Introduction
Malignant tumours are considered to be in a hypoxic
state (1). Under hypoxic
conditions, cancer cells switch from oxygen-dependent glucose
metabolism to oxygen-independent glycolysis (2). Malignant cells show increased glucose
uptake in vitro and in vivo. This process is
considered to be mediated by glucose transporters (GLUTs). Among
GLUTs, GLUT-1 is one of the most significant mediators of increased
glucose influx into cells (3). The
rate of glucose transport via GLUT-1 may be altered under
conditions in which the metabolic rate must be adjusted, including
during cell division (mitosis and meiosis), differentiation,
transformation and nutrient starvation (4). In previous studies (5–11),
Glut-1 expression has been correlated with lymph node metastasis,
poor survival rate and clinical stage of head and neck carcinoma
(HNC).
Hypoxia-inducible factor-1α (HIF-1α), a
transcription factor associated with the cellular response to
hypoxia (12), upregulates the
expression of several hypoxia response genes, including GLUT-1
(1). The overexpression of GLUT-1
increases glucose transport to meet the energy requirements of
malignant tumour cells. In numerous types of human cancer, the
correlation between HIF-1α and GLUT-1 expression may promote tumour
progression, leading to a poor outcome (1,13–18),
although the correlation between HIF-1α and GLUT-1 expression in
colorectal cancer is controversial (19,20).
To the best of our knowledge, only one study exists
in the English-language literature with regard to the correlation
between HIF-1α and GLUT-1 expression in laryngeal carcinoma and
their association with clinicopathological features (21). This study identified no correlation
between GLUT-1 overexpression and clinical outcome parameters of
laryngeal carcinoma. A small quantity of studies have investigated
HIF-1α (22,23) or GLUT-1 expression alone (24,25) in
laryngeal carcinoma. The present study investigated the correlation
between HIF-1α and GLUT-1 expression with respect to various
clinical and pathological features of laryngeal carcinoma.
Patients and methods
Patients and tissues
A total of 49 paraffin-embedded archival tissue
blocks from patients with laryngeal squamous cell carcinomas were
obtained between 2002 and 2009; six blocks were from the surgical
pathological files at The People’s Hospital of Deqing County
(Zhejiang, China) and 43 blocks were from the surgical pathological
files at The First Affiliated Hospital, College of Medicine,
Zhejiang University (Deqing City, China). A total of 15
paraffin-embedded archival tissue blocks from patients with vocal
cord polyps and 15 paraffin-embedded archival tissue blocks from
patients with vocal cord leukokeratosis were also obtained.
Formalin-fixed, paraffin-embedded archival tissues were obtained
from institutional and consultation files. One representative
paraffin block from each tumour was selected for the
immunohistochemical study. Diagnosis was confirmed following blind
review of all haematoxylin- and eosin-stained sections. None of the
patients had received preoperative radiotherapy or chemotherapy.
Demographic and clinicopathological data, including gender, age, T
and N category (as established by the International Union Against
Cancer TNM classification 2007, 7th edition) and current and past
tobacco and alcohol use were retrospectively collected by reviewing
the patient charts. Institutional Review Board approvals were
obtained through The People’s Hospital of Deqing County and The
First Affiliated Hospital, College of Medicine, Zhejiang
University.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue blocks
from primary lesions were cut into 4-μm sections and
representative sections were analysed immunohistochemically
(EliVision™ Plus IHC kit; Fuzhou Maixin Biotechnology Development
Co., Ltd., Fuzhou, China) for HIF-1α (1:100; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and GLUT-1 (1:50, rabbit
polyclonal; Santa Cruz Biotechnology) expression (22). Briefly, the sections were
deparaffinised in xylene and rehydrated through graded
concentrations of alcohol. Antigen retrieval was performed in a
microwave oven for two cycles of 10 min each. Endogenous peroxidase
activity was blocked by incubating the sections in 1.5% hydrogen
peroxide in absolute methanol at room temperature for 10 min.
Primary antibodies were applied for 1 h at room temperature and the
sections were washed three times with 0.05 M Tris-buffered saline
(TBS, pH 7.2). Then, 50 μl of polymer enhancer were added
and following 20 min, 50 μl of polymerised horseradish
peroxidase anti-mouse immunoglobulin G were added, followed by
incubation for 30 min at room temperature. The sections were washed
three times with TBS and the reaction products were visualised with
diaminobenzidine (DAB kit; Maixin Biological Technology Ltd.,
Fujian, China). The sections were counterstained with haematoxylin
and eosin, dehydrated and evaluated under a light microscope. As a
negative control, samples were incubated using 10 mM TBS (pH 7.4)
instead of a primary antibody. Erythrocytes in the tissue sections
served as the positive control for GLUT-1 expression (5). Nuclear and cytoplasmic HIF-1α staining
was scored as reported previously (22).
