Introduction
Matrix metalloproteinases (MMPs), a group of
zinc-dependent endopeptidases, are involved in the degradation of
the extracellular matrix (ECM) under normal physiological
conditions and during the metastatic process (1). While MMP activity is normally tightly
regulated, dysregulation of MMP activity has been linked to cancer
progression and metastasis via the degradation of the basement
membrane and the induction of angiogenesis (2). The degree of overexpression of certain
MMPs has been noted to correlate with the stage of disease and/or
prognosis (3). The majority of MMPs
are secreted as inactive zymogens that are activated
extracellularly and their functions are tightly regulated by
several mechanisms (4). MMP-9 is a
key effector molecule that promotes tumor cell invasion through
type-IV collagen degradation-dependent extracellular matrix
remodeling (5). MMP-9 expression
has been observed in tumors of various organs, including the
prostate, bladder, brain, liver and pancreas (6). The growth and metastasis of prostate
cancer, as well as other tumors, is dependent on the formation of
new blood vessels from preexisting ones via angiogenesis. Growing
evidence suggests that prostate cancer cells secrete high levels of
growth factors and matrix-degrading proteases, thus allowing
metastasis to distant organs, including the liver, lungs, spine,
bladder, bone and lymph nodes (7).
Therefore, targeting MMP-9 inhibition for treating prostate cancers
is considered to be an effective strategy.
A previous study has shown that the nuclear factor
κB (NF-κB) pathway tightly regulates MMP-9 expression in several
types of cancer cells (8). NF-κB is
normally located in the cytoplasm as an inactive dimer. The
activity of NF-κB is regulated by interaction with inhibitory IκB
proteins, which repress the potential of NF-κB to translocate to
the nucleus and bind with DNA (9).
Upon activation, IκB is phosphorylated, which marks the inhibitor
for ubiquitination and degradation via a proteasome-dependent
pathway (10). A previous study
demonstrated that the inhibition of NF-κB activity in human
prostate cancer cells decreases their tumorigenic and metastatic
abilities by suppressing angiogenesis and invasion via the
downregulation of MMP-9 (11).
Conversely, TNF-α stimulation results in NF-κB activation in
prostate cancer cells and induces tumor invasion (12). Therefore, a potential strategy to
suppress MMP-9-mediated tumor invasion is via the NF-κB
pathway.
Piceatannol (3,5,3′,4′-tetrahydroxytrans-stilbene),
a phenolic compound and an analog of resveratrol, which naturally
occurs in grapes and red wine, has been shown to possess anticancer
properties via reduction in the expression of the anti-apoptotic
protein Bcl-2 and inhibitors of apoptosis (IAP) family, in addition
to anti-inflammatory activity via the downregulation of NF-κB
(13–15). In a previous study, Kang et
al reported the mechanism of piceatannol in terms of tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced
apoptosis (16). However, the
effects of piceatannol on MMP-9 gene expression and invasion in
cancer cells have not been evaluated.
The current study examined the effects of
piceatannol on MMP-9 expression and invasion in TNF-α-stimulated
DU145 prostate cancer cells. It was demonstrated that piceatannol
downregulates TNF-α-induced MMP-9 mRNA and protein expression by
suppressing NF-κB activation. Furthermore, the regulation of NF-κB
activity by piceatannol is associated with the inhibition of the
phosphorylation of Akt.
Materials and methods
Reagent and antibodies
Piceatannol was purchased from Tocris (St. Louis,
MO, USA) and dissolved in DMSO (vehicle). Antibodies against p65,
p50, β-actin, phospho (p)-Akt and Akt were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). A specific NF-κB
inhibitor pyrrolidine dithiocarbamate (PDTC) and a specific Akt
inhibitor LY294002 were purchased from Calbiochem (San Diego, CA,
USA). MMP-9 inhibitor I was obtained from Merck (Darmstadt,
Germany). 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium
bromide (MTT) and propidium iodine (PI) were obtained from Sigma
(St. Louis, MO, USA).
Cell culture and viability
Human prostate cancer DU145 cells were obtained from
the American Type Culture Collection (Manassas, VA, USA). Cells
were maintained in DMEM (WelGENE, Inc., Daegu, Korea) supplemented
with 10% heat-inactivated FBS (WelGENE, Inc.) and 1%
penicillin-streptomycin (WelGENE, Inc.) in 5% CO2 at
37°C. Cells were seeded at 1×105 cells/ml and treated
with the indicated concentrations of piceatannol. Following a 24-h
incubation, the viability was determined by an MTT assay.
Flow cytometric analysis
Cell cycle distribution was analyzed by PI-stained
cells. Briefly, cells (1×106) were fixed in 70% ethanol
overnight at 4°C. The cells were washed in phosphate-buffered
saline (PBS) with 0.1% BSA. Cells were incubated with 1 U/ml RNase
A (DNase free, Sigma) and 10 μg/ml PI overnight at room temperature
in the dark. Cells were analyzed using a FACS Calibur flow
cytometer (Becton Dickenson, San Jose, CA, USA). The levels of
apoptotic cells with sub-G1 DNA were determined as a percentage of
the total number of cells.
DNA fragmentation assay
Cells were treated with the indicated chemicals and
then lysed on ice in a buffer containing 10 mM Tris-HCl (pH 7.4),
150 mM NaCl, 5 mM EDTA and 0.5% Triton X-100 for 30 min. Lysates
were vortexed and cleared by centrifugation at 10,000 x g for 20
min. Fragmented DNA in the supernatant was extracted with an equal
volume of neutral phenol:chloroform:isoamylalcohol (25:24:1, v/v/v)
and analyzed electrophoretically on a 1.5% agarose gel containing
ethidium bromide.
