Introduction
Deacetylase inhibitors (DACis) represent novel
therapeutic options for human cancer. As previously demonstrated by
our group (1,2), DACis, as epigenetic modulators, have
promising results in various human cancer types. The antitumor
efficacy of DACis has been extensively studied with respect to
their antiproliferative and proapoptotic activity in a number of
cancers (3,4), and additional effects of DACis have
now been identified that act through alternative mechanisms of cell
demise (5,6).
With the exception of proliferation and apoptosis,
the status of transdifferentiation and dedifferentiation appears to
play a key role in tumor initiation, progression and metastasis.
Transdifferentiation defines the process of converting one
differentiated cell type into another (7–9), while
dedifferentiation defines the phenomena of reexpressing genes
related to embryonic development and stem cell characteristics
(10). The ability of cells to
transdifferentiate and dedifferentiate plays a key role in invasion
and metastasis by the process of epithelial-mesenchymal-transition
(EMT) (11,12). This phenomenon refers to a number of
important mechanisms within current carcinogenesis models and has
been fully integrated in the updated ‘Hallmarks of cancer’ by
Hanahan and Weinberg (13,14).
Liver cells are characterized by a genetic pattern
conferring them the capability to extensively regenerate following
liver resection and differentiation during chronic inflammatory
conditions. Due to these properties, liver cells may undergo
neoplastic transformations, rendering liver cancer as the second
and sixth most common cause of cancer-related mortality in males
and females, respectively (15).
Although several compounds with various modes of action have been
recently introduced for liver cancer treatment, the overall
response rates remain dissatisfactory (16). In recent years, DACis revealed a
strong efficacy in the treatment of liver cancer as well as other
solid and hematopoietic malignancies (2). Since EMT is extremely
well-characterized in the liver (17,18)
and the knowledge of epigenetic regulation in EMT is growing
(19), the influence of DACis
should be investigated (20).
Our earlier studies on human liver (21), biliary tract (22) and pancreatic cancer cell lines
(23) revealed various
morphological patterning in association with the molecular
expression of differentiation in xenograft models. We previously
detected altered patterns of differentiation in pancreatic cancer
models following treatment with the histone deacetylase (HDAC)
inhibitor SAHA and the methyltransferase inhibitor zebularine
(24). The cinnamic hydroxamic acid
pan-DACi panobinostat (LBH589) is a novel potent inhibitor of all
HDAC enzymes (25) and has already
entered clinical development, particularly for hematological
diseases including multiple myeloma, Hodgkin’s lymphoma and AML
(26).
Here, we investigate the influence of panobinostat
on the differentiation status in human hepatocellular carcinoma
(HCC) cell lines (27). We analyzed
the expression of transdifferentiation markers including
cytokeratin (Ck) 7, Ck8, Ck18, Ck19 and Ck20, and dedifferentiation
markers including β-catenin, vimentin, members of the hedgehog
pathway, Oct4 and CD133, in vitro and in vivo (in
xenografts).
Material and methods
Cell culture
Human HCC cell lines HepG2 (p53wt) and Hep3B
(p53null) were cultured under standard conditions as described in a
previous study (27). Cells were
treated with 0.1 μM panobinostat, kindly provided by
Novartis Pharma AG (Basel, Switzerland) and prepared as described
previously (27), and analyzed or
processed for further experiments after 6–72 h. Animal experiments
complied with the institute’s guidelines and were approved by the
Government of Lower Franconia (Würzburg, Germany) prior to the
experiment.
Xenograft specimens
Formalin-fixed and paraffin-embedded specimens of
HepG2 xenografts treated daily with intraperitoneal panobinostat
injections of 2.5 mg/kg of body weight (BW) or 10 mg/kg of BW were
obtained from a previous study (27).
Quantitative real-time
reverse-transcription polymerase chain reaction (RT-PCR)
For quantitative real-time RT-PCR analysis of mRNA
using SYBR-Green detection, total cellular RNA was extracted using
the RNeasy mini kit (Qiagen, Hilden, Germany) according to the
manufacturer’s instructions, and reverse transcription (RT) was
conducted using the QuantiTect Reverse Transcription kit (Qiagen).
QuantiTect primers for human Ck7 (NM_005556), Ck8 (NM_002273), Ck18
(NM_000224), Ck19 (NM_002276), vimentin (NM_003380), β-catenin
(NM_001904), sonic hedgehog homolog (SHH) (NM_000193), patched
(Ptc) (NM_000264), smoothened (Smo) (NM_005631), glioma-associated
oncogene homolog 1 (Gli1) (NM_005269), Oct4 (NM_203289), CD133
(NM_006017) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
(NM_002046) were purchased from Qiagen and run with the SsoFast™
EvaGreen Supermix (Bio-Rad Laboratories GmbH, Munich, Germany) on a
CFX96™ real-time PCR detection system (Bio-Rad Laboratories GmbH).
Results were analyzed using the CFX Manager version 2.0 and Rest
2008 software, which was normalized to GAPDH mRNA content for each
sample.
Tissue preparation and
immunohistochemistry
All specimens were fixed in 10% buffered formalin,
routinely processed and embedded in paraffin wax.
Immunohistochemistry was conducted using routine diagnostic methods
as recently published (28), and
immunohistochemical stainings were obtained using an autostainer
system (Dako, Glostrup, Denmark) according to the manufacturer’s
instructions. Antigen retrieval was conducted by heat induced
epitope retrieval in antigen retrieval buffer (pH 9.0) (Dako) at
95°C for 40 min. The primary antibodies for Ck7, Ck8, Ck18, Ck19,
Ck20, vimentin, SHH, Ptc and β-catenin were used to clarify the
differentiation status (type of antibodies, vendors, pretreatment
conditions and dilutions) as previously published (21). Additionally, primary polyclonal
rabbit antibodies of connective tissue growth factor (CTGF; 1:50
dilution; pH 9; antigen retrieval; Abcam, Cambridge, UK) were used
to characterize EMT interactions. Tonsils and lymph nodes served as
positive controls. Negative control experiments were performed
using phosphate-buffered saline in place of the primary or
secondary antibodies and the same processing as previously
described (not shown).