Scoring of immunopositive cells
Positive staining for HIF-1α and GLUT-1 was assessed
in 10 high-power fields of each tumour by two pathologists using
light microscopy. The mean rate of positive tumour cells was
calculated. Positive expression and negative expression were
defined as immunostaining of >10% and <10% of the cancer
cells, respectively.
Statistical analysis
Associations between HIF-1α and GLUT-1
immunostaining and other parameters (age, T and N category, tobacco
and alcohol use) were analysed using a χ2 test and
Fisher’s exact test. P≤0.05 was considered to indicate a
statistically significant difference. The correlation between
HIF-1α and GLUT-1 was analysed by Spearman’s correlation. Overall
survival rate (OS), defined as the time from surgery until
mortality from any cause, was plotted as a Kaplan-Meier curve.
Univariate survival rate analysis was performed using a log-rank
test and multivariate analysis was performed using Cox
proportional-hazards regression analysis. All analyses were
conducted using SPSS for Windows (ver. 19.0; SPSS, Inc., Chicago,
IL, USA).
Results
Patient characteristics
Of the 49 laryngeal carcinoma tissue samples, 43
were from male patients and 6 were from female patients, yielding a
male to female ratio of ∼7:1. The median age of the 49 patients was
60.8 years (range, 32–81). Of the 49 laryngeal carcinomas, 32
(65.3%) were located in the glottic area; 14 (28.6%) in the
supraglottic area and three (6.1%) in the subglottic area. At the
time of diagnosis, 12 patients (24.5%) presented with lymph node
metastases. Further details of the patients and tumours are
presented in Table I.
 | Table IClinicopathological findings of 49
laryngeal carcinomas. |
Table I
Clinicopathological findings of 49
laryngeal carcinomas.
| Characteristics | No. (%) | HIF-α-positive
(%) | χ2 | P-value | GLUT-1-positive
(%) | χ2 | P-value |
|---|
| Gender | | | | | | | |
| Male | 43 (87.8) | 29 (59.2) | 1.37 | 0.24 | 24 (49.0) | 0.07 | 0.56 |
| Female | 6 (12.2) | 2 (4.1) | | | 3 (6.1) | | |
| Age (years) | | | | | | | |
| <60 | 25 (51.0) | 14 (28.6) | 0.61 | 0.44 | 12 (24.5) | 1.05 | 0.23 |
| ≥60 | 24 (49.0) | 17 (34.7) | | | 15 (30.6) | | |
| Primary tumour
site | | | | | | | |
| Supraglottic | 14 (28.6) | 10 (20.4) | 0.64 | 0.73 | 10 (20.4) | 2.61 | 0.28 |
| Glottic | 32 (65.3) | 19 (38.8) | | | 15 (30.6) | | |
| Subglottic | 3 (6.1) | 2 (4.1) | | | 2 (4.1) | | |
| Tumour
classification | | | | | | | |
| T1 | 18 (36.7) | 8 (16.3) | 0.136a | | 8 (16.3) | 0.53a | |
| T2 | 23 (46.9) | 16 (32.7) | | | 13 (26.5) | | |
| T3 | 7 (14.3) | 6 (12.2) | | | 5 (10.2) | | |
| T4a | 1 (2.0) | 1 (2.0) | | | 1 (2.0) | | |
| Lymph node
metastasis | | | | | | | |
| Yes | 12 (24.5) | 11 (22.4) | 6.50 | 0.018 | 9 (18.4) | 2.66 | 0.10 |
| No | 37 (75.5) | 20 (40.8) | | | 18 (36.7) | | |
| Pathological
type | | | | | | | |
| SCC | 46 (93.9) | 30 (61.2) | 0.30a | | 26 (53.1) | 0.61 | 0.42 |
| ACC | 3 (6.1) | 1 (2.0) | | | 1 (2.0) | | |
| Histological
grade | | | | | | | |
|
Well-differentiated | 24 (49.0) | 13 (26.5) | 3.19 | 0.20 | 12 (24.5) | 2.71 | 0.29 |
| Moderately
differentiated | 13 (26.5) | 8 (16.3) | | | 6 (12.2) | | |
| Poorly
differentiated | 12 (24.5) | 10 (20.4) | | | 9 (18.4) | | |
| Recurrence | | | | | | | |
| Yes | 20 (40.8) | 17 (34.7) | 5.38 | 0.02 | 15 (30.6) | 5.59 | 0.02 |
| No | 29 (59.2) | 14 (28.6) | | | 12 (24.5) | | |
| Metastasis | | | 0.031a | | | 0.01a | |
| Yes | 7 (14.3) | 7 (14.3) | | | 7 (14.3) | | |
| No | 42 (85.7) | 24 (49.0) | | | 20 (40.8) | | |
The average follow-up period was 42.6 months (range,
13–181) and 4 patients were lost to follow-up. A total of 20
patients (40.8%) developed recurrence and seven (14.3%) developed
distant metastases. In total, 6 patients (12.2%) succumbed to
distant metastasis and local recurrence and seven (14.3%) succumbed
to local recurrence of the disease. Among the 13 deceased patients,
9 had tumours in the supraglottic area and 4 had tumours in the
glottic area (P<0.001). In total, 36 were alive at the last
follow-up.
