Introduction
Hepatocellular carcinoma (HCC), which accounts for
90% of all primary liver cancers, is the fifth most common
malignancy worldwide and the third most common cause of
cancer-related mortality globally (1). Due to its asymptomatic nature, early
HCC is difficult to detect and numerous patients present with
advanced or unresectable forms of the disease at diagnosis. Thus,
the prognosis for such patients remains poor. Previously, the
treatment of advanced HCC with conventional antineoplastic drugs
has not resulted in satisfactory outcomes, whilst the mean survival
time of an untreated HCC patient is 7–8 months (2). Sorafenib (Nexavar®, Bayer
Healthcare Pharmaceuticals, USA) is an oral multikinase inhibitor
that mainly targets Raf kinases, vascular endothelial growth factor
receptors 1, 2 and 3, and platelet-derived growth factor receptor
beta. It has been demonstrated to improve overall survival in
patients with advanced HCC in two randomized, double-blinded,
placebo-controlled trials (3,4). This
drug has been approved as the first-line therapy for such patients
(5). Observations of the tumor
response and its clinical course under treatment with sorafenib
were markedly different from those of conventional cytotoxic
agents. Notably, the majority of patients who responded to
sorafenib exhibited stable disease (SD) in both of the
aforementioned studies, and sorafenib seldom induced the
dimensional tumor shrinking typically observed with conventional
cytotoxic agents. Therefore, it has been suggested that sorafenib
prolongs survival by delaying disease progression. However,
long-term progression-free survival for almost five years with a
reduced dose of sorafenib in metastatic HCC is extremely rare. We
describe a case of a 74-year-old patient with hepatitis B,
cirrhosis and HCC, who was treated with a reduced dose of sorafenib
(200 mg twice a day) and achieved progression-free survival for
almost five years. Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images. The study was approved by the ethics committee of
Drum-Tower Hospital, Nanjing, China.
Case report
A 74-year-old Chinese man who had suffered from
hepatitis B virus-related cirrhosis since 1987 was referred to the
Drum Tower Hospital, Nanjing, China, due to the discovery of a
liver mass during an ultrasonographic examination in October 2005.
Enhanced computed tomography (CT) confirmed the presence of a
lesion approximately 55x50 mm in size, located in the inferior
segment of the right side of the liver. This mass was characterized
by its indistinct margins and infiltration, with wash-in in
arterial phase and wash-out in late phase, which are typical of
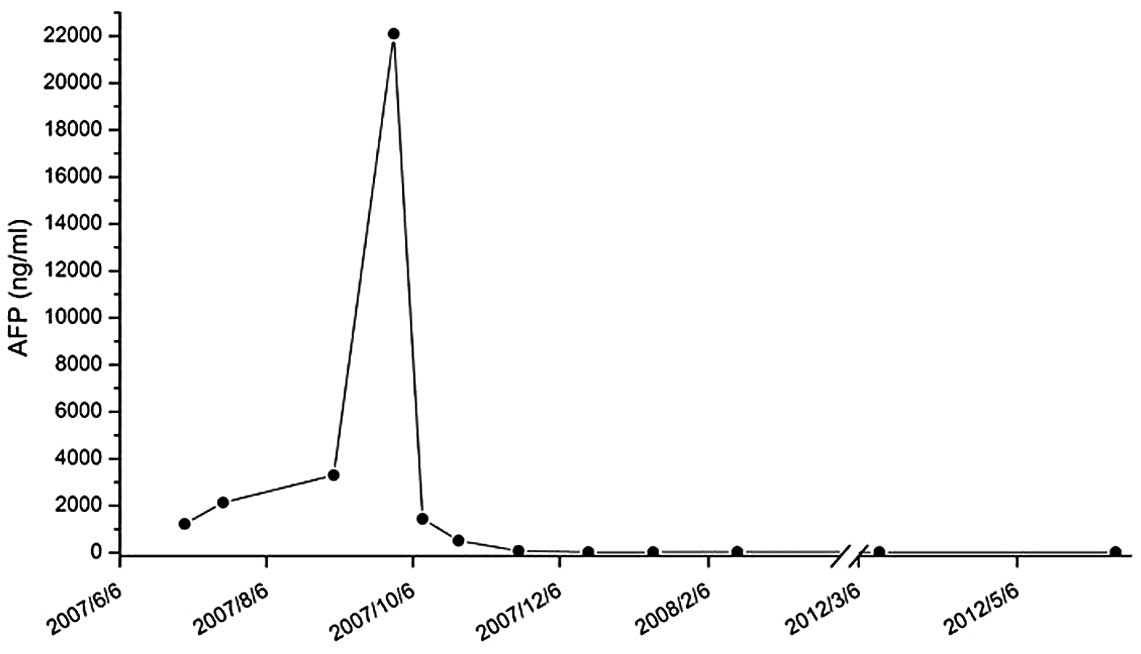
HCC. The patient’s alpha-fetoprotein (AFP) serum level was elevated
to 120 ng/ml (normal level is below 10 ng/ml). The pathological
examination of fine needle aspiration (FNA) confirmed the presence
of a moderate differentiated hepatocellular carcinoma. The patient
was otherwise healthy (ECOG PS 0) and hepatic synthesis was
well-retained without clinical signs of liver impairment
(Child-Pugh class A). Additionally, extrahepatic metastasis was not
observed. The patient was submitted to transarterial
chemoembolization in November 2005 and his AFP serum level was
reduced to a normal level following treatment.
