA-3′ |
Statistical analysis
Comparisons between groups were performed with a
Chi-square test (or Fisher’s exact test) for continuous variables.
The least significant difference (LSD) pair-wise multiple
comparison was used for multivariate analysis of associated
factors. All statistical analyses were performed using the SPSS
version 17.0 (Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant result.
Results
No tumors were observed in the livers in group A
mice at any point in this study. At the end of the second week
following injection of ML-1 cells, tumors developed successfully in
left lobe of the liver of all the mice in groups B, C and D. At the
end of the study, the reduction of the tumor size in group D was
found to be significant, from 0.152±0.219 cm2 (mean ±
SD) before treatment to 0.003±0.009 cm2 (mean ± SD)
after treatment (P=0.047). The tumor size in group C reduced from
0.152±0.219 cm2 to 0.071±0.187 cm2 without
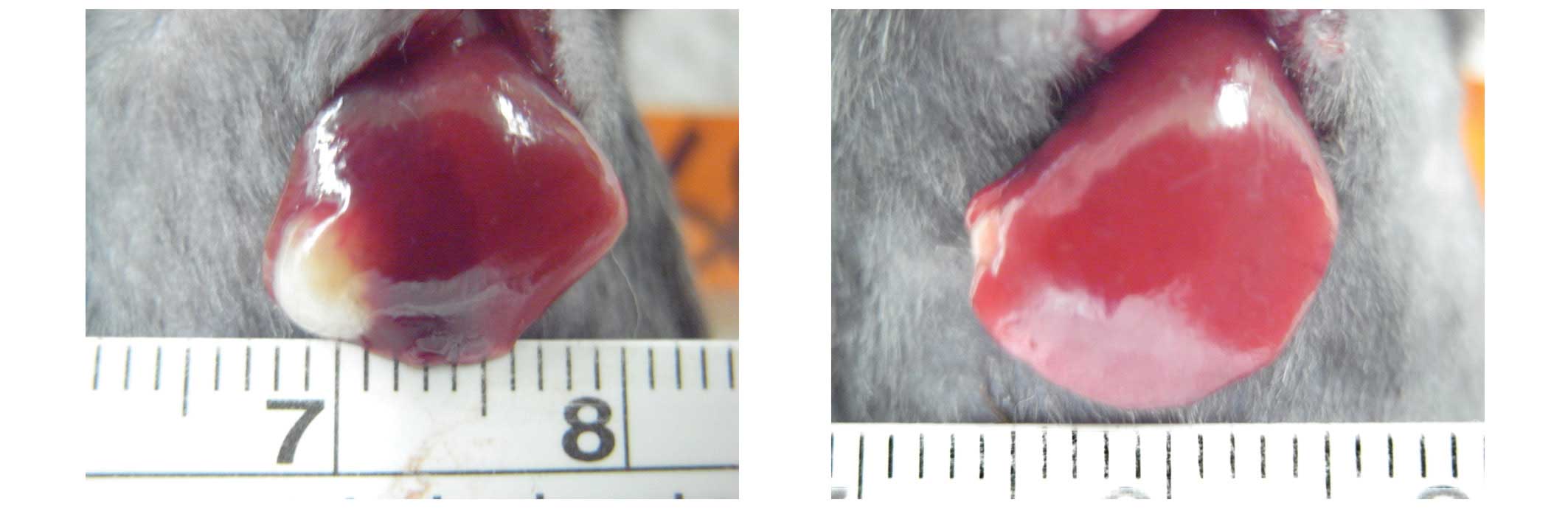
statistical significance (P=0.267). Fig. 1 shows the decrease of tumor size in
one mouse from group D.
Table II shows the
mRNA expression of Shh, Ptch-1, Gli-1 and Smoh in the
4 groups. Compared with group B, the value of Shh mRNA of
HCC in groups C and D decreased. However, the difference of each
had only borderline significance (P=0.062 and 0.071; Table II). Compared with group B, the
decrease of Ptch-1 mRNA expression in groups C and D also
had no statistical significance. However, compared with group B,
the decrease of Gli-1 mRNA in group D had statistical
significance (P=0.044). Compared with group B, the decrease of
Smoh mRNA in groups C and D was not statistically
significant (Table II).
 | Table II.Comparison of the mRNA expression of
Shh, Ptch-1, Gli-1 and Smoh of liver (group A) and
HCC (groups B, C and D). |
Table II.