HIF-1α and GLUT-1 expression in laryngeal
carcinoma and associations with clinicopathological variables and
prognosis
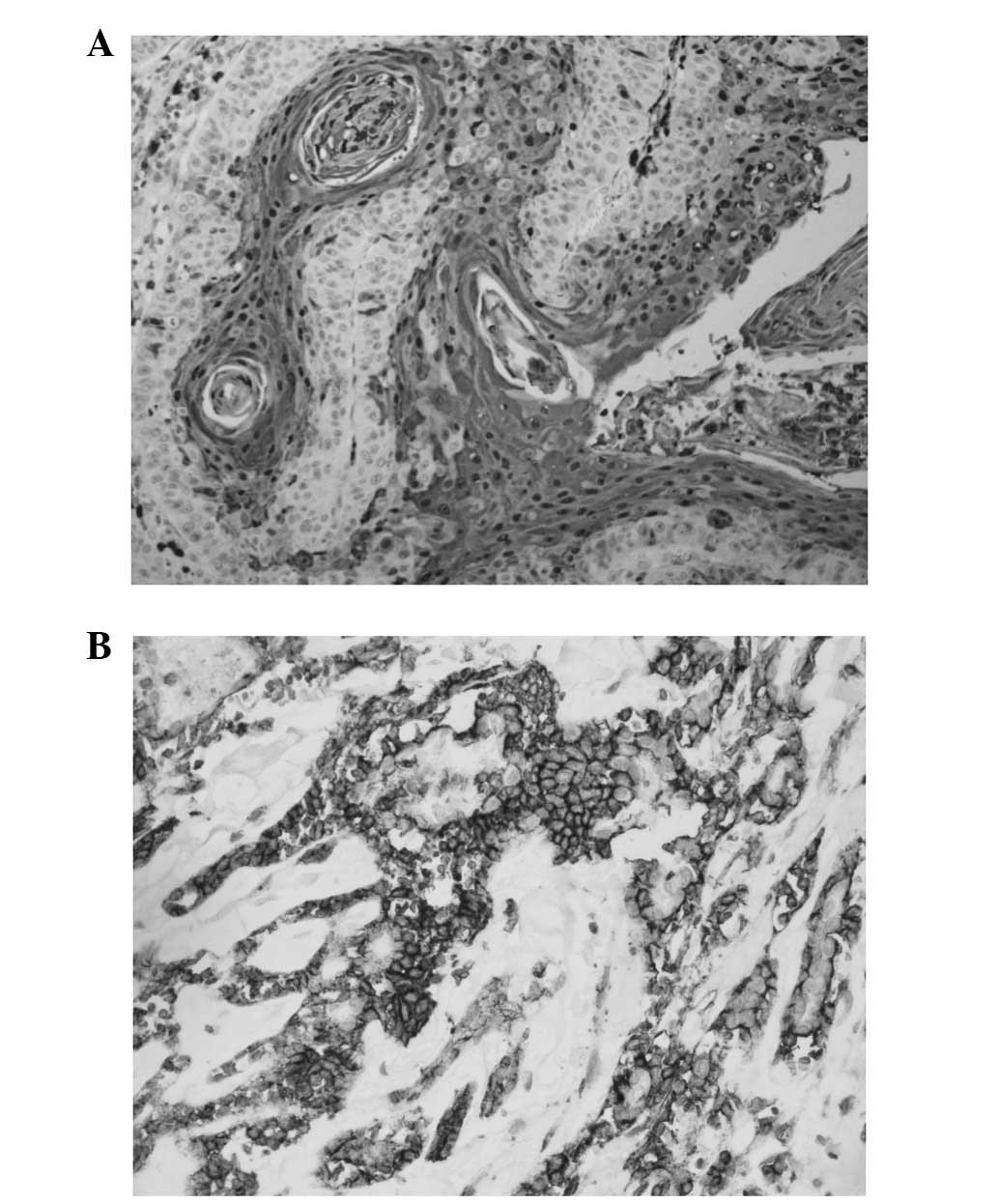
Staining for HIF-1α occurred in a granular and
diffuse pattern localised mainly in the cytoplasm of cancer cells,
although staining was occasionally nuclear and cytoplasmic
(Fig. 1A). HIF-1α expression was
detected in 63.3% (31/49) of the tumours. HIF-1α expression was
higher in laryngeal carcinoma than in cord polyp or vocal cord
leukokeratosis (P<0.05; Table
II). HIF-1α expression did not differ significantly according
to patient gender, age, tumour site, T classification, pathological
type or histological grade (Table
I). However, HIF-1α expression was significantly correlated
with lymph node classification (P=0.018), recurrence (P=0.02) and
metastasis (P=0.031) (Table I).