Interpretation of
immunohistochemistry
The stained slides were digitalized using the
ImageAccess 11 Enterprise software (Imagic Bildverarbeitung,
Glattbrugg, Switzerland). The percentage of positive cells
(extensity) was detected by evaluating the images per high-power
field (HPF; magnification, ×400) using the particle analysis module
with optimized binarization method. The level of staining intensity
(0, none; 1, low; 2, moderate; and 3, strong) was assessed by two
independent pathologists (R.K. and D.N.). Finally, an expression
score was calculated by multiplying the extensity and intensity
score (22).
Statistical analysis
Statistical analysis was conducted using SPSS 18.0
(SPSS Inc., Chicago, IL, USA). Univariate analysis of variance
(ANOVA) was used to test for differences between the groups of
tissue samples using the least significant difference (LSD)
post-hoc test to adjust for multiple comparisons. The Pearson’s
product-moment correlation coefficient test was used to measure
correlation. P<0.05 was considered to indicate a statistically
significant difference.
Results
In vitro expression pattern of
transdifferentiation and dedifferentiation markers following
panobinostat treatment
As shown in Fig. 1,
treatment with panobinostat markedly decreased the mRNA level of
Ck7 in Hep3B after 6 h and in HepG2 after 48 h. Ck8 and Ck18
transcripts revealed unpredictable patterns demonstrating no
correlation during the time points of both cell lines, while mRNA
levels of Ck19 increased early in both cell lines. The level of
vimentin mRNA significantly increased in both cell lines with a
marked upregulation after 48 h. With regards to the hedgehog
pathway components, a heterogeneous expression pattern was observed
over the treatment time periods: mRNA of SHH significantly
increased in HepG2 cells, while the opposite effect was observed in
Hep3B cells. Transcript levels of Smo were extremely low in both
cell lines with a stable decrease occurring after 72 h. In contrast
to Smo, Ptc and Gli transcripts were highly expressed and revealed
the highest expression 6 and 72 h after panobinostat treatment.
Overall, the mRNA levels of β-catenin were stable in HepG2 cells
with a slight decrease observed 24 h after treatment, and were
stable in Hep3B cells with a slight decrease observed 48 h after
panobinostat treatment. Finally, Oct4 was not detectable in either
cell line, and CD133 revealed low expression in Hep3B cells and no
expression in HepG2 cells (data not shown).
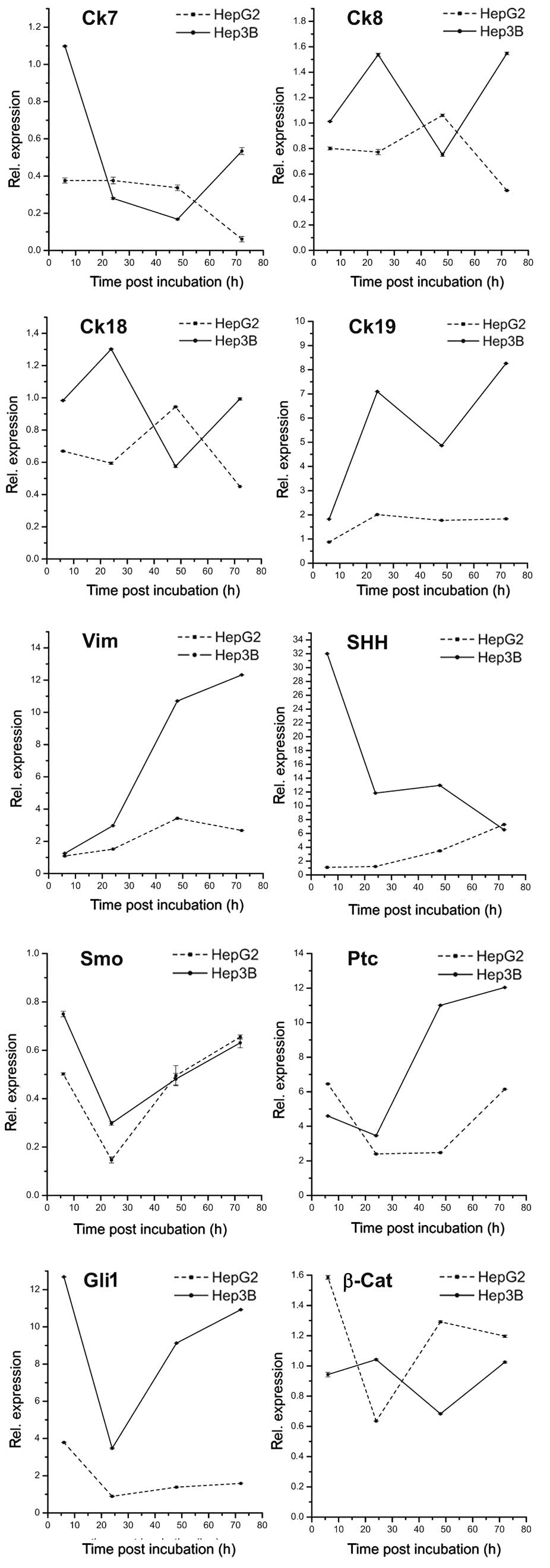 | Figure 1mRNA expression patterns of
transdifferentiation and dedifferentiation markers in human hepatic
cancer cell lines HepG2 and Hep3B in vitro at 6, 24, 48 and
72 h after 0.1 μmol panobinostat treatment. mRNA expression
was normalized to GAPDH and all results are expressed relative to
untreated controls set at 1.0. Results are expressed as mean ± SEM
of three independent experiments conducted in triplicate. Ck,
cytokeratin; Vim, vimentin; SHH, sonic hedgehog homolog; Ptc,
patched; Smo, smoothened; Gli1, glioma-associated oncogene homolog
1; β-Cat, β-catenin; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; SEM, standard error of mean. |
Correlation analysis of
transdifferentiation and dedifferentiation markers in vitro
Post-treatment correlation analysis (Table I) between differentiation markers in
HepG2 cells analyzed over time revealed a significant inverse
correlation between Ck7 and SHH markers as well as Ck19 and Gli
markers (P<0.001). A heterogeneous correlative association was
found between all investigated markers, whereby the majority of
transdifferentiation markers were negatively associated with
markers of dedifferentiation (e.g. Ck7 vs. vimentin and
Ck8/Ck18/Ck19 vs. Smo or Ptc).