Comparison of the mRNA expression of
Shh, Ptch-1, Gli-1 and Smoh of liver (group A) and
HCC (groups B, C and D).
| Group | Shh | Ptch-1 | Gli-1 | Smoh |
|---|
| Group A | | | | |
| Median | 0.58 | 1.715 | 0.74 | 2.87 |
| Mean ± SD | 0.5575±0.0810 | 1.7325±0.4918 | 0.7125±0.3165 | 3.1333±1.2952 |
| 95% CI | 0.4286–0.6864 | 0.9500–2.5150 | 0.2089–1.2161 | −0.0842–6.3509 |
| Range | 0.45–0.62 | 1.22–2.28 | 0.31–1.06 | 1.99–4.54 |
| Group B | | | | |
| Median | 1.23 | 1.29 | 1.22 | 2.905 |
| Mean ± SD | 1.2720±0.4535 | 1.9920±1.8249 | 1.2825±0.1839 | 3.0875±1.5321 |
| 95% CI | 0.7090–1.8350 | −0.2739–4.2579 | 0.9898–1.5752 | 0.6496–5.5254 |
| Range | 0.67–1.89 | 0.85–5.22 | 1.15–1.54 | 1.59–4.95 |
| Group C | | | | |
| Median | 0.595 | 0.685 | 1.07 | 2.145 |
| Mean ± SD | 0.7175±0.4310 | 0.7950±0.3397 | 1.1600±0.2762 | 3.2125±3.2488 |
| 95% CI | 0.0317–1.4033 | 0.2545–1.3355 | 0.4738–1.8462 | −1.9571–8.3821 |
| Range | 0.36–1.32 | 0.52–1.29 | 0.94–1.47 | 0.75–7.81 |
| Group D | | | | |
| Median | 0.77 | 0.61 | 0.94 | 1.4475 |
| Mean ± SD | 0.7700±0.2993 | 0.6840±0.3818 | 0.9380±0.2128 | 0.8640±0.6661 |
| 95% CI | 0.3984–1.1416 | 0.2099–1.1581 | 0.6738–1.2022 | 0.0369–1.6911 |
| Range | 0.43–1.12 | 0.23–1.10 | 0.71–1.15 | 0.30–1.82 |
| P-value | B vs C: 0.062 | B vs C: 0.145 | B vs C: 0.484 | B vs C: 0.932 |
| B vs D: 0.071 | B vs D: 0.097 | B vs D: 0.044 | B vs D: 0.130 |
| C vs D: 0.848 | C vs D: 0.887 | C vs D: 0.200 | C vs D: 0.112 |
Discussion
In this study, we found that cyclopamine treatment,
either at a low or high dose, may decrease the size of liver tumors
in mice in vivo. The effect of high-dose therapy was
significant.
We successfully established the growth of ML-1
hepatoma in the livers of groups B, C and D mice. A higher
expression level of Shh, Gli-1 and Smoh mRNA
was observed in group B when compared with group A, which suggests
that the activation of the Shh pathway occurred during HCC
development in mice. This corresponds with certain authors’
findings that compared with paired adjacent noncancerous liver
tissue, Shh, Ptch-1, Gli-1 and Smoh
were overexpressed in human HCC tissues (17,20).
Similarly, Patil et al used quantitative real-time RT-PCR
and revealed an increased level of expression of Gli-1 and
Smoh in HCC samples compared with non-tumor liver tissues
(16). Che et al found that
in over 50% of human HCC, the mRNA of Shh pathway target
genes Ptch-1, Gli-1 and Smoh were expressed
(17). Tada et al
demonstrated that hedgehog signaling components were expressed in
hepatoma cell lines in various degrees (21). These findings suggested that the
hedgehog pathway was frequently activated or deregulated in human
HCCs (14–17,21).
In vitro, certain authors considered that some hedgehog
signal-responsive progenitor cells function as cancer stem cells,
leading to carcinogenesis (22–24).