 | Table IIImmunohistochemical results of HIF-1α
and GLUT-1 in laryngeal carcinomas, cord polyp and vocal cord
leukokeratosis. |
Table II
Immunohistochemical results of HIF-1α
and GLUT-1 in laryngeal carcinomas, cord polyp and vocal cord
leukokeratosis.
| | HIF-1α
| | GLUT-1
| |
|---|
| Group | n | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| Cord polyp | 15 | 0 | 15a | 0.000a | 0 | 15a | 0.000a |
| Leukokeratosis | 15 | 2 | 13b | 0.001b | 3 | 12b | 0.02b |
| Laryngeal
carcinomas | 49 | 31 | 18 | | 27 | 22 | |
Staining for GLUT-1 occurred in a diffuse pattern
localised in the membrane of cancer cells (Fig. 1B). GLUT-1 expression was detected in
55.1% (27/49) of the tumours and was higher in laryngeal carcinoma
than in cord polyp or vocal cord leukokeratosis (P<0.05;
Table II). GLUT-1 expression did
not differ significantly according to patient age, tumour site, T
classification, pathological type, histological grade or lymph node
classification. However, GLUT-1 expression was significantly
correlated with recurrence (P=0.02) and metastasis (P=0.01)
(Table I).
The median OS was 134 months [95% confidence
interval (CI), 113–154]. The 3- and 5-year OS rates were 77.0%