 | Table ICorrelation analysis of
transdifferentiation and dedifferentiation markers following
panobinostat treatment in vitro. |
Table I
Correlation analysis of
transdifferentiation and dedifferentiation markers following
panobinostat treatment in vitro.
| Ck8 | Ck18 | Ck19 | Vim | SHH | Smo | Ptc | Gli1 | CD133 | β-Cat |
|---|
| Ck7 | | | | | | | | | | |
| HepG2 | 0.77 | 0.57 | −0.31 | −0.42 | −0.96a | −0.68 | −0.48 | 0.21 | n.a. | −0.06 |
| Hep3B | −0.05 | 0.18 | −0.64 | −0.52 | 0.81 | 0.83 | −0.35 | 0.69 | 0.96a | 0.33 |
| Ck8 | | | | | | | | | | |
| HepG2 | | 0.99a | −0.12 | 0.23 | −0.58 | −0.30 | −0.63 | 0.00 | n.a. | 0.14 |
| Hep3B | | 0.83 | 0.74 | −0.01 | −0.48 | −0.27 | −0.16 | −0.41 | −0.29 | 0.91a |
| Ck18 | | | | | | | | | | |
| HepG2 | | | −0.11 | 0.43 | −0.37 | −0.07 | −0.55 | 0.00 | n.a. | 0.27 |
| Hep3B | | | 0.32 | −0.56 | −0.02 | −0.30 | −0.68 | −0.50 | 0.04 | 0.92a |
| Ck19 | | | | | | | | | | |
| HepG2 | | | | 0.52 | 0.39 | −0.33 | −0.65 | −0.99a | n.a. | −0.82 |
| Hep3B | | | | 0.58 | −0.95a | −0.52 | 0.39 | −0.51 | −0.83 | 0.39 |
| Vim | | | | | | | | | | |
| HepG2 | | | | | 0.64 | 0.44 | −0.34 | −0.53 | n.a. | 0.05 |
| Hep3B | | | | | −0.74 | 0.01 | 0.97a | 0.18 | −0.61 | −0.34 |
| Shh | | | | | | | | | | |
| HepG2 | | | | | | 0.72 | 0.32 | −0.31 | n.a. | 0.10 |
| Hep3B | | | | | | 0.57 | −0.57 | 0.48 | 0.94 | −0.07 |
| Smo | | | | | | | | | | |
| HepG2 | | | | | | | 0.68 | 0.38 | n.a. | 0.76 |
| Hep3B | | | | | | | 0.22 | 0.97a | 0.78 | −0.01 |
| Ptc | | | | | | | | | | |
| HepG2 | | | | | | | | 0.74 | n.a. | 0.65 |
| Hep3B | | | | | | | | 0.40 | −0.41 | −0.42 |
| Gli | | | | | | | | | | |
| HepG2 | | | | | | | | | n.a. | 0.82 |
| Hep3B | | | | | | | | | 0.67 | −0.21 |
| CD133 | | | | | | | | | | |
| HepG2 | | | | | | | | | | n.d. |
| Hep3B | | | | | | | | | | n.d. |
Correlation analysis for Hep3B cells revealed
overall positive correlations between markers of dedifferentiation.
Notably, a positive correlation of Cks to β-catenin was also
identified. Comparable to HepG2 cells, the majority of
transdifferentiation markers were negatively associated with
markers of dedifferentiation, whereby the statistical analysis of
vimentin mRNA were diametric to HepG2.
In vivo expression pattern of
transdifferentiation and dedifferentiation markers following
panobinostat treatment
The investigated HCC xenografts displayed a
predominantly solid growth pattern. As previously published,
proliferation and apoptosis levels were significantly decreased and
increased, respectively, in xenografts treated with panobinostat
(27).
mRNA levels of Cks were also evaluated in HepG2
xenografts 4 weeks after treatment with 10 mg/kg BW panobinostat.
As shown in Fig. 2, panobinostat
caused a marked transient reduction of Ck7 mRNA transcript after 1
week, and restored it to the basal level after 4 weeks. In
comparison, the mRNA of Ck19 was stably expressed and revealed an
upregulation 4 weeks after panobinostat treatment. Panobinostat
induced similar effects on the Ck8 and Ck18 levels; both were
upregulated after 2 weeks but were slightly reduced after 4 weeks
of treatment. Notably, vimentin mRNA was significantly decreased
after 1 week, with exorbitant increase 2 and 4 weeks following
panobinostat treatment. We also investigated the mRNA expression
pattern of hedgehog pathway components and revealed a notable
decrease of all investigated members of the pathway (SHH, Smo, Ptc
and Gli), which were no longer detectable after 4 weeks of
treatment with panobinostat. The mRNA of β-catenin was stable until
the second week, and increased slightly after that. Finally, mRNA
levels of Oct4 and CD133 were not detectable at any time point
in vivo (data not shown).