Between March 2006 and June 2007 different therapies
of transarterial chemoembolization, percutaneous ethanol injection
and radiofrequency ablation were conducted due to local tumor
recurrence. The patient’s AFP serum level returned to normal after
each treatment. However, in July 2007, the patient’s AFP serum
level rose to 1,220 ng/ml and did not decrease following either
percutaneous ethanol injection or radio frequency ablation therapy.
By September 2007, the patient’s AFP serum level had increased to
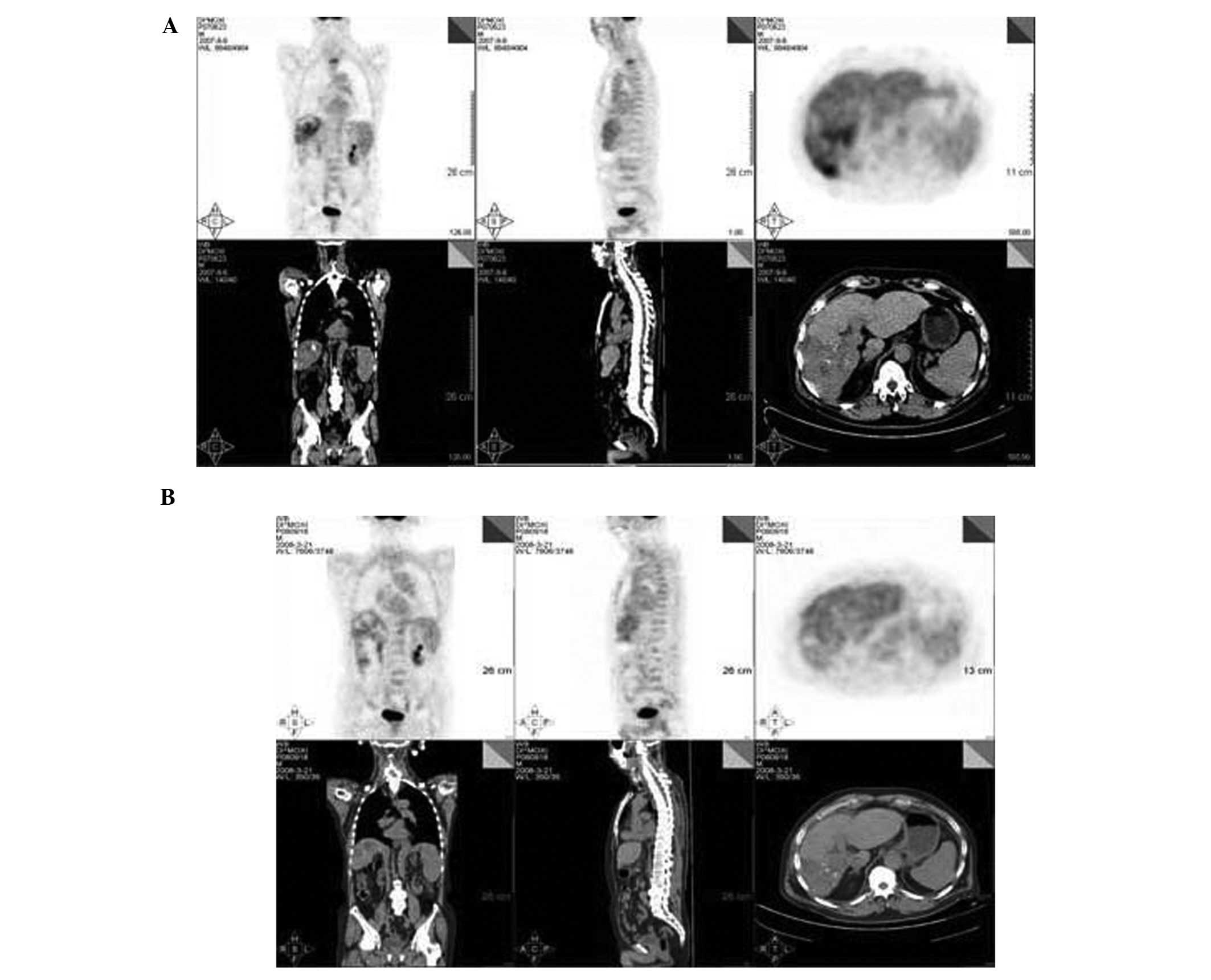
22,100 ng/ml. A surveillance computed tomography with positron