Wound-healing assay
DU145 cells were grown to 90% confluence in a 6-well
plate at 37°C in a 5% CO2 incubator. A wound was created
by scratching cells with a sterile 200-μl pipette tip, cells were
washed twice with PBS to remove floating cells and then added to a
medium without serum. Images of the wound were captured under an
×100 magnitude microscope.
Gelatin zymography
Cultured DU145 cells were harvested and washed with
serum-free DMEM three times and incubated for 24 h at
5×105 cells/ml of serum-free DMEM. The cells were
stimulated with piceatannol in the presence of TNF-α (20 ng/ml).
MMP-9 activity was determined by gelatin zymography using 0.1%
gelatin as a substrate. The conditioned medium was mixed with
SDS-PAGE sample buffer in the absence of reducing agent and
electrophoresed in 8% polyacrylamide gel. Following
electrophoresis, the gels were washed three times with 2.5% Triton
X-100 in water and then incubated overnight in a closed container
at 37°C in 0.2% Brij 35.5 mM CaCl2, 1 mM NaCl and 50 mM
Tris, pH 7.4. The gels were stained for 30 min with 0.25% Coomassie
Blue R-250 in 10% acetic acid and 45% methanol and then destained
for 30 min using an aqueous mix of 20% acetic acid, 20% methanol
and 17% ethanol. Areas of protease activity appeared as clear
bands.
RNA extraction and RT-PCR
Total RNA was isolated using TRIzol reagent
(GIBCO-BRL, Gaithersburg, MD, USA) according to the manufacturer’s
recommendations. Genes of interest were amplified from cDNA that
was reverse-transcribed from 1 μg of total RNA using the One-Step
RT-PCR Premix (iNtRON Biotechnology, Sungnam, Korea). Primers for
MMP-9 sense (5′-CCTGGAGACCTGAGAACCAATCT-3′) and antisense
(5′-CCACCCGAGTGTAACCATAGC-3′) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) sense (5′-CCACCCATGGCAAATTCCATGGCA-3′) and
antisense (5′-TCTAGACGGCAGGTCAGGTCCACC-3′) were used. The PCR was
initiated at 94°C for 2 min followed by 31 cycles of 94°C for 30
min, 30-min annealing temperature, 72°C for 30 min followed by
final extension at 72°C for 5 min. The annealing temperatures for
MMP-9 and GAPDH were 63°C and 62°C, respectively. Following
amplification, PCR products were separated on 1.5% agarose gels and
visualized by ethidium bromide fluorescence.
Invasion assay
Cells (5×104/chamber) were used for each
invasion assy. Invasion assays were performed using modified Boyden
chambers with polycarbonate nucleopore membrane (Corning, Corning,
NY, USA). Precoated filters (6.5 mm in diameter, 8 μm pore-size,
Matrigel 100 μg/cm2) were rehydrated and
5×104 cells in medium with or without piceatannol or
MMP-9 inhibitor I (5 nM) in the presence of TNF-α were seeded into
the upper part of each chamber. Following 24 h incubation,
nonmigratory cells on the upper surface of the filter were wiped
with a cotton swab and migrated cells on the lower surface of the
filter were fixed and stained with 0.125% Commassie Blue in a
methanol:acetic acid:water mixture (45:10:45, v/v/v). Random fields
were counted under a light microscope.
Luciferase assay
NF-κB reporter construct was purchased from
Clonetech (Palo Alto, CA, USA) and MMP-9 promoter was obtained from
Professor Y.H. Choi (Oriental Medicine, Dongeui University, Busan,
Korea). Briefly, DU145 cells were plated onto six-well plates at a
density of 5×105 cells/well and grown overnight. Cells
were transfected with 2 μg of each plasmid construct for 6 h by the
Lipofectamine method. Following transfection, the cells were
cultured in 10% FBS containing DMEM with the indicated
concentrations of piceatannol in the presence of 20 ng/ml TNF-α for
24 h. Cells were lysed with lysis buffer (20 mM Tris-HCl, pH 7.8,
1% Triton X-100, 150 mM NaCl and 2 mM DTT). The cell lysate (5 μl)
was mixed with luciferase activity assay reagent (25 μl) and
luminescence produced for 5 sec was measured using GLOMAX
luminometer (Promega, Madison, WI, USA).
Electrophoretic mobility shift assay
(EMSA)
The preparation of cytoplasmic and nuclear extracts
was conducted using the NE-PER nuclear and cytoplasmic extraction
reagents (Pierce, Rockford, IL, USA). DNA-protein binding assays
were carried out with nuclear extract. Synthetic complementary
NF-κB-binding oligonucleotides (5′-AGTTGAGGGGACTTTCCCAGGC-30′;
Santa Cruz Biotechnology) were biotinylated using the biotin 30-end
DNA labeling kit (Pierce) according to the manufacturer’s
instructions and annealed for 1 h at room temperature. Binding
reactions were carried out for 20 min at room temperature in the
presence of 50 ng/ml poly(dI-dC), 0.05% Nonidet P-40, 5 mM
MgCl2, 10 mM EDTA and 2.5% glycerol in 1X binding buffer
(LightShift™ chemiluminescent EMSA kit) with 20 fmol of
biotin-end-labeled target DNA and 10 μg of nuclear extract. Assays
were loaded onto native 4% polyacrylamide gels pre-electrophoresed
for 60 min in 0.5X Tris borate/EDTA and transferred onto a
positively charged nylon membrane (HybondTM-N+) in 0.5X Tris
borate/EDTA at 100 V for 30 min. Transferred DNAs were cross-linked
to the membrane at 120 mJ/cm2 and detected using
horseradish peroxidase-conjugated streptavidin according to the
manufacturer’s instructions.