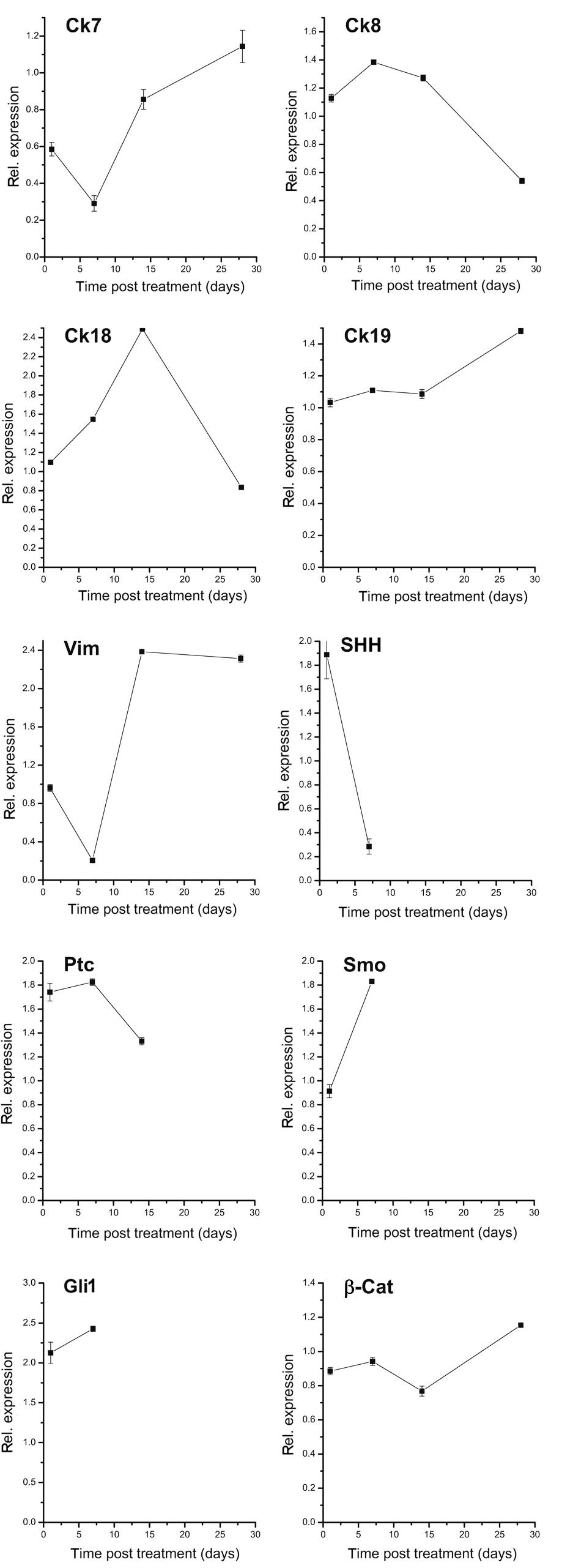 | Figure 2mRNA expression pattern of
transdifferentiation and dedifferentiation markers under 10 mg/kg
BW panobinostat in HepG2 xenografts at 1 day, 1 week, 2 weeks and 4
weeks after treatment. Where values are missing, mRNA could not be
detected. mRNA expression was normalized to GAPDH and all results
are expressed relative to untreated controls set at 1.0. Results
are expressed as mean ± SEM of three independent experiments
conducted in triplicate. Ck, cytokeratin; Vim, vimentin; SHH, sonic
hedgehog homolog; Ptc, patched; Smo, smoothened; Gli1,
glioma-associated oncogene homolog 1; β-Cat, β-catenin; BW, body
weight; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SEM,
standard error of mean. |
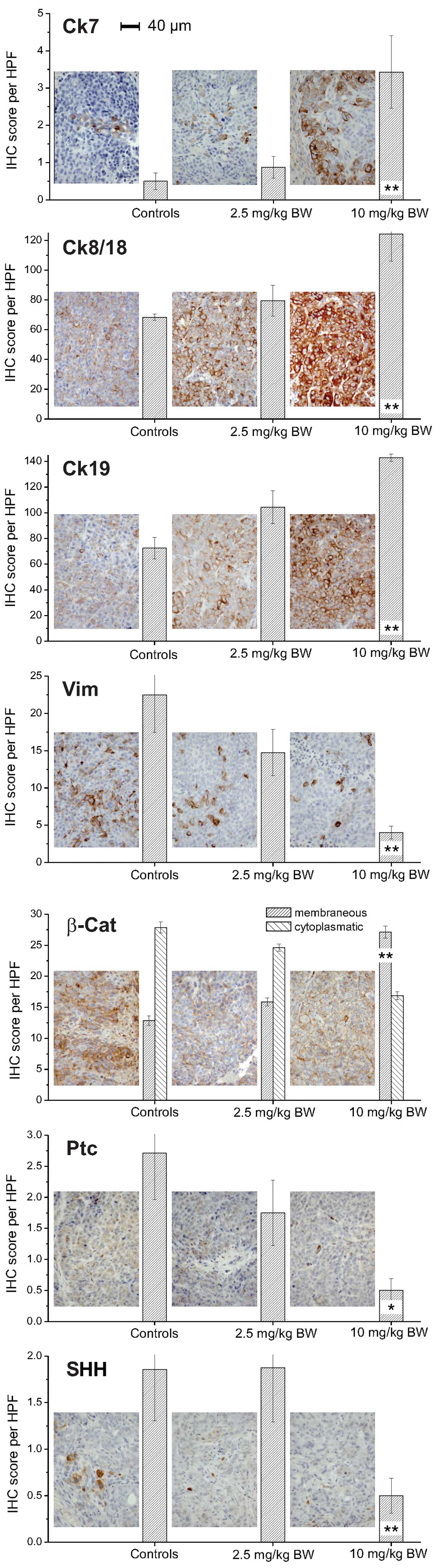
HepG2 xenografts implanted in nude mice treated with
10 mg/kg BW panobinostat demonstrated a significant increase of Ck7
protein levels compared with control xenografts (vehicle only;
Fig. 3). Ck8 and Ck18 also
demonstrated a significant increase following treatment with 10
mg/kg BW panobinostat in contrast to vimentin, a mesenchymal
marker, which was significantly decreased. A lower dose of
panobinostat (2.5 mg/kg BW) was able to induce a significant
increase of Ck19 protein expression; while the protein expression
of Ck20 minimally increased following panobinostat treatment at
high dose (<1 cell/HPF; data not shown). Immunostaining for
β-catenin revealed a different cellular distribution, highlighting
a reduction of its cytoplasmic level and a slight increase of its
cellular membrane localization, particularly following treatment
with 10 mg/kg BW panobinostat. Although the expression levels of
hedgehog pathway members SHH and Ptc were low in the untreated
control xenografts, a significant reduction of the protein levels
was observed, particularly following treatment with 10 mg/kg BW
panobinostat.