[standard error (SE), 0.06] and 69.0% (SE, 0.07), respectively.
Univariate analysis revealed that improved survival rate was
significantly associated with a primary cancer site in the glottic
area (χ2=15.5, P<0.001), well-differentiated
carcinoma (χ2=8.4, P=0.004), early T classification
(T1+T2; χ2=10.2, P=0.001), no lymph node involvement
(χ2=33.1, P<0.001), no recurrence
(χ2=31.0, P<0.001) and no metastasis
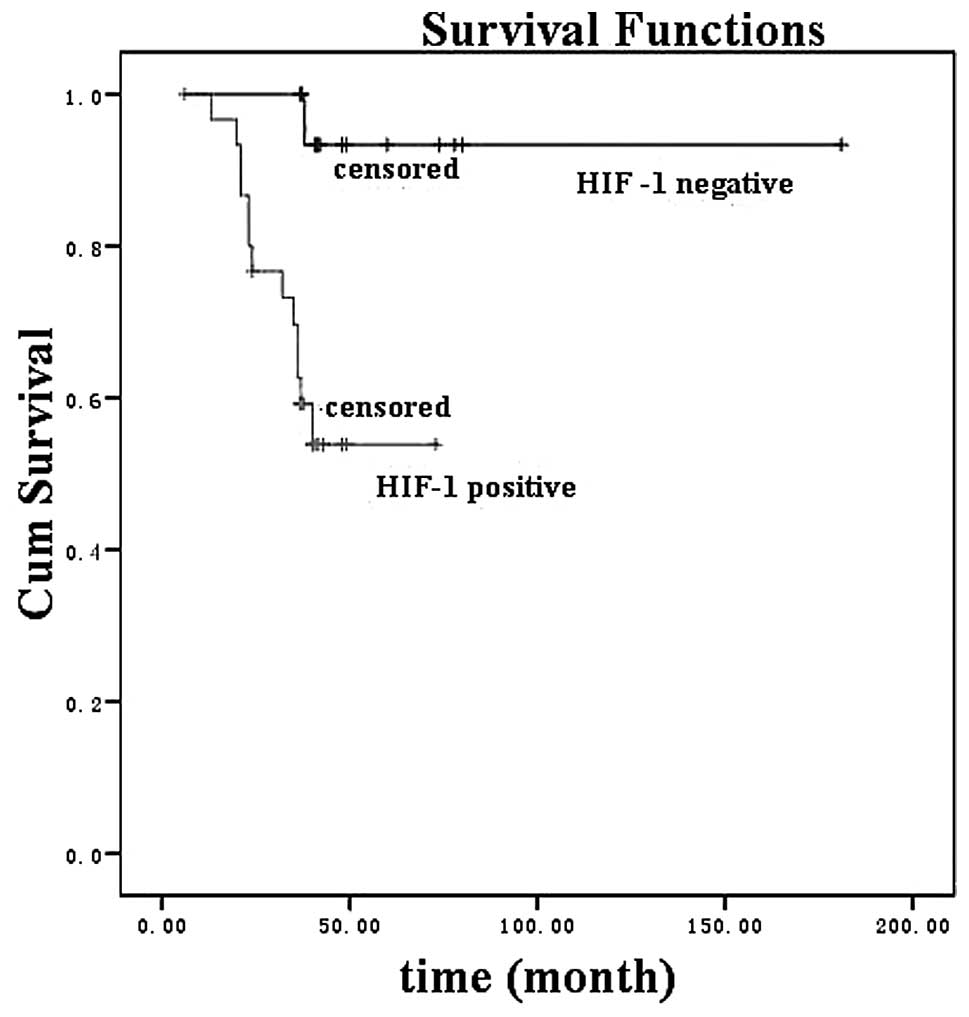
(χ2=20.9, P<0.001). HIF-1α expression (Fig. 2; χ2 =8.2, P=0.004) and
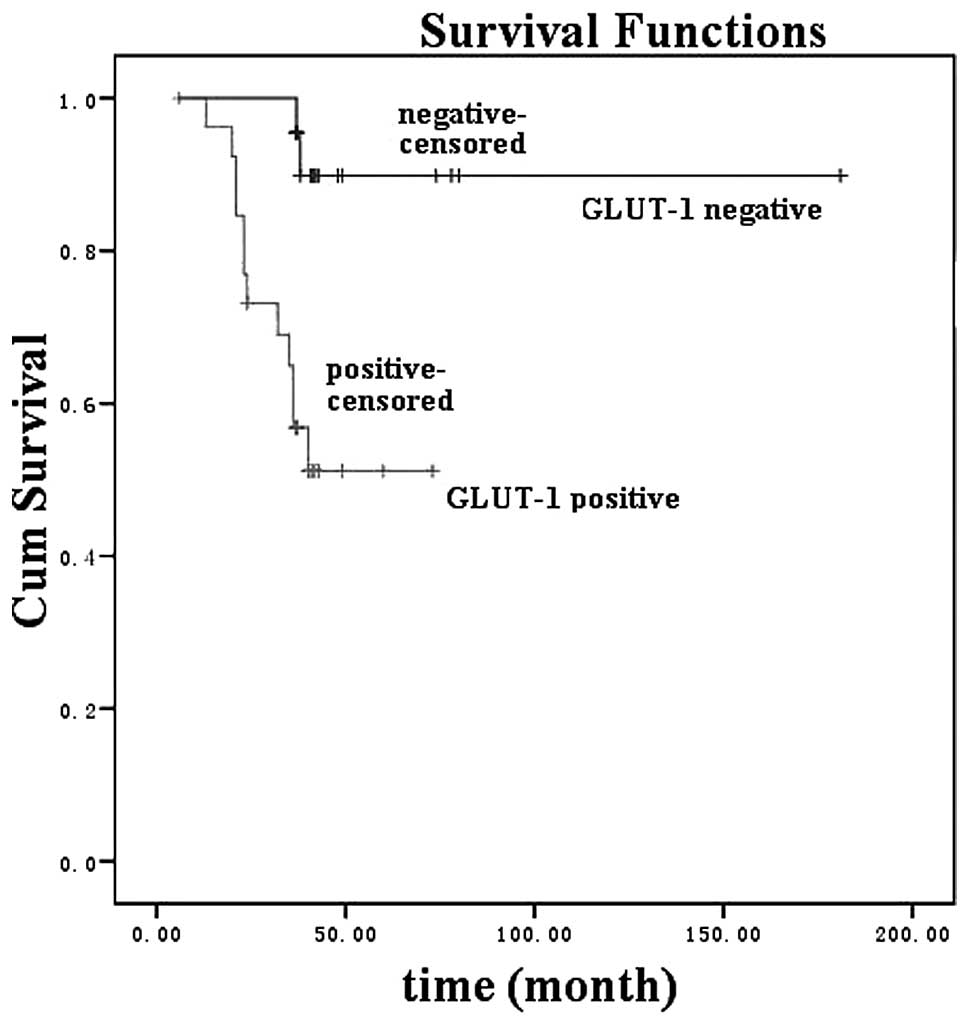
GLUT-1 expression (Fig. 3;
χ2 =9.0, P= 0.003) were significantly associated with a
poorer survival rate in a univariate analysis. In a multivariate
analysis, significant predictors of poor survival rate included a
primary cancer site in the supraglottic and subglottic areas (P=
0.038), lymph node invasion (P= 0.007), distant metastasis (P=
0.006) and GLUT-1 expression (P=0.006).
Correlation between HIF-1α and GLUT-1
expression
Spearman’s analysis revealed a significant
correlation between GLUT-1 and phosphatidylinositol 3-kinase (PI3K)
expression (r=0.504, P=0.000).