Western blot analysis
Total cell extracts were prepared using PRO-PREP
protein extraction solution (iNtRON Biotechnology). Proteins were
separated by SDS-PAGE and electrotransferred to nitrocellulose
membranes (Amersham, Arlington Heights, IL, USA). The detection of
specific proteins was carried out with an ECL western blotting kit
(Amersham) according to the manufacturer’s instructions.
Statistical analysis
All data were derived from at least three
independent experiments. The images were visualized with
Chemi-Smart 2000 (Vilber Lourmat, Cedex, France). Images were
captured using Chemi-Capt (Vilber Lourmat) and transported into
Adobe Photoshop (version 8.0). All data are presented as mean ± SE.
Significant differences between the groups were determined using
one-way ANOVA test. P<0.05 was considered to indicate a
statistically significant difference.
Results
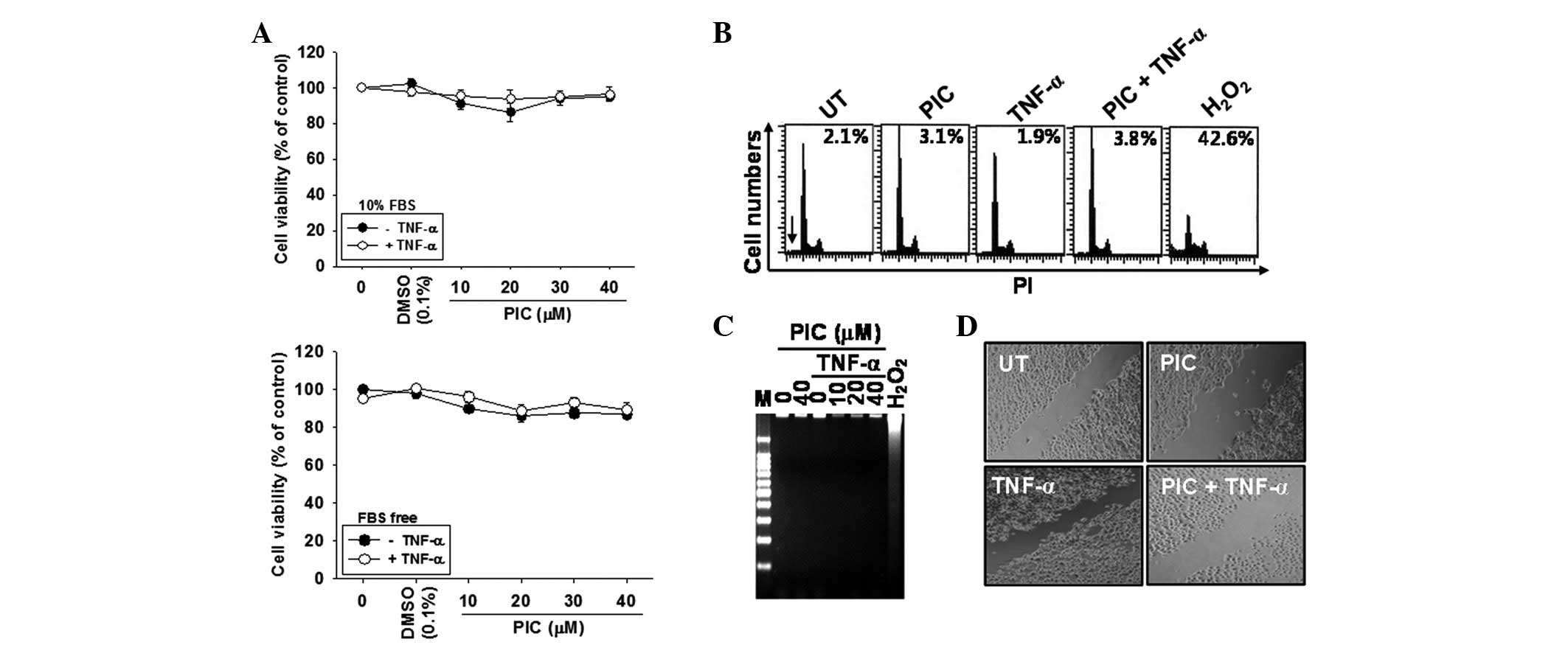
Piceatannol does not affect DU145 cell
viability
In order to determine the cytotoxic potential of
piceatannol on DU145 cells, an MTT assay was performed at 24 h
following treatment with various concentrations (0–40 μM) of
piceatannol in the presence or absence of TNF-α, regardless of the
presence of FBS. Piceatannol alone, or in conjunction with TNF-α,
was not cytotoxic at any of the concentrations tested in this study
(Fig. 1A). Therefore, piceatannol
levels of <40 μM were selected for subsequent experiments.