Correlation analysis of
transdifferentiation and dedifferentiation markers in vivo
As listed in Table
II, Cks as markers of transdifferentiation demonstrated a
constant, partly significant, negative correlation with
proliferation rate (Ki-67), mitosis rate, microvessel density, size
of tumor and necrosis, indicating an inverse temporal correlation
following panobinostat treatment. Overall, the most significant
correlations were identified between markers of differentiation and
the rate of mitosis and microvessel density. We revealed that those
morphological parameters were negatively associated with the
expression of Ck7, Ck8/18 and Ck19, but positively associated with
markers of dedifferentiation, including vimentin and members of the
hedgehog pathway. In line with these results, membranous and
cytoplasmic levels of β-catenin demonstrated an inverse correlation
pattern; changes of membranous β-catenin are positively correlated
with changes of Ck expression, while cytoplasmic levels of
β-catenin are inversely correlated with changes of Ck expression.
Finally, the expression of Ck19, Ck20 and membranous β-catenin were
significantly associated with CTGF expression, while an opposite
correlation was identified for the expression of vimentin and
cytoplasmic β-catenin.
 | Table IICorrelation analysis of
transdifferentiation and dedifferentiation markers following
panobinostat treatment in vivo (HepG2 xenografts). |
Table II
Correlation analysis of
transdifferentiation and dedifferentiation markers following
panobinostat treatment in vivo (HepG2 xenografts).
| | | | | | | | β-catenin
|
|---|
| Ck7 | Ck8/18 | Ck19 | Ck20 | Vim | SHH | Ptc | mb | cp |
|---|
| Ck7 | | 0.84b | 0.46a | 0.43 | −0.49a | −0.30 | −0.32 | 0.68a | −0.68a |
| Ck8/18 | | | 0.34 | 0.44a | −0.33 | −0.32 | −0.36 | 0.64a | −0.66a |
| Ck19 | | | | 0.43a | −0.60a | −0.23 | −0.37 | 0.72a | −0.73a |
| Ck20 | | | | | −0.35 | −0.22 | −0.24 | 0.57a | −0.45a |
| Vim | | | | | | 0.01 | 0.06 | 0.71a | 0.66a |
| SHH | | | | | | | 0.88b | −0.38 | 0.41a |
| Ptc | | | | | | | | −0.48a | 0.49a |
| Ki-67 | −0.42 | −0.38 | −0.73b | −0.25 | 0.61a | 0.23 | 0.44a | −0.67a | 0.74b |
| Mitosis | −0.63a | −0.55a | −0.67a | −0.35 | 0.62b | 0.47a | 0.54a | −0.77a | 0.93b |
| MVD | −0.54a | −0.50a | −0.63a | −0.45a | 0.66b | 0.45a | 0.59b | −0.89b | 0.89b |
| Tumor size
(mm) | −0.31 | −0.28 | −0.22 | −0.26 | 0.37 | 0.33 | 0.47a | −0.49a | 0.59a |
| Tumor necrosis
(mm) | −0.24 | −0.19 | −0.02 | −0.15 | 0.25 | 0.24 | 0.36 | −0.33 | 0.37 |
| CTGF | 0.29 | 0.21 | 0.54 | 0.44a | −0.42a | −0.38 | −0.29 | 0.74b | −0.54a |
Discussion
We demonstrated that with the exception of
proliferation and apoptosis (27)
the differentiation status of human HCC cell lines in vitro
and in vivo (using a xenograft model) may be influenced by
the pan-DACi panobinostat (LBH589). This suggests that DACis may
also influence cellular processes of differentiation and EMT
transition, leading to the reversion of a malignant,
undifferentiated phenotype into a more benign and differentiated
phenotype.
As discussed by a number of authors, the processes
of differentiation, transdifferentiation and dedifferentiation are
essentially involved in the cellular development, maintenance and
regeneration of different biological systems (7–9).
In the last few years, the impact of differentiation
in carcinogenesis has gained increasing attention and it has been
included as a basic mechanism in the cancer stem cell model
(14). Tumor-specific stem cell
signatures were proven experimentally (29), predicting clinical endpoints
including time of tumor progression or outcome (30). Here, we reveal that untreated human
HCC cell lines (HepG2 and Hep3B) express markers of
dedifferentiation rather than of transdifferentiation. Differences
between the in vitro and in vivo situation were
identified, which may be induced by the effect of extra-cellular
matrix interactions in the xenograft model (21) since matrix stiffness influences
proliferation and chemotherapeutic resistance as well as cellular
dormancy and stem cell characteristics in HCC (31). Notably, CD133 expression was only
observed in Hep3B cells, which confirms the results of a recent
study revealing that CD133 expression levels are associated with
the upregulation of invasion- and EMT-associated genes leading to
greater cell migration in various human hepatic cancer cell lines
(32). This reflects the whole
differentiation capacity of human liver cancer (17,18),
which should be the concept for further experimental investigations
and the new future approach for patient risk stratification and
therapeutic decision, as it has assumed strong relevance in other
solid malignancies including breast cancer (33,34).