Discussion
Hypoxia, a common feature of malignancy and
particularly of solid tumours, is thought to promote tumour
invasiveness and metastasis (26).
HIF is a key regulator of cellular responses to hypoxia (12). It targets the genes involved in
tumour cell energy metabolism, angiogenesis, tumour metastasis, ion
metabolism and catecholamine metabolism, thereby influencing the
expression of proteins, including erythropoietin, vascular
endothelial growth factor, GLUT-1, glyceraldehyde 3-phosphate
dehydrogenase, inducible nitric oxide synthase, insulin-like growth
factor-2, tyrosine hydroxylase and glycolytic enzymes (27). HIF-1α and GLUT-1 are the intrinsic
hypoxia markers that have been studied the most in various tumours
(13–18). Nevertheless, few studies have
investigated the value of HIF-1α (22,23,28,29) or
GLUT-1 expression (24,25) alone for the prediction of clinical
outcome and survival rate of laryngeal carcinoma and, to the best
of our knowledge, there is only one study in the English-language
literature with regard to the correlation between HIF-1α and GLUT-1
expression in laryngeal carcinoma (21).
In a previous study, 67.5% (27/40) of patients with
laryngeal carcinoma were immunopositive for HIF-1α and HIF-1α
expression was associated with T stage and lymph node metastasis
(22). Similarly, in the present
study, the overexpression of HIF-1α was immunohistochemically
detected in 63.3% (31) of another 49 laryngeal carcinomas and
HIF-1α expression was significantly correlated with lymph node
classification, recurrence and metastasis. Yu et al revealed
that HIF-1α was correlated with the clinical stage of laryngeal
cancer and lymph node metastasis (23). Wildeman et al suggested that
the effect of hypoxia-related proteins, including carbonic
anhydrase IX and HIF-1α, on an increased risk for local recurrence
of laryngeal carcinoma is stronger following radiotherapy (29). Cabanillas et al reported a
significant positive correlation between HIF-1α and T
classification, but no association was observed with other
clinicopathological variables or with the prognosis of supraglottic
laryngeal squamous cell carcinoma (28). Although HIF-1α expression was
significantly associated with a poorer survival rate in a
univariate model in the present study, multivariate analysis
revealed no significant association. These apparent discrepancies
concerning the correlation between HIF-1α and survival rate may be
attributable to variations in therapeutics or in patient
populations, as the disease course and prognosis of head and neck
squamous cell carcinoma are known to differ regionally and
different patients may carry distinct genetic alterations (28). These limitations may make studies of
HIF-1α in head and neck squamous cell carcinoma susceptible to bias
(28).
In a previous study of GLUT-1 expression in
laryngeal carcinoma, the expression of GLUT-1 mRNA and protein was
increased in laryngeal carcinoma Hep-2 cells and an antisense
oligonucleotide against GLUT-l mRNA reduced the expression of
GLUT-1 mRNA and protein, thus inhibiting glucose uptake and cell
growth in Hep-2 cells (25). Based
on these results, it was suggested that GLUT-1 is a potential
therapeutic target for strategies designed to inhibit the
progression of laryngeal cancer (25). The present study also demonstrated
the overexpression of GLUT-1 in laryngeal carcinoma tissues and
there was a significant correlation between GLUT-1 expression and
recurrence and metastasis. In univariate and multivariate analyses,
increased GLUT-1 expression was significantly associated with a
poorer survival rate. GLUT-1 may serve as an independent survival
rate predictor, similar to the primary tumour site, lymph node
invasion and distant metastasis of laryngeal carcinoma in the
present series. The present study provides further support for
considering GLUT-1 as a new therapeutic target for laryngeal
carcinoma.
In normal cells, HIF-1α upregulates GLUT-1
expression in response to hypoxic injury (20). In cancer cells, the HIF-1α-induced
increase in GLUT-1 serves to provide for the energy requirements of
malignant tumour cells. However, certain studies do not reflect
this phenomenon. Schrijvers et al reported that there was no
significant correlation between GLUT-1 and HIF-1α expression
detected immunohistochemically in 91 stage T1-T2 glottic laryngeal
carcinomas treated with radiotherapy and that only HIF-1α was a
predictor of poor survival rate (21). This is contrary to the results of
the present study. Contradictory results have also been revealed
among studies of other types of cancer and the reason for this
remains unknown. Yasuda et al suggested that GLUT-1
expression is not fully regulated by HIF-1α in ovarian
adenocarcinoma. Heterogeneous GLUT-1 expression cannot be
satisfactorily explained solely through regulation by HIF-1α and
GLUT-1 overexpression may be more strongly affected by
micro-environmental conditions (1).