The detailed molecular mechanisms and the effect of
the timing of Shh pathway activation upon HCC are not well
understood. Some authors have hypothesized that activation of the
Shh pathway is important both in the development and the
progression of HCC (14–18). Cheng et al found that the
Shh signaling pathway correlated with the proliferation and
invasiveness of HCC cells (20). In
addition, some authors reported an association between the factors
of Shh signaling pathways and invasiveness of human HCC
(17,20).
Tada et al regarded the overexpression of
Smoh or Shh as being positive regulators and the
major trigger for the activation of this signaling pathway
(21). The authors demonstrated
that overexpression and/or tumorigenic activation of the
Smoh protooncogene mediates c-myc overexpression, which
plays a critical role in hepatocarcinogenesis (21). Smoh has been suggested as
being a prognostic factor in hepatocarcinogenesis (21).
Cyclopamine is the inhibitor of Smoh.
Cyclopamine has been reported to inhibit the growth of HCC cells or
hepatoblastoma cells (19,25,26).
Chen et al revealed that cyclopamine markably decreased cell
viability, induced apoptosis and downregulated Bcl-2 expression in
HCC cells (19). Kim et al
treated three hepatoma cell lines with KAAD-cyclopamine, resulting
in a decrease of the expression of hedgehog target genes and cell
growth, leading to apoptosis (25).
Cheng et al showed that the blockade of the Shh
signaling pathway by KAAD-cyclopamine induced a reduction of DNA
synthesis leading to a marked inhibition of cell growth and a
significant attenuation in invasiveness and motility of HCC cells
(20). Collectively, the studies
support the hypothesis that inhibition of the Shh pathway by
cyclopamine may inhibit both the development and invasiveness of
HCC.
However, the majority of these studies were carried
out in vitro. By contrast, our present study is in
vivo.
From our study, high-dose cyclopamine therapy not
only effectively decreases the tumor size but also significantly
decreases the expression of Gli-1 mRNA in the tumors. The
reason for the significant decrease of Gli-1 mRNA and not
the mRNA of Smoh, Ptch-1 or Smoh is unknown.
We attribute this result to three possible mechanisms.
The first is that the interactions among these
factors of the Shh pathways are complex. Ptch-1
activation predisposes a cell to proliferative and expansive
behavior (22,27). Some elements of the interaction
between Smoh and Ptch-1 are not fully understood.
Smoh is an intracellular substrate that migrates to the
cellular membrane where it is activated following engagement of
Ptch-1 by Shh. At the cellular membrane, the
activated Smoh triggers the downstream transcription of
Gli-1 proteins (22,27). Aberrant activation of the Shh
pathway leads Gli-1 into the nucleus to promote gene
transcription and to maintain the biological behaviors of cancer
cells.
However, the change of the mRNA expression may be
dynamic. The timing of tumor harvesting affects the values of the
factor.
The second mechanism may be that the significance of
Shh pathway activation may be different among different
stages of the same cancer and among different malignancies at the
same stage. For example, a previous study reported that the
proliferation of extrahepatic biliary tract cancer cell lines could
also be suppressed by inhibition of the Shh pathway
(28). However, the degrees of
Shh and Gli-1 expression were independent of tumor
stage and cancer cell differentiation (28). Activation of the Shh pathway
also occurs in different stages of the same cancer. Huang et
al suggested that the activation occurs in the early stage of
HCC (14), whereas Thayer et
al considered the hedgehog is both an early and late mediator
in pancreatic carcinogenesis (13).
The activation of the Shh pathway occurring in advanced
stages of other cancers is also noted (8,10,13).
The detailed cause of these discrepancies needs further
elucidation.
The third possibility affecting the level of
expression of Shh pathway factors is the hypothesized
concept of cancer stem cells which have the capacity of
self-renewal and unlimited replication (29–31).
Bailey et al also identified the so-called cancer stem cell
of the pancreas (32) and Tian
et al studied lung cancer and observed that the Shh
pathway is activated mainly in the cancer stem cells and not in
every cancer cell (23). The effect
of cyclopamine upon the Shh pathway may have occurred only
in the cancer stem cells of our HCC mice and not in all cancer
cells. Cyclopamine may affect the mRNA expression. The key target
factor of the Shh pathway in the inhibition of cancer
remains controversial (24,33,34).