emission tomography (CT/PET) scan revealed a radioactive abnormal
uptake shadow mass of 86x57 mm in the inferior segment of the right
side of the liver that demonstrated increased uptake of FDG
(average SUV, 5.5). Additionally, a radioactive abnormal uptake
shadow mass was observed in the second thoracic vertebra with the
same increased uptake of FDG (Fig.
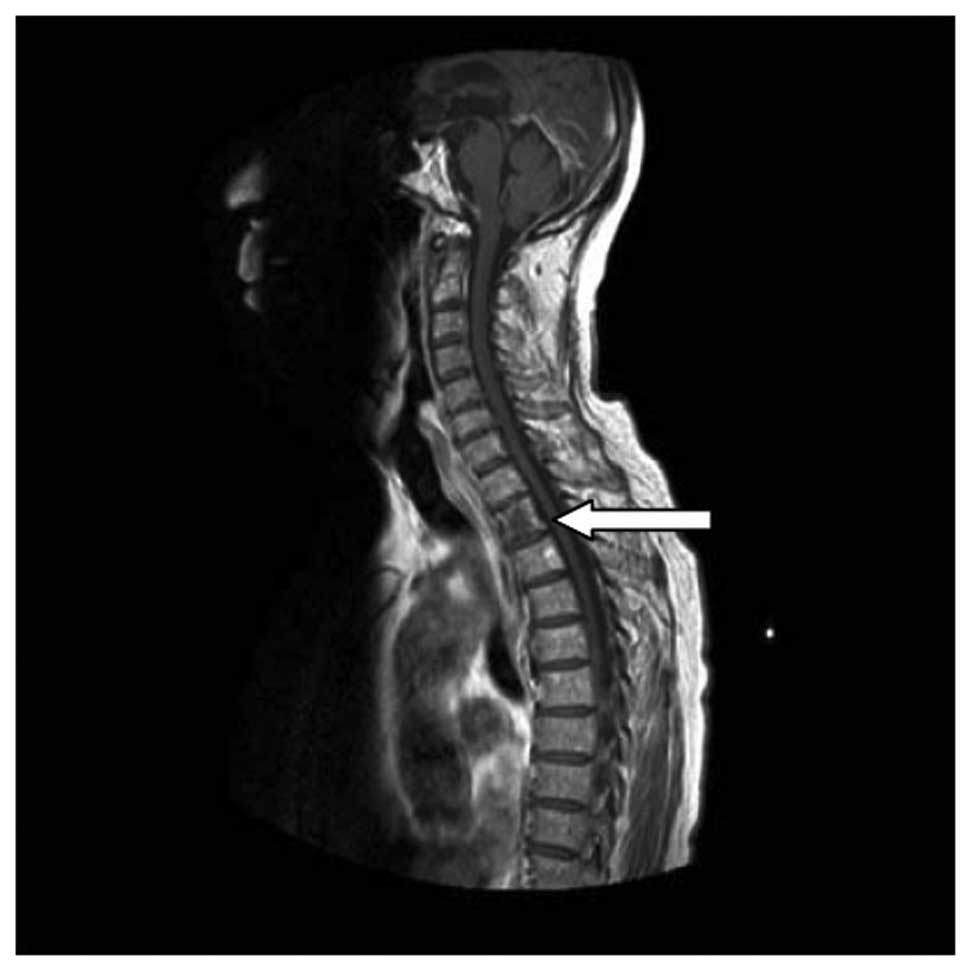
1A). Magnetic resonance imaging (MRI) revealed that the second
thoracic vertebra signal intensity was uneven and enhancement was
visible in contrast-enhanced scans, confirming vertebral metastasis
(Fig. 2).