Koukourakis et al identified no association between HIF-1α
and GLUT-1 expression and clinicopathological characteristics in
colorectal cancer, reporting GLUT-1 immunoreactivity in not only
cancer cells but also the endothelium (19). Wincewicz et al reported that
HIF-1α expression was correlated with GLUT-1 expression in
colorectal cancer and suggested that HIF-1α-dependent induction of
GLUT-1 is difficult to demonstrate, given that HIF-1α is detected
mainly in the cytoplasm, while it exerts its transcriptional
activity in the nucleus (20). In
renal cell carcinoma, Lidgren et al revealed that HIF-1α and
GLUT-1 expression levels were significantly correlated with
chromophobe renal cell carcinoma (cRCC), but not with papillary
renal cell carcinoma (pRCC) (17).
In addition, GLUT-1 was overexpressed mainly in cRCC, but not in
pRCC, suggesting that other pathways for glucose metabolism are
involved in various types of RCC (17).
Thus, numerous factors, including histopathological
type, immunohistochemical techniques, tumour stage, sample number
and other transcriptional regulators, may affect the correlation
between HIF-1α and GLUT-1 expression in types of cancer and the
correlation between their expression and clinicopathological
variables and cancer prognosis. HIF-1α and GLUT-1 expression in
carcinomas requires further study.
The current study reports the first finding of a
significant correlation between GLUT-1 and HIF-1α expression in
laryngeal carcinoma. Overexpression of HIF-1α was significantly
correlated with lymph node classification, recurrence and
metastasis. Increased GLUT-1 expression was significantly
associated with recurrence and metastasis of laryngeal carcinoma
and may serve as an independent survival rate predictor. The
present results further indicate that GLUT-1 may be a potential new
therapeutic target for laryngeal carcinoma.
Acknowledgements
This study was supported by the
Science and Technology Bureau of Deqing County, Zhejiang Province,
China (No. 2009Ny01), Department of Science and Technology of
Zhejiang Provincial (contract grant number: 2009C33026), Health
Bureau of Zhejiang Province (contract grant number: 2010KYA062) and
National Natural Science Foundation of China (No. 81172562).
References
|
1
|
Yasuda M, Miyazawa M, Fujita M, et al:
Expression of hypoxia inducible factor-1α (HIF-1α) and glucose
transporter-1 (GLUT-1) in ovarian adenocarcinomas: difference in
hypoxic status depending on histological character. Oncol Rep.
19:111–116. 2008.
|
|
2
|
Havelund BM, Sørensen FB, Lindebjerg J,
Spindler KL and Jakobsen A: Pretreatment HIF-1α and GLUT-1
expressions do not correlate with outcome after preoperative
chemoradiotherapy in rectal cancer. Anticancer Res. 31:1559–1565.
2011.
|
|
3
|
Gu J, Yamamoto H, Fukunaga H, et al:
Correlation of GLUT-1 overexpression, tumor size, and depth of
invasion with 18F-2-fluoro-2-deoxy-D-glucose uptake by positron
emission tomography in colorectal cancer. Dig Dis Sci. 51:198–205.
2006.PubMed/NCBI
|
|
4
|
Merrall NW, Plevin R and Gould GW: Growth
factors, mitogens, oncogenes and the regulation of glucose
transport. Cell Signal. 5:667–675. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou S, Wang S, Wu Q, Fan J and Wang Q:
Expression of glucose transporter-1 and -3 in the head and neck
carcinoma - the correlation of the expression with the biological
behaviors. ORL J Otorhinolaryngol Relat Spec. 70:189–194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eckert AW, Lautner MH, Taubert H, Schubert
J and Bilkenroth U: Expression of Glut-1 is a prognostic marker for
oral squamous cell carcinoma patients. Oncol Rep. 20:1381–1385.
2008.PubMed/NCBI
|
|
7
|
Deron P, Vermeersch H, Mees G, Vangestel
C, Pauwels P and Van de Wiele C: Expression and prognostic value of
glucose transporters and hexokinases in tonsil and mobile tongue
squamous cell carcinoma. Histol Histopathol. 26:1165–1172.
2011.PubMed/NCBI
|
|
8
|
Nakajo M, Nakajo M, Tani A, et al:
Clinical significance of primary lesion FDG uptake for choice
between oesophagectomy and endoscopic submucosal dissection for
resectable oesophageal squamous cell carcinomas. Eur Radiol.
21:2396–2407. 2011. View Article : Google Scholar
|
|
9
|
Kondo Y, Yoshikawa K, Omura Y, et al:
Clinicopathological significance of carbonic anhydrase 9, glucose
transporter-1, Ki-67 and p53 expression in oral squamous cell
carcinoma. Oncol Rep. 25:1227–1233. 2011.PubMed/NCBI
|
|
10
|
Deron P, Vangestel C, Goethals I, et al:
FDG uptake in primary squamous cell carcinoma of the head and neck.