Subsequently, the effect of piceatannol on cell viability and
cytotoxicity was analyzed in detail. According to the percentages
of sub-G1 DNA content as measured by flow cytometry, no
apoptotic cell death was observed compared with the positive
H2O2-treated group (Fig. 1B). Furthermore, DNA fragmentation
was analyzed to determine the level of fragmented DNA. No
fragmented DNA was identified in the piceatannol alone or the
TNF-α-treated groups compared with the positive
H2O2-treated group (Fig. C). To determine whether piceatannol
also inhibited TNF-α-induced migration, a scratch wound healing
assay was performed. Cell migration was significantly increased by
TNF-α treatment and was inhibited by the presence of piceatannol
(Fig. 1D). Overall, these data
indicate that piceatannol inhibited TNF-α-induced migration, and
hence tumor invasion, without any cytotoxicity.
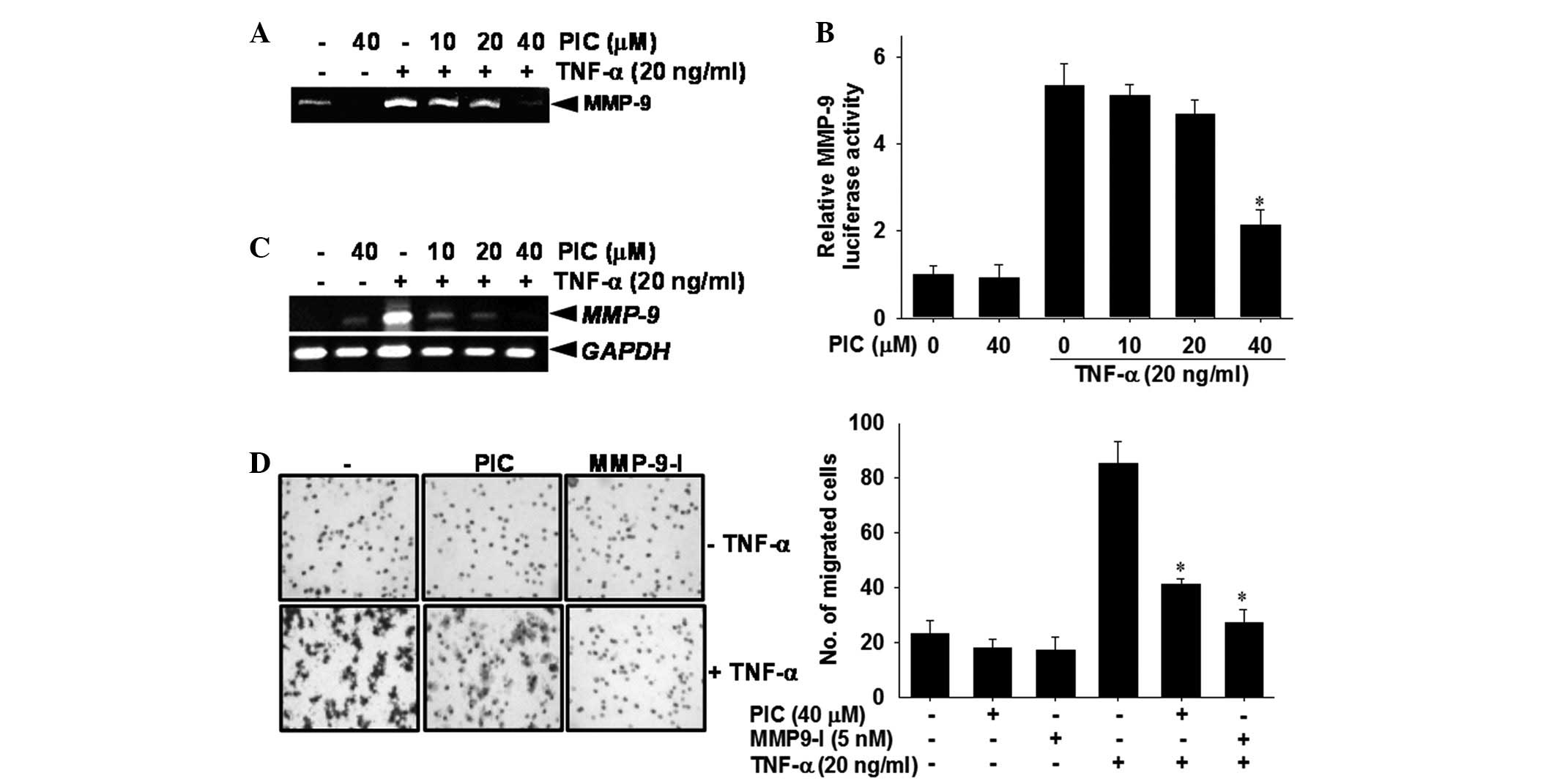
Piceatannol inhibits TNF-α-induced
invasion of DU145 cells by suppressing MMP-9 activation
Zymography, RT-PCR and measurement of luciferase
reporter activity were conducted to assess whether piceatannol
regulates TNF-α-induced MMP-9 expression. The zymography data
revealed that the secretion of MMP-9 was significantly increased by
TNF-α treatment compared with the untreated control group.
Piceatannol decreased TNF-α-induced MMP-9 activity in a
dose-dependent manner (Fig. 2A). In
a subsequent experiment, the luciferase activity of MMP-9 was
assayed using transient transfection with reporter vectors that
included MMP-9 promoters. Treatment with TNF-α significantly
increased MMP-9 luciferase activity, whereas piceatannol
downregulated TNF-α-induced MMP-9 luciferase reporter activity
(Fig. 2B). In addition, RT-PCR
analysis revealed that steady-state MMP-9 mRNA levels are
undetectable in piceatannol-treated cells compared with the
untreated control group (Fig. 2C).