Interference with Shh-Gli-1 signaling may inhibit the
proliferation of prostate cancer cells (35). Chen et al considered that the
downregulation of Bcl-2 was important in HCC following cyclopamine
treatment (19). Kim et al
reported that the suppression of Gli-2 expression is
significant (25).
There are some limitations of the present study. One
is that the most effective and tolerable dose of cyclopamine for
the treatment of HCC in mice requires further study. The second is
that the side-effects of this drug at higher doses in humans need
to be understood. The third is that it remains unknown whether the
treatment outcome would be improved if a longer treatment period
was used.
We conclude that cyclopamine may effectively inhibit
HCC in mice in vivo. The results also indicate that blockade
of the Shh signaling pathway may potentially be an effective
treatment target for HCC.
Acknowledgements
The authors are grateful for the
support of the Far Eastern Memorial Hospital and the financial
support from a research grant from the National Science Council,
Executive Yuan, Taiwan (NSC-97-2314-B-195-008-MY3).
References
|
1.
|
FX BoschJ RibesJ BorràsEpidemiology of
primary liver cancerSemin Liver
Dis19271285199910.1055/s-2007-100711710518307
|
|
2.
|
DM ParkinF BrayJ FerlayP PisaniEstimating
the world cancer burden: Globocan 2000Int J
Cancer94153156200110.1002/ijc.144011668491
|
|
3.
|
AP VenookC PapandreouJ FuruseLL de
GuevaraThe incidence and epidemiology of hepatocellular carcinoma:
a global and regional
perspectiveOncologist15513201010.1634/theoncologist.2010-S4-0521115576
|
|
4.
|
MF ChenTL HwangLB JengPostoperative
recurrence of hepatocellular carcinoma: 205 consecutive patients
who underwent hepatic resection in 15 yearsArch
Surg12973874219947517662
|
|
5.
|
KS JengIS SheenYC TsaiDoes the presence of
circulating hepatocellular carcinoma cells indicate a risk of
recurrence after resection?Am J
Gastroenterol9915031509200410.1111/j.1572-0241.2004.30227.x15307868
|
|
6.
|
AE BaleKP YuThe hedghog pathway and basal
cell carcinomasHum Mol
Genet10757762200110.1093/hmg/10.7.75711257109
|
|
7.
|
O LupiCorrelations between the sonic
hedgehog pathway and basal cell carcinomaInt J
Dermatol4611131117200710.1111/j.1365-4632.2007.03391.x17988327
|
|
8.
|
SS KarhadkarGS BovaN AbdallahHedgehog
signaling in prostate regeneration, neoplasia and
metastasisNature431707712200410.1038/nature0296215361885
|
|
9.
|
DN WarkinsCD PeacockHedgehog signalling in
foregut malignancyBiochem
Pharmacol6810551060200410.1016/j.bcp.2004.04.02515313401
|
|
10.
|
X MaK ChenS HuangFrequent activation of
the hedgehog pathway in advanced gastric
adenocarcinomasCarcinogenesis2616981705200510.1093/carcin/bgi13015905200
|
|
11.
|
X MaT ShengY ZhangHedgehog signaling is
activated in subsets of esophageal cancersInt J
Cancer118139148200610.1002/ijc.2129516003737
|
|
12.
|
NS ChariTJ McDonnellThe sonic hedgehog
signaling network in development and neoplasiaAdv Anat
Pathol14344352200710.1097/PAP.0b013e3180ca8a1d17717435
|
|
13.
|
SP ThayerMP di MaglianoPW HeiserHedgehog
is an early and late mediator of pancreatic cancer
tumorigenesisNature425851856200310.1038/nature0200914520413
|
|
14.
|
S HuangJ HeX ZhangActivation of the
hedgehog pathway in human hepatocellular
carcinomasCarcinogenesis2713341340200610.1093/carcin/bgi37816501253
|
|
15.
|
JK SicklickYX LiA JayaramanDysregulation
of the hedgehog pathway in human
hepatocarcinogenesisCarcinogenesis27748757200610.1093/carcin/bgi29216339184
|
|
16.
|
MA PatilJ ZhangC HoST CheungHedgehog
signaling in human hepatocellular carcinomaCancer Biol
Ther5111117200610.4161/cbt.5.1.237916397407
|
|
17.