The relationship between overexpression of glucose transporters and
hexokinases, tumour proliferation and apoptosis. Nuklearmedizin.
50:15–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ayala FR, Rocha RM, Carvalho KC, et al:
GLUT1 and GLUT3 as potential prognostic markers for oral squamous
cell carcinoma. Molecules. 15:2374–2387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pez F, Dayan F, Durivault J, et al: The
HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway
in a positive regulation loop and synergizes with HIF-1 in
promoting tumor cell growth. Cancer Res. 71:1647–1657. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eckert AW, Lautner MH, Schütze A, Taubert
H, Schubert J and Bilkenroth U: Coexpression of hypoxia-inducible
factor-1α and glucose transporter-1 is associated with poor
prognosis in oral squamous cell carcinoma patients. Histopathology.
58:1136–1147. 2011.
|
|
14
|
Ogane N, Yasuda M, Shimizu M, et al:
Clinicopathological implications of expressions of hypoxia-related
molecules in esophageal superficial squamous cell carcinoma. Ann
Diagn Pathol. 14:23–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sulkowska M, Wincewicz A, Sulkowski S,
Koda M and Kanczuga-Koda L: Relations of TGF-beta1 with HIF-1alpha,
GLUT-1 and longer survival of colorectal cancer patients.
Pathology. 41:254–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iida T, Yasuda M, Miyazawa M, et al:
Hypoxic status in ovarian serous and mucinous tumors: relationship
between histological characteristics and HIF-1α/GLUT-1 expression.
Arch Gynecol Obstet. 277:539–546. 2008.PubMed/NCBI
|
|
17
|
Lidgren A, Bergh A, Grankvist K, Rasmuson
T and Ljungberg B: Glucose transporter-1 expression in renal cell
carcinoma and its correlation with hypoxia inducible factor-1
alpha. BJU Int. 101:480–484. 2008.PubMed/NCBI
|
|
18
|
Palit V, Phillips RM, Puri R, Shah T and
Bibby MC: Expression of HIF-1α and Glut-1 in human bladder cancer.
Oncol Rep. 14:909–913. 2005.
|
|
19
|
Koukourakis MI, Giatromanolaki A, Harris
AL and Sivridis E: Comparison of metabolic pathways between cancer
cells and stromal cells in colorectal carcinomas: a metabolic
survival role for tumor-associated stroma. Cancer Res. 66:632–637.
2006. View Article : Google Scholar
|
|
20
|
Wincewicz A, Sulkowska M, Koda M and
Sulkowski S: Clinicopathological significance and linkage of the
distribution of HIF-1α and GLUT-1 in human primary colorectal
cancer. Pathol Oncol Res. 13:15–20. 2007.PubMed/NCBI
|
|
21
|
Schrijvers ML, van der Laan BF, de Bock
GH, et al: Overexpression of intrinsic hypoxia markers HIF1α and
CA-IX predict for local recurrence in stage T1-T2 glottic laryngeal
carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys.
72:161–169. 2008.PubMed/NCBI
|
|
22
|
Wu XH, Lu YF, Hu XD, et al: Expression of
hypoxia inducible factor-1α and its significance in laryngeal
carcinoma. J Int Med Res. 38:2040–2046. 2010.
|
|
23
|
Yu L, Liu Y and Cui Y: Expression of
hypoxia inducible factor-1alpha and its relationship to apoptosis
and proliferation in human laryngeal squamous cell carcinoma. J
Huazhong Univ Sci Technolog Med Sci. 24:636–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo XM, Zhou SH and Fan J: Glucose
transporter-1 as a new therapeutic target in laryngeal carcinoma. J
Int Med Res. 38:1885–1892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou SH, Fan J, Chen XM, Cheng KJ and Wang
SQ: Inhibition of cell proliferation and glucose uptake in human
laryngeal carcinoma cells by antisense oligonucleotides against
glucose transporter-1. Head Neck. 31:1624–1633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weljie AM and Jirik FR: Hypoxia-induced
metabolic shifts in cancer cells: moving beyond the Warburg effect.
Int J Biochem Cell Biol. 43:981–989. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wan J, Chai H, Yu Z, et al: HIF-1α effects
on angiogenic potential in human small cell lung carcinoma. J Exp
Clin Cancer Res. 30:772011.
|
|
28
|
Cabanillas R, Rodrigo JP, Secades P, et
al: The relation between hypoxia-inducible factor (HIF)-1α
expression with p53 expression and outcome in surgically treated
supraglottic laryngeal cancer. J Surg Oncol. 99:373–378. 2009.
|
|
29
|
Wildeman MA, Gibcus JH, Hauptmann M, et
al: Radiotherapy in laryngeal carcinoma: can a panel of 13 markers
predict response? Laryngoscope. 119:316–322. 2009. View Article : Google Scholar : PubMed/NCBI
|