As it was no longer possible to utilize regional
therapy, treatment with sorafenib tablets at a dose of 400 mg twice
a day was initiated. The first assessment of the efficacy of the
treatment regimen took place when the patient had undergone
treatment for two weeks. The patient’s AFP serum level had
significantly decreased from 22,100 to 1,436 ng/ml. However, the
patient required a dose reduction of sorafenib to 200 mg twice a
day, due to a grade 3 hand-foot skin reaction, grade 2 diarrhea and
significant deterioration in performance status.
The second assessment took place after 2 months of
treatment. The AFP serum level had decreased to within the normal
range. The patient remained stable on sorafenib at a dose of 200 mg
twice a day and the main adverse side-effect was a grade 1
hand-foot skin reaction, which was simply controlled with local
treatment. No other obvious adverse reactions were evident.
Following 6 months of treatment, a third assessment
took place. A follow-up CT/PET scan revealed that liver
radioactivity was distributed uniformly; on the right lobe,
numerous round/oval radioactive distribution defects of various
sizes were observed, which were considered to be tumor necrosis
based on the local treatment history. The radioactive abnormal
uptake shadows on the right lobe of the liver and the second
thoracic vertebra had disappeared (Fig.
1B).
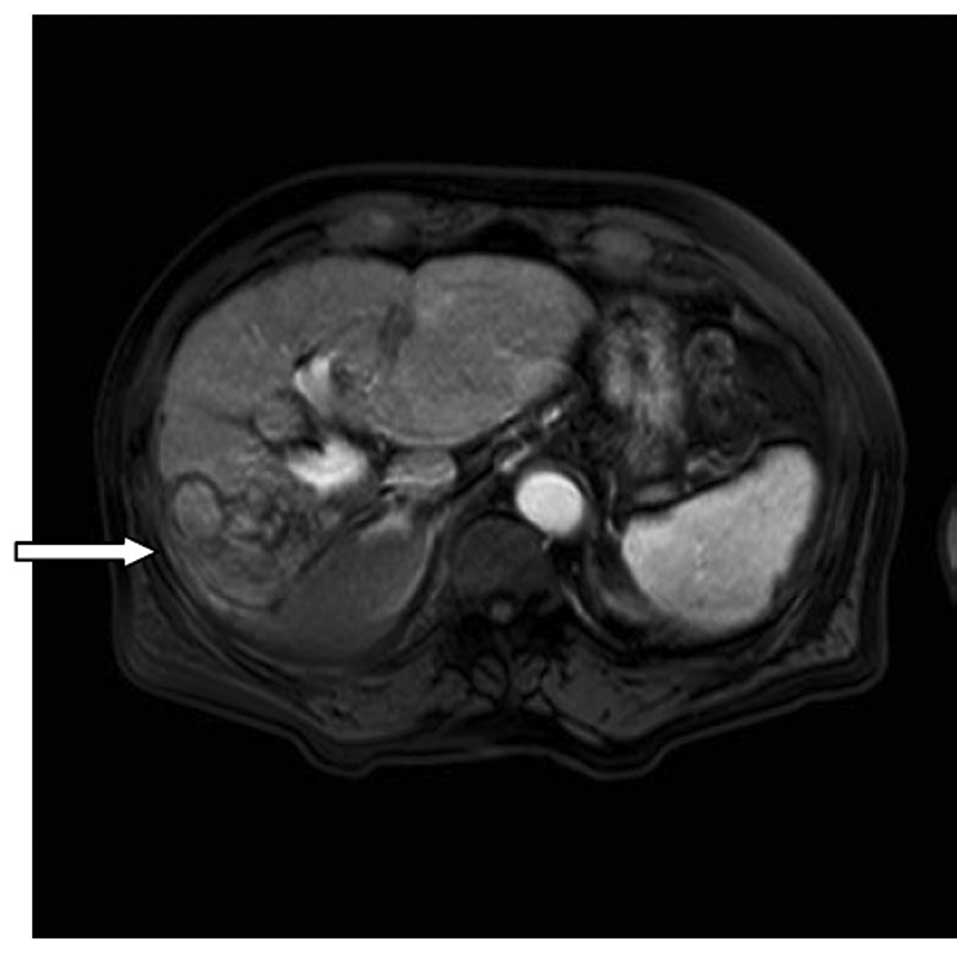
A fourth assessment took place after the patient had
undergone treatment for 20 months. The patient’s AFP serum level
remained steady within the normal range. MRI revealed the presence
of multiple nodules on the right lobe of the liver; however, the
contrast-enhanced scans did not identify hypervascular lesions in
the liver parenchyma tissue (Fig.