Until now, the therapeutic effectiveness following
chemotherapy and surgical tumor resection is routinely evaluated by
investigating the tumor volume and the signs of regression. The
estimation of tumor volume or its reduction is semi-quantitatively
assessed as previously reviewed (35), and the signs of regression include
tumor cell pyknosis, cytoplasmic vacuolization/fragmentation and
necrosis. Comparable to our previous study, we identified that
tumor size and tumor necrosis of the xenografts decreased and
increased, respectively, following panobinostat treatment (27), which was recently confirmed in
combination with sorafenib (36).
Other approaches characterized the tumor-associated inflammation to
identify therapy-associated prognostic factors (37). Nevertheless, it is worth
characterizing the tumor differentiation status since it is known
that tumors change their morphological and molecular phenotype due
to the onset of chemotherapy resistance or by shifting the
differentiation status and selecting specific tumor subgroups
including tumor stem cells (38).
Gastrointestinal stromal tumors present different phenomena of
transdifferentiation and dedifferentiation, including loss of c-kit
expression or rhabdomyoid differentiation following targeted
therapy with kinase inhibitors (imatinib mesylate) (39). Analysis of breast cancer following
neoadjuvant chemotherapy revealed a different expression of the
estrogen and progesterone receptors compared to untreated cancer
(40). In contrast to classical Ck
profiling, these markers are not lineage- or
differentiation-specific, demonstrating only an on-off switch
phenomenon of activation inside these tumor cells (41). Additionally, hematopoietic cancer
cells revealed a loss of their surface markers, including CD20,
following target therapy with retuximab, a specific antibody
against CD20 (42). Furthermore,
treatment of MDS and AML with the DNA methylation inhibitor
decitabine vidaza induced different morphological changes,
including colony formation, and expression of hematopoietic
differentiation markers (43).
Our data demonstrates that human liver cancer cell
lines change their status shifting from a dedifferentiated acquired
pattern to a well-differentiated status following treatment with
panobinostat. These results are in line with earlier studies in a
human pancreatic cancer cell model using a combination of the
histone deacetylase inhibitor SAHA and the methyltransferase
inhibitor Zebularine (24). The
effect of panobinostat demonstrated a time- and dose-dependent
activity (particularly in vitro), whereby heterogeneous
effects were observed in vitro and in vivo as
previously discussed. Overall, we identified that treatment with
panobinostat not only caused a decrease of dedifferentiation
markers, but also an increase of differentiation markers including
Cks. This supports the idea of a well-differentiated pattern having
a similar morphological and molecular plasticity of human liver
cells and preventing the EMT in the process of tumor migration and
metastasis (11,12). Further experiments using invasion
assays are required to support this hypothesis. Notably, the
expression pattern of differentiation could significantly be linked
to the expression of CTGF contributing to HCC cell
dedifferentiation (44). Therefore,
differentiation patterning may be used as an additional prognostic
and predictive indicator for therapeutic effectiveness as recently
discussed (45). We could
demonstrate that the expression of markers, including Cks, vimentin
and β-catenin, correlates with the effectiveness of photodynamic
therapy (46) or experimental Wnt
pathway inhibitors (22,47) in a biliary tract cancer model.
Additionally, the importance of differentiation status is supported
by our results, which demonstrate that markers of differentiation
are significantly inversely correlated to classical prognostic
tumor markers, including tumor size, tumor necrosis, mitosis rate,
microvessel density and Ki-67-associated proliferation rate in the
xenograft model. This should be taken into account since targeted
therapies are often underestimated by conventional parameters like
RECIST or WHO radiology. Therefore, functional biomarkers like
differentiation markers should be established.
Finally, we are aware that we descriptively present
the effect of the pan-DACi panobinostat on EMT in a human liver
cancer model, which should be proven and supported by further
functional investigations using siRNA or small molecules
interacting with intermediary filaments (48,49).
In conclusion, the pan-DACi panobinostat influences
not only the classical markers of cancerogenesis including
proliferation and apoptosis, but also the differentiation status of
human hepatic cancer cell lines HepG2 and Hep3B. Since the
epigenetic-associated shift of differentiation is paralleled by
morphological markers such as tumor size and proliferation, more
effort should be focused on the differentiation status of tumors in
order to provide additional information for therapeutic
effectiveness and success.
Abbreviations:
|
BW
|
body weight
|
|
CTGF
|
connective tissue growth factor
|
|
Ck
|
cytokeratin
|
|
DACi
|
deacetylase inhibitor
|
|
Ptc
|
patched
|
|
Smo
|
smoothened
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HCC
|
human hepatocellular carcinoma
|
|
SHH
|
sonic hedgehog homolog
|
Acknowledgements
The expert technical assistance of Mrs
Berta Lechner is gratefully acknowledged. T.K. was supported by a
research grant from the research fund of the Paracelsus Medical
University in Salzburg (Grant No. R-10/04/17-KIE). M.O. was
supported by the Wissenschaftlicher Verein der Pathologie
Salzburg/Austria grant of the University Hospital Medical Center
Giessen and Marburg (UKGM), as well as a grant from Novartis Pharma
GmbH (Germany). Additionally, P.D.F. received a Dame Sheila
Sherlock Post-Doctoral Fellowship from the European Association for
the Study of the Liver (EASL).