TNF-α stimulation of cells resulted in a significant increase in
MMP-9 mRNA expression compared with the untreated control; however,
piceatannol completely reversed TNF-α-induced MMP-9 mRNA levels to
the levels of the untreated control. Since MMP-9 is thought to be
critically involved in the processes of tumor invasion (25), the effects of piceatannol on the
invasion of DU145 cells was examined. In the invasion assay, when
the cells were treated with TNF-α alone, a marked 4-fold increase
in cell invasion was observed compared with the untreated control.
However, the addition of piceatannol resulted in an ∼50% reduction
in penetration through a Matrigel-coated membrane when compared
with the TNF-α-treated group (Fig.
2D). Addition of MMP-9 inhibitor I significantly blocked
TNF-α-induced cell invasion. These results confirm that piceatannol
suppressed the upregulation of TNF-α-stimulated MMP-9 expression at
the transcription level and inhibited TNF-α-induced invasion of
DU145 carcinoma cells.
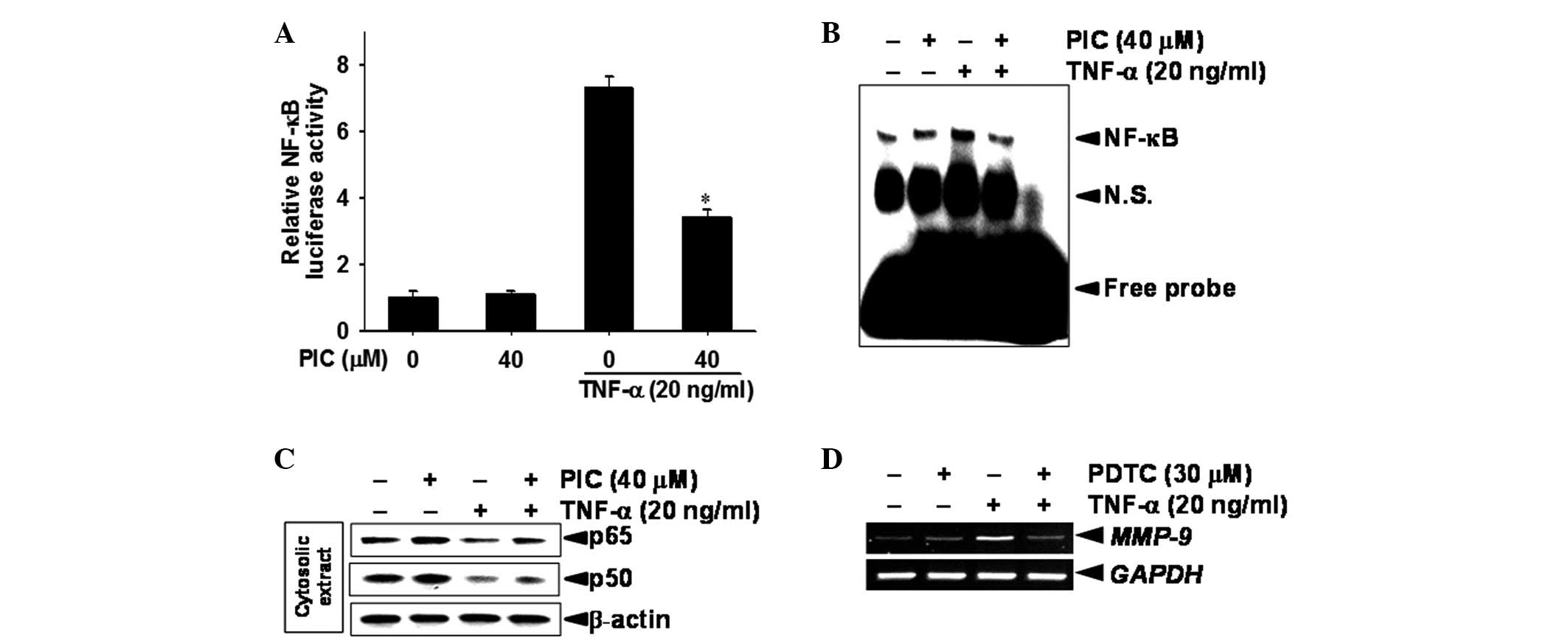
Piceatannol inhibits TNF-α-induced
expression of the MMP-9 gene by suppression of NF-κB activity
In order to determine whether MMP-9 mRNA expression
was linked to NF-κB activity, a promoter assay was conducted using
DU145 cells that had been transiently transfected with a luciferase
reporter vector, which included the NF-κB-binding sites. NF-κB
luciferase activity was significantly increased, ∼7.4-fold, in
TNF-α-stimulated DU145 cells compared with the untreated control
group (Fig. 3A). However, the
TNF-α-stimulated luciferase activity in the cells containing the
NF-κB construct was significantly reduced to ∼50%, by treatment
with piceatannol. An EMSA was conducted to evaluate the effect of
piceatannol on the DNA-binding activity of NF-κB to assess its
inhibitory effect on the transcriptional activity of MMP-9. Nuclear
extracts from control and piceatannol-treated DU145 cells were
subjected to analysis for the DNA-binding activity of NF-κB. This
reached a maximum at 30 min and was sustained for 1 h following
treatment with TNF-α (data not shown). EMSA data revealed that
treatment with piceatannol downregulated the TNF-α-induced
DNA-binding activity of NF-κB at 30 min (Fig. 3B). As shown in the western blot
analysis, treatment with TNF-α decreased the levels of p65 and p50
in the cytosolic extract, whereas the addition of piceatannol
resulted in sustainment of the levels of p65 and p50. This suggests
that piceatannol inhibits the nuclear translocation of NF-κB
subunits p65 and p50 (Fig. 3C). In
addition, our data indirectly demonstrated that a specific NF-κB
inhibitor PDTC has the ability to inhibit TNF-α-induced expression
of MMP-9 mRNA (Fig. 3D). These
results indicate that treatment with piceatannol suppresses MMP-9
gene expression in TNF-α-induced DU145 cells via inhibition of
NF-κB activity.