3).
A fifth assessment took place after four years of
treatment. The patient’s AFP serum level remained within the normal
range. Additionally, the contrast-enhanced MRI scans did not reveal
any abnormal enhancement lesions in the whole spine.
At the last visit, in May 2012, almost five years
after the diagnosis of bone metastasis, the patient’s AFP serum
level remained within the normal range (Fig. 4). MRI confirmed response by the
liver and thoracic vertebra lesions, whilst no new lesions were
found.
Until now, treatment with sorafenib has been
continued and a follow-up program to evaluate the duration of the
response is in progress. The patient also maintained a relatively
active lifestyle and no additional adverse events have been
identified.
Discussion
The present case concerns long-term progression-free
survival, extensively supported by imaging and the evaluation of
tumor markers, which occurred in a patient suffering from advanced
HCC with bone metastasis that was treated with sorafenib.
The approval of sorafenib and the active development
of numerous other molecularly targeted agents in HCC have presented
challenges to understand the mechanism of action of sorafenib and
to identify predictive biomarkers capable of selecting patients who
are more likely to benefit from sorafenib. Raf kinase is
overexpressed in a high percentage of human HCC tumors (6), and the RAF/MEK/ERK pathway can be
activated by key etiologic factors including hepatitis B virus
(HBV) and hepatitis C virus (HCV) infection (7). A previous study provides preliminary
evidence that baseline phosphorylated extracellular
signaling-regulated kinase (pERK) may be a relevant marker to
reflect the level of constitutive activation of the RAF/MAPK kinase
(MEK)/ERK signaling pathway, and has the potential value to predict
responses to sorafenib (8). The
clinical data from the initial single-arm phase II study and the
preliminary findings from the randomized phase III study also
suggest a correlation between baseline archived tumor pERK levels
and time to tumor progression in HCC patients. It is likely that
baseline pERK will become a useful predictive biomarker of the
response to, and the clinical benefits of, sorafenib in HCC;
however, this requires validation in future large-prospective
studies. The response observed in our patient was unique and the
underlying mechanism is not completely understood. We hypothesize
that the patient’s HCC may represent a rare condition where only a
single or few pathway(s) are mainly responsible for tumor
formation, including the Raf or vascular endothelial growth factor
(VEGF) pathway. Therefore, sorafenib, as a Raf or VEGF inhibitor,
may have completely blocked such growth signals, thus generating an
exceedingly optimized effect.
Sorafenib is the only effective systemic therapy for
the treatment of HCC; however, side effects may lead to treatment
discontinuation in certain patients. The described case highlights
how, in the case of sorafenib-related side effects, reductions in
the administered dose permit long-term treatment. The standard dose
of 400 mg twice a day was selected as the maximum tolerated dose
(MTD) based on a phase I study by Strumberg et al(9). Whether low-dose therapy is as
effective as high-dose therapy is not clear, but this was true in
the case of our patient. The efficacy of conventional cytotoxic
agents is positively correlated with the administered dose. With
new targeted agents, the length of treatment (as opposed to the
dose intensity) may be fundamental for tumor control. The most
effective strategy may involve managing the side-effects of
treatment and tailoring the anticancer regimen to the
characteristics of the patients, rather than discontinuing
treatment at the appearance of signs of intolerance. Notably,
although the recommended sorafenib dose is 400 mg twice a day, in
certain cases, dose reductions that limit the side effects may
offer a better quality of life and may allow for long-term
administration and maintenance of tumor control. Data concerning
the blood drug levels that are required to achieve and maintain
target inhibition are inadequate. The multi-target nature of
sorafenib is one additional challenge, as it implies that various
factors play a role in the activity of this agent (10).