References
|
1
|
Stintzing S, Kemmerling R, Kiesslich T,
Alinger B, Ocker M and Neureiter D: Myelodysplastic syndrome and
histone deacetylase inhibitors: ‘to be or not to be acetylated’? J
Biomed Biotechnol. 2011:2141432011.
|
|
2
|
Miller CP, Singh MM, Rivera-Del Valle N,
Manton CA and Chandra J: Therapeutic strategies to enhance the
anticancer efficacy of histone deacetylase inhibitors. J Biomed
Biotechnol. 2011:5142612011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jana S and Paliwal J: Apoptosis: potential
therapeutic targets for new drug discovery. Curr Med Chem.
14:2369–2379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schneider-Stock R and Ocker M: Epigenetic
therapy in cancer: molecular background and clinical development of
histone deacetylase and DNA methyltransferase inhibitors. IDrugs.
10:557–561. 2007.
|
|
5
|
Ellis L, Atadja PW and Johnstone RW:
Epigenetics in cancer: targeting chromatin modifications. Mol
Cancer Ther. 8:1409–1420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ocker M: Deacetylase inhibitors - focus on
non-histone targets and effects. World J Biol Chem. 1:55–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones PA and Taylor SM: Cellular
differentiation, cytidine analogs and DNA methylation. Cell.
20:85–93. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones PA, Taylor SM and Wilson V: DNA
modification, differentiation, and transformation. J Exp Zool.
228:287–295. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eguchi G and Kodama R:
Transdifferentiation. Curr Opin Cell Biol. 5:1023–1028. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neureiter D, Herold C and Ocker M:
Gastrointestinal cancer - only a deregulation of stem cell
differentiation? (Review). Int J Mol Med. 17:483–489.
2006.PubMed/NCBI
|
|
11
|
Gavert N and Ben-Ze’ev A:
Epithelial-mesenchymal transition and the invasive potential of
tumors. Trends Mol Med. 14:199–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sabbah M, Emami S, Redeuilh G, Julien S,
Prevost G, Zimber A, Ouelaa R, Bracke M, De Wever O and Gespach C:
Molecular signature and therapeutic perspective of the
epithelial-to-mesenchymal transitions in epithelial cancers. Drug
Resist Updat. 11:123–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
14
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
16
|
Greten TF, Korangy F, Manns MP and Malek
NP: Molecular therapy for the treatment of hepatocellular
carcinoma. Br J Cancer. 100:19–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi SS and Diehl AM:
Epithelial-to-mesenchymal transitions in the liver. Hepatology.
50:2007–2013. 2009. View Article : Google Scholar
|
|
18
|
Rountree CB, Mishra L and Willenbring H:
Stem cells in liver diseases and cancer: recent advances on the
path to new therapies. Hepatology. 55:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McDonald OG, Wu H, Timp W, Doi A and
Feinberg AP: Genome - scale epigenetic reprogramming during
epithelial-to-mesenchymal transition. Nat Struct Mol Biol.
18:867–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marquardt JU, Factor VM and Thorgeirsson
SS: Epigenetic regulation of cancer stem cells in liver cancer:
current concepts and clinical implications. J Hepatol. 53:568–577.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jabari S, Meissnitzer M, Quint K, Gahr S,
Wissniowski T, Hahn EG, Neureiter D and Ocker M: Cellular
plasticity of trans- and dedifferentiation markers in human
hepatoma cells in vitro and in vivo. Int J Oncol. 35:69–80.
2009.PubMed/NCBI
|
|
22
|
Kiesslich T, Alinger B, Wolkersdorfer GW,
Ocker M, Neureiter D and Berr F: Active Wnt signalling is
associated with low differentiation and high proliferation in human
biliary tract cancer in vitro and in vivo and is sensitive to
pharmacological inhibition. Int J Oncol. 36:49–58. 2010.PubMed/NCBI
|
|
23
|
Neureiter D, Zopf S, Dimmler A, Stintzing
S, Hahn EG, Kirchner T, Herold C and Ocker M: Different
capabilities of morphological pattern formation and its association
with the expression of differentiation markers in a xenograft model
of human pancreatic cancer cell lines. Pancreatology. 5:387–397.
2005. View Article : Google Scholar
|
|
24
|
Neureiter D, Zopf S, Leu T, Dietze O,
Hauser-Kronberger C, Hahn EG, Herold C and Ocker M: Apoptosis,
proliferation and differentiation patterns are influenced by
Zebularine and SAHA in pancreatic cancer models. Scand J
Gastroenterol. 42:103–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maiso P, Carvajal-Vergara X, Ocio EM,
Lopez-Perez R, Mateo G, Gutierrez N, Atadja P, Pandiella A and San
Miguel JF: The histone deacetylase inhibitor LBH589 is a potent
antimyeloma agent that overcomes drug resistance. Cancer Res.
66:5781–5789. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Atadja P: Development of the pan-DAC
inhibitor panobinostat (LBH589): successes and challenges. Cancer
Lett. 280:233–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Fazio P, Schneider-Stock R, Neureiter
D, Okamoto K, Wissniowski T, Gahr S, Quint K, Meissnitzer M,
Alinger B, Montalbano R, Sass G, Hohenstein B, Hahn EG and Ocker M:
The pan-deacetylase inhibitor panobinostat inhibits growth of
hepatocellular carcinoma models by alternative pathways of
apoptosis. Cellular Oncology. 32:285–300. 2010.PubMed/NCBI
|
|
28
|
Kemmerling R, Stintzing S, Muhlmann J,
Dietze O and Neureiter D: Primary testicular lymphoma: A strictly
homogeneous hematological disease? Oncol Rep. 23:1261–1267.