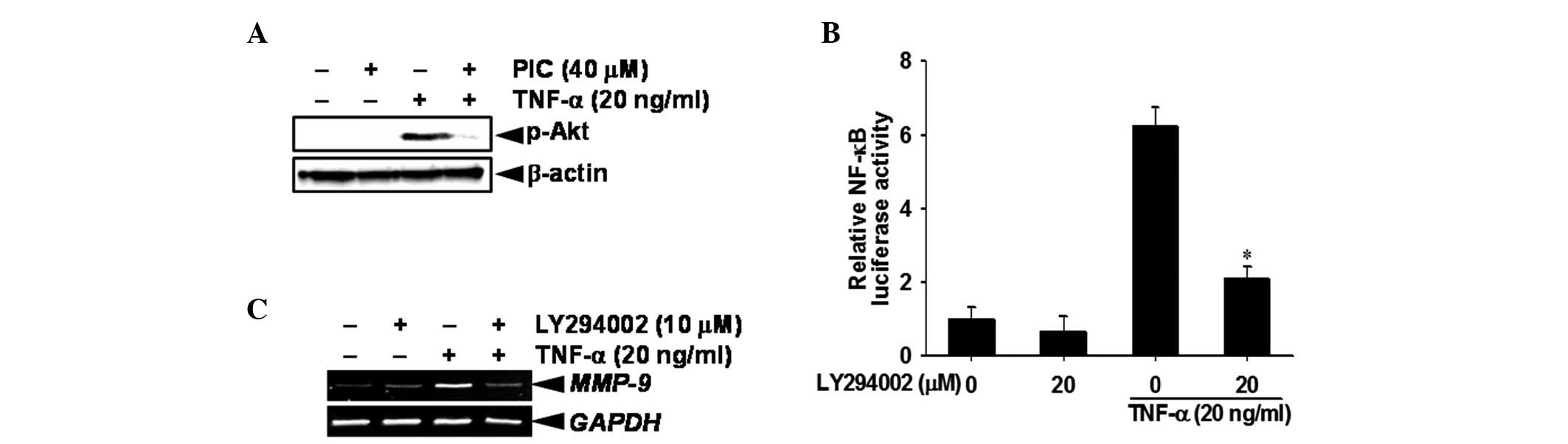
Piceatannol downregulates Akt
phosphorylation, which regulates NF-κB-dependent MMP-9 gene
expression
As Akt is known to be an upstream regulator of
NF-κB, we investigated whether NF-κB activity is regulated by
piceatannol-induced Akt phosphorylation or dephosphorylation.
Western blot analysis revealed that TNF-α stimulation induced the
phosphorylation of Akt at 15 min, whereas treatment with
piceatannol suppressed the phosphorylation of Akt (Ser 473,
Fig. 4A). It was also demonstrated
that a specific Akt inhibitor LY294002 completely eliminated the
TNF-α-induced NF-κB promoter activity, suggesting that inactivation
of Akt by piceatannol regulates TNF-α-induced NF-κB (Fig. 4B). Moreover, RT-PCR analysis
demonstrated that pretreatment of DU145 cells with LY294002
suppressed TNF-α-induced MMP-9 activity at the transcription level
(Fig. 4C). These results confirmed
that piceatannol regulated TNF-α-induced MMP-9 gene expression by
blocking Akt phosphorylation-dependent NF-κB activity.
Discussion
Our previous study determined that piceatannol
considerably suppresses lipopolysaccharide-induced pro-inflammatory
cytokines and nitric oxide expression in BV2 microglial cells
(15). Furthermore, it has been
reported that piceatannol enhances TRAIL-induced apoptosis in human
leukemia THP-1 cells via Sp1- and ERK-dependent DR5 upregulation
(16). It is not fully known how
piceatannol regulates anticancer activity during the invasion
process. Therefore, we investigated whether piceatannol inhibits
TNF-α-induced MMP-9, leading to decreased invasion of DU145
prostate cancer cells, by suppression of NF-κB activity. This study
provides substantial evidence that piceatannol inhibits
TNF-α-induced MMP-9 expression in DU145 prostate cancer cells by
suppressing Akt-mediated NF-κB activity.
MMPs are capable of digesting various components of
the extracellular matrix and other molecules, including cell
surface receptors, growth factors and cell adhesion molecules
(17,18). Thus, they play a significant role in
tissue repair, tumor invasion and metastasis (19,20).
The generation and analysis of transgenic and knockout mice for
MMPs have confirmed that MMPs play key roles in the process of
carcinogenesis and invasion (21).
One of these, MMP-9, is regarded as a critical molecule in tumor
progression and invasion (22).
Therefore, inhibition of MMP-9 appears to be an ideal strategy to
control tumor growth, invasion and metastasis. Our results
demonstrate that piceatannol inhibits TNF-α-induced MMP-9 activity
accompanied by the suppression of MMP-9 gene transcription in DU145
prostate cancer cells. Notably, the Matrigel assay revealed that
piceatannol significantly suppresses cell invasion when compared
with an MMP-9 inhibitor. Previous studies have also identified the
signal transduction pathways involved in regulating MMP-9
expression in various tumor cells (23,24).