|
L CheJ RenYH YuanExpression of genes
related to sonic hedgehog signaling in human hepatocellular
carcinomasBeijing Da Xue Xue Bao406166232008(In Chinese).
|
|
18.
|
X FuQ WangX ChenExpression patterns and
polymorphisms of PTCH in Chinese hepatocellular carcinoma
patientsExp Mol
Pathol84195199200810.1016/j.yexmp.2008.04.00218538319
|
|
19.
|
XL ChenQY ChengMR SheExpression of sonic
hedgehog signaling components in hepatocellular carcinoma and
cyclopamine-induced apoptosis through Bcl-2 downregulation in
vitroArch Med
Res41315323201010.1016/j.arcmed.2010.06.00320851287
|
|
20.
|
WT ChengK XuDY TianZG ZhangLJ LiuY
ChenRole of Hedgehog signaling pathway in proliferation and
invasiveness of hepatocellular carcinoma cellsInt J
Oncol34829836200919212688
|
|
21.
|
M TadaF KanaiY TanakaDown-regulation of
hedgehog-interacting protein through genetic and epigenetic
alterations in human hepatocellular carcinomaClin Cancer
Res1437683776200810.1158/1078-0432.CCR-07-118118559595
|
|
22.
|
J TaipalePA BeachyThe Hedgehog and Wnt
signaling pathways in
cancerNature411349354200110.1038/3507721911357142
|
|
23.
|
F TianJ MysliwietzJ EllwartEffects of the
hedgehog pathway inhibitor GDC-0449 on lung cancer cell lines are
mediated by side populationsClin Exp
Med122530201210.1007/s10238-011-0135-821519961
|
|
24.
|
FC KelleherHedgehog signaling and
therapeutics in pancreatic
cancerCarcinogenesis32445451201110.1093/carcin/bgq28021186299
|
|
25.
|
Y KimJW YoonX XiaoNM DeanBP MoniaEG
MarcussonSelective down-regulation of glioma-associated oncogene 2
inhibits the proliferation of hepatocellular carcinoma cellsCancer
Res6735833593200710.1158/0008-5472.CAN-06-304017440069
|
|
26.
|
M EichenmüllerI GrunerB HaglBlocking the
hedgehog pathway inhibits hepatoblastoma
growthHepatology49482490200919177589
|
|
27.
|
C AdolpheR HetheringtonT EllisB
WainwrightPatched1 functions as a gatekeeper by promoting cell
cycle progressionCancer
Res6620812088200610.1158/0008-5472.CAN-05-214616489008
|
|
28.
|
YJ KimJY ParkSW ParkThe sonic hedgehog
pathway as a treatment target for extrahepatic biliary tract
cancerMol Med Report51216201221946948
|
|
29.
|
MH TaiCC ChangM KiupelOct4 expression in
adult human stem cells: evidence in support of the stem cell theory
of carcinogenesisCarcinogenesis26495502200515513931
|
|
30.
|
CT JordanSearching for leukemia stem cells
- not yet the end of the road?Cancer
Cell10253254200610.1016/j.ccr.2006.09.01017045202
|
|
31.
|
M ShackletonE QuintanaER FearonSJ
MorrisonHeterogeneity in cancer: cancer stem cells versus clonal
evolutionCell138822829200910.1016/j.cell.2009.08.01719737509
|
|
32.
|
JM BaileyAM MohrMA HollingsworthSonic
hedgehog paracrine signaling regulates metastasis and
lymphangiogenesis in pancreatic
cancerOncogene2835133525200910.1038/onc.2009.22019633682
|
|
33.
|
M NakamuraM KuboS NagaiNew therapeutic
strategy for cancer targeting the hedgehog signaling pathwayGan To
Kagaku Ryoho34191419162007(In Japanese).
|
|
34.
|
RLH BigelowNS ChariAB UndenTranscriptional
regulation of bcl-2 mediated by the sonic hedgehog signaling
pathway through gli-1J Biol Chem279119712052004
|
|
35.
|
P SanchezAM HernándezB SteccaInhibition of
prostate cancer proliferation by interference with sonic
hedgehog-Gli-1 signalingProc Natl Acad Sci
USA1011256112566200410.1073/pnas.040495610115314219
|