2010.PubMed/NCBI
|
|
29
|
Alison MR, Lim SM and Nicholson LJ: Cancer
stem cells: problems for therapy? J Pathol. 223:147–161. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ailles LE and Weissman IL: Cancer stem
cells in solid tumors. Curr Opin Biotechnol. 18:460–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schrader J, Gordon-Walker TT, Aucott RL,
van Deemter M, Quaas A, Walsh S, Benten D, Forbes SJ, Wells RG and
Iredale JP: Matrix stiffness modulates proliferation,
chemotherapeutic response, and dormancy in hepatocellular carcinoma
cells. Hepatology. 53:1192–1205. 2011. View Article : Google Scholar
|
|
32
|
Na DC, Lee JE, Yoo JE, Oh BK, Choi GH and
Park YN: Invasion and EMT-associated genes are up-regulated in B
viral hepatocellular carcinoma with high expression of CD133-human
and cell culture study. Exp Mol Pathol. 90:66–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van ’t Veer LJ, Dai H, van de Vijver MJ,
He YD, Hart AA, Mao M, Peterse HL, van der KK, Marton MJ, Witteveen
AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R
and Friend SH: Gene expression profiling predicts clinical outcome
of breast cancer. Nature. 415:530–536. 2002.PubMed/NCBI
|
|
34
|
Perou CM, Sorlie T, Eisen MB, van de RM,
Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE,
Borresen-Dale AL, Brown PO and Botstein D: Molecular portraits of
human breast tumours. Nature. 406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wittekind C and Tannapfel A: Regression
grading of colorectal carcinoma after preoperative
radiochemotherapy. An inventory Pathologe. 24:61–65.
2003.PubMed/NCBI
|
|
36
|
Lachenmayer A, Toffanin S, Cabellos L,
Alsinet C, Hoshida Y, Villanueva A, Minguez B, Tsai HW, Ward SC,
Thung S, Friedman SL and Llovet JM: Combination therapy for
hepatocellular carcinoma: additive preclinical efficacy of the HDAC
inhibitor panobinostat with sorafenib. J Hepatol. 56:1343–1350.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zitvogel L, Kepp O and Kroemer G: Immune
parameters affecting the efficacy of chemotherapeutic regimens. Nat
Rev Clin Oncol. 8:151–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Diaz DM, Hernandez AA, Pereira GS,
Jaramillo S, Virizuela Echaburu JA and Gonzalez-Campora RJ:
Gastrointestinal stromal tumors: morphological, immunohistochemical
and molecular changes associated with kinase inhibitor therapy.
Pathol Oncol Res. 17:455–461. 2011. View Article : Google Scholar
|
|
40
|
Lee J, Im YH, Lee SH, Cho EY, Choi YL, Ko
YH, Kim JH, Nam SJ, Kim HJ, Ahn JS, Park YS, Lim HY, Han BK and
Yang JH: Evaluation of ER and Ki-67 proliferation index as
prognostic factors for survival following neoadjuvant chemotherapy
with doxorubicin/docetaxel for locally advanced breast cancer.
Cancer Chemother Pharmacol. 61:569–577. 2008. View Article : Google Scholar
|
|
41
|
Moll R, Divo M and Langbein L: The human
keratins: biology and pathology. Histochem Cell Biol. 129:705–733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goteri G, Olivieri A, Ranaldi R, Lucesole
M, Filosa A, Capretti R, Pieramici T, Leoni P, Rubini C, Fabris G
and Lo ML: Bone marrow histopathological and molecular changes of
small B-cell lymphomas after rituximab therapy: comparison with
clinical response and patients outcome. Int J Immunopathol
Pharmacol. 19:421–431. 2006.
|
|
43
|
Ryningen A, Stapnes C and Bruserud O:
Clonogenic acute myelogenous leukemia cells are heterogeneous with
regard to regulation of differentiation and effect of epigenetic
pharmacological targeting. Leuk Res. 31:1303–1313. 2007. View Article : Google Scholar
|
|
44
|
Urtasun R, Latasa MU, Demartis MI, Balzani
S, Goni S, Garcia-Irigoyen O, Elizalde M, Azcona M, Pascale RM, Feo
F, Bioulac-Sage P, Balabaud C, Muntane J, Prieto J, Berasain C and
Avila MA: Connective tissue growth factor autocriny in human
hepatocellular carcinoma: oncogenic role and regulation by
epidermal growth factor receptor/yes-associated protein-mediated
activation. Hepatology. 54:2149–2158. 2011. View Article : Google Scholar
|
|
45
|
Hoshida Y, Toffanin S, Lachenmayer A,
Villanueva A, Minguez B and Llovet JM: Molecular classification and
novel targets in hepatocellular carcinoma: recent advancements.
Semin Liver Dis. 30:35–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kiesslich T, Neureiter D, Alinger B,
Jansky GL, Berlanda J, Mkrtchyan V, Ocker M, Plaetzer K and Berr F:
Uptake and phototoxicity of meso-tetrahydroxyphenyl chlorine are
highly variable in human biliary tract cancer cell lines and
correlate with markers of differentiation and proliferation.
Photochem Photobiol Sci. 9:734–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wachter J, Neureiter D, Alinger B, Pichler
M, Fuereder J, Oberdanner C, Di Fazio P, Ocker M, Berr F and
Kiesslich T: Influence of five potential anticancer drugs on wnt
pathway and cell survival in human biliary tract cancer cells. Int
J Biol Sci. 8:15–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kiesslich T, Berr F, Alinger B, Kemmerling
R, Pichler M, Ocker M and Neureiter D: Current status of
therapeutic Targeting of developmental signalling pathways in
oncology. Curr Pharm Biotechnol. 13:2184–2220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Finn RS, Dering J, Ginther C, Wilson CA,
Glaspy P, Tchekmedyian N and Slamon DJ: Dasatinib, an orally active
small molecule inhibitor of both the src and abl kinases,
selectively inhibits growth of basal-type/‘triple-negative’ breast
cancer cell lines growing in vitro. Breast Cancer Res Treat.
105:319–326. 2007.PubMed/NCBI
|