In particular, NF-κB is a significant transcription factor for
regulating the MMP-9 gene promoter and contains NF-κB-binding sites
(12). This study revealed that
piceatannol regulates NF-κB activity by inhibiting p65 and p50
protein translocation. The results of the current study suggest
that the downregulation of NF-κB by piceatannol potentiates
antitumor and antimetastatic activities via the downregulation of
MMP-9 expression. A previous study has illustrated that the
suppression of NF-κB activity in human prostate cancer cells
inhibits their tumorigenic and metastatic properties in nude mice
by suppressing angiogenesis and invasion via the downregulation of
MMP-9 (25). It has also been
reported that several transcription factors in the human MMP-9
promoter region, including AP-1 and Sp1, regulate MMP-9 expression
in response to PMA and TNF-α induction (26,27).
Therefore, further studies are required to determine whether other
transcriptional factors are inhibited in piceatannol-induced MMP-9
downregulation. In addition, we investigated whether piceatannol
inhibits Akt phosphorylation, as Akt is the main molecule upstream
of NF-κB that has a significant role in cellular growth, adhesion
and differentiation (28).
Treatment with piceatannol completely suppressed Akt
phosphorylation. This was further confirmed by conducting
luciferase reporter assays and RT-PCR following treatment of the
cells with an Akt inhibitor LY294002. Therefore, the NF-κB-mediated
Akt pathway revealed the absolute effects on TNF-α-induced
MMP-9-dependent invasion in this study.
The role of MAPKs in the regulation of MMP-9
expression is particularly well understood in TNF-α-stimulated
cancer cells (29–31). Previously, the regulation of
TNF-α-induced MMP-9 activity has been reported via ERK, p38 and JNK
phosphorylation, whereas non-phosphorylated ERK, p38 and JNK kinase
expression was unaffected (32).
However, Lee et al have reported that TNF-α-induced p38 is
an effector MAPK that induces MMP-9 expression regardless of the
activation of ERK1/2 and JNK (23).
Therefore, additional studies are necessary to elucidate the
precise signaling mechanism that controls NF-κB activity and its
regulatory kinases during piceatannol-induced MMP-9 inhibition in
DU145 prostate cells.
In summary, the current study suggests that MMP-9
plays a considerable part in the regulation of MMP-9, resulting in
an inhibition of invasion in DU145 prostate cancer cells. Finally,
these results demonstrate that piceatannol is a potent inhibitor of
TNF-α-induced MMP-9 expression and invasion by suppressing the
Akt-mediated NF-κB pathway.
Acknowledgements
This study was supported by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (grant no. NRF-2012R1A1B4000508), Republic of Korea.
References
|
1
|
Koutroulis I, Zarros A and Theocharis S:
The role of matrix metalloproteinases in the pathophysiology and
progression of human nervous system malignancies: a chance for the
development of targeted therapeutic approaches? Expert Opin Ther
Targets. 12:1577–1586. 2008. View Article : Google Scholar
|
|
2
|
Himelstein BP, Canete-Soler R, Bernhard
EJ, Dilks DW and Muschel RJ: Metalloproteinases in tumor
progression: the contribution of MMP-9. Invasion Metastasis.
14:246–258. 1994.PubMed/NCBI
|
|
3
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heo SJ, Park EJ, Lee KW and Jeon YJ:
Antioxidant activities of enzymatic extracts from brown seaweeds.
Bioresour Technol. 96:1613–1623. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khan MN, Choi JS, Lee MC, Kim E, Nam TJ,
Fujii H and Hong YK: Anti-inflammatory activities of methanol
extracts from various seaweed species. J Environ Biol. 29:465–469.
2008.PubMed/NCBI
|
|
6
|
Brehmer B, Biesterfeld S and Jakse G:
Expression of matrix metalloproteinases (MMP-2 and -9) and their
inhibitors (TIMP-1 and -2) in prostate cancer tissue. Prostate
Cancer Prostatic Dis. 6:217–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dasgupta S, Srinidhi S and Vishwanatha JK:
Oncogenic activation in prostate cancer progression and metastasis:
Molecular insights and future challenges. J Carcinog. 11:42012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshida S, Ono M, Shono T, Izumi H,
Ishibashi T, Suzuki H and Kuwano M: Involvement of interleukin-8,
vascular endothelial growth factor, and basic fibroblast growth
factor in tumor necrosis factor alpha-dependent angiogenesis. Mol
Cell Biol. 17:4015–4023. 1997.
|
|
9
|
Ohshima H and Bartsch H: Chronic
infections and inflammatory processes as cancer risk factors:
possible role of nitric oxide in carcinogenesis. Mutat Res.
305:253–264. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palayoor ST, Youmell MY, Calderwood SK,
Coleman CN and Price BD: Constitutive activation of IkappaB kinase
alpha and NF-kappaB in prostate cancer cells is inhibited by
ibuprofen. Oncogene. 18:7389–7394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lü L, Tang D, Wang L, Huang LQ, Jiang GS,
Xiao XY and Zeng FQ: Gambogic acid inhibits TNF-α-induced invasion
of human prostate cancer PC3 cells in vitro through PI3K/Akt and
NF-κB signaling pathways. Acta Pharmacol Sin. 33:531–541. 2012.
|
|
13
|
Roupe KA, Remsberg CM, Yanez JA and Davies
NM: Pharmacometrics of stilbenes: segueing towards the clinic. Curr
Clin Pharmacol. 1:81–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuo PL and Hsu YL: The grape and wine
constituent piceatannol inhibits proliferation of human bladder
cancer cells via blocking cell cycle progression and inducing
Fas/membrane bound Fas ligand-mediated apoptosis pathway. Mol Nutr
Food Res. 52:408–418. 2008. View Article : Google Scholar
|
|
15
|
Jin CY, Moon DO, Lee KJ, Kim MO, Lee JD,
Choi YH, Park YM and Kim GY: Piceatannol attenuates
lipopolysaccharide-induced NF-kappaB activation and
NF-kappaB-related proinflammatory mediators in BV2 microglia.
Pharmacol Res. 54:461–467. 2006. View Article : Google Scholar
|
|
16
|
Kang CH, Moon DO, Choi YH, Choi IW, Moon
SK, Kim WJ and Kim GY: Piceatannol enhances TRAIL-induced apoptosis
in human leukemia THP-1 cells through Sp1- and ERK-dependent DR5
up-regulation. Toxicol In Vitro. 25:605–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong Z, Bonfil RD, Chinni S, Deng X,
Trindade JC, Bernardo M, Vaishampayan U, Che M, Sloane BF, Sheng S,
Fridman R and Cher ML: Matrix metalloproteinase activity and
osteoclasts in experimental prostate cancer bone metastasis tissue.
Am J Pathol. 166:1173–1186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curran S and Murray GL: Matrix
metalloproteinases: molecular aspects of their roles in tumour
invasion and metastasis. Eur J Cancer. 36:1621–1630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
John A and Tuszynski G: The role of matrix
metalloproteinases in tumor angiogenesis and tumor metastasis.
Pathol Oncol Res. 7:14–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong D, Li Y, Wang Z, Banerjee S and
Sarkar FH: Inhibition of angiogenesis and invasion by
3,3′-diindolymethane is mediated by the nuclear factor-kappaB
downstream target genes MMP-9 and uPA that regulated
bioavailability of VEGF in prostate cancer. Cancer Res.
67:3310–3319. 2007.
|
|
21
|
Bhoopathi P, Chetty C, Kunigal S, Vanamala
SK, Rao JS and Lakka SS: Blockade of tumor growth due to matrix
metalloproteinase-9 inhibition is mediated by sequential activation
of beta1-integrin, ERK, and NF-kappaB. J Biol Chem. 283:1545–1552.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ganesan P, Matsubara K, Ohkubo T, Tanaka
Y, Noda K, Sugawara T and Hirata T: Anti-angiogenic effect of
siphonaxanthin from green alga, Codium fragile.
Phytomedicine. 17:1140–1144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SJ, Park SS, Lee US, Kim WJ and Moon
SK: Signaling pathway for TNF-α-induced MMP-9 expression: mediation
through p38 MAP kinase, and inhibition by anti-cancer molecule
magnolol in human urinary bladder cancer 5637 cells. Int
Immunopharmacol. 8:1821–1826. 2008.
|
|
24
|
Genersch E, Hayess K, Neuenfeld Y and
Haller H: Sustained ERK phosphorylation is necessary but not
sufficient for MMP-9 regulation in endothelial cells: involvement
of Ras-dependent and -independent pathways. J Cell Sci.
23:4319–4330. 2000.PubMed/NCBI
|
|
25
|
Mimeault M and Batra SK: Potential
applications of curcumin and its novel synthetic analogs and
nanotechnology-based formulations in cancer prevention and therapy.
Chin Med. 6:312011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin AW, Chang CC and Mccormick CC:
Molecular cloning and expression of an avian macrophage
nitric-oxide synthase cDNA and the analysis of the genomic
5′-flanking region. J Biol Chem. 271:11911–11919. 1996.PubMed/NCBI
|
|
27
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carpenter CL and Cantley LC:
Phosphoinositide kinases. Curr Opin Cell Biol. 8:153–158. 1996.
View Article : Google Scholar
|
|
29
|
Olson CM, Hedrick MN, Izadi H, Bates TC,
Olivera ER and Anguita J: p38 mitogen-activated protein kinase
controls NF-kappaB transcriptional activation and tumor necrosis
factor alpha production through RelA phosphorylation mediated by
mitogen- and stress-activated protein kinase 1 in response to
Borrelia burgdorferi antigens. Infect Immun. 75:270–277.
2007. View Article : Google Scholar
|
|
30
|
Roberts JR, Rowe PA and Demaine AG:
Activation of NF-kappaB and MAP kinase cascades by hypothermic
stress in endothelial cells. Cryobiology. 44:161–169. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Behera AK, Thorpe CM, Kidder JM, Smith W,
Hildebrand E and Hu LT: Borrelia burgdorferi-induced
expression of matrix metalloproteinases from human chondrocytes
requires mitogen-activated protein kinase and Janus kinase/signal
transducer and activator of transcription signaling pathways.
Infect Immun. 72:2864–2871. 2004. View Article : Google Scholar
|
|
32
|
Jayasooriya RG, Choi YH, Moon SK, Kim WJ
and Kim GY: Methanol extract of Hydroclathrus clathratus
suppresses matrix metalloproteinase-9 in T24 bladder carcinoma
cells by suppressing the NF-κB and MAPK pathways. Oncol Rep.
27:541–546. 2012.
|