The radiological features of responding lesions also
require investigation. When assessing the efficacy of targeted
therapies by imaging, a gradual change in tumor density and blood
flow may be observed prior to tumor shrinkage. In certain cases,
these lesions have been known to respond and become substituted by
residual scars (11). However,
uncommon radiological patterns may lead to late recognition of
responses or even to misleading evaluations. This issue requires
serious consideration, along with new, more appropriate methods of
appraisal. With targeted therapies, traditional methods of
quantitative evaluation, including WHO criteria or Response
Evaluation Criteria in Solid Tumors (RECIST), may not be optimal
and the requirement for qualitative standardized measurements
becomes more imminent. Recently, modified RECIST (mRECIST) were
developed based on measurements of viable tumors with arterial
enhancement on a computed tomography (CT) scan, which should be
used for the standard assessment of treatment efficacy,
particularly in patients who are receiving antiangiogenic drugs
(12). Moreover, functional 18
fluorodeoxyglucose (18FDG)-positron emission tomography (PET)
imaging provides an additional tool to assess tumor activity. The
integration of 18FDG-PET, MRI and CT allows for more precise
characterization of drug responses in this disease, which enhances
the evaluation of oncologic patients treated with molecularly
targeted drugs and accelerates drug development in numerous types
of tumors (13,14). In the described case, lesions
appeared to be unchanged and would initially have been considered
to be active. However, abnormal enhancement lesions disappeared
following treatment, so these lesions may have been substituted by
residual scars.
To our knowledge, although sorafenib has proven
effective in prolonging survival of advanced or unresectable HCC in
numerous clinical trials, there are currently no reported cases of
long-term progression-free survival for five years. In our case,
the patient’s follow-up CT/PET and MRI scans demonstrated that no
new lesions were present in the body and the AFP serum level has
remained within the normal range for almost five years, thus
demonstrating progression-free survival. This is the first
documented case of an advanced HCC patient with bone metastasis who
achieved long-term progression-free survival due to the
administration of a reduced dose of sorafenib. The duration of
overall survival and the time to progression of the patient have
been significantly prolonged, his quality of life has been elevated
and no markedly adverse side-effects are evident.
In summary, these data suggest that sorafenib may be
a well-tolerated treatment option, even in cases where the tumor
has spread extrahepatically. This unexpected outcome raises certain
questions and may become a matter of debate. For example, it may be
assumed that in tumor cells, mutations or alterations of the
genomic profile that cause the cells to become more sensitive to
sorafenib may have occurred. If this hypothesis is true, it
represents a valid reason to increase new investigations aiming to
study in-depth further genetic and molecular features in order to
identify more selective (or even individual) patterns of
response.
References
|
1
|
Bosch FX, Ribes J, Cléries R, et al:
Epidemiology of hepatocellular carcinoma. Clin Liver Dis.
9:191–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM and Bruix J: Novel advancements
in the management of hepatocellular carcinoma in 2008. J Hepatol.
48(Suppl 1): S20–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gish RG, Porta C, Lazar L, et al: Phase
III randomized controlled trial comparing the survival of patients
with unresectable hepatocellular carcinoma treated with nolatrexed
or doxorubicin. J Clin Oncol. 25:3069–3075. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshida T, Hisamoto T, Akiba J, et al:
Spreds, inhibitors of the Ras/ERK signal transduction, are
dysregulated in human hepatocellular carcinoma and linked to the
malignant phenotype of tumors. Oncogene. 25:6056–6066. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huitzel-Melendez FD, Saltz LB, Song J, et
al: Retrospective analysis of outcome in hepatocellular carcinoma
(HCC) patients with hepatitis C (C+) versus B (B+) treated with
sorafenib (abstract). In: Presented at the 2007 ASCO
Gastrointestinal Cancers Symposium. (abstract 173); Orlando, FL.
2007;
|
|
8
|
Zhu AX: Predicting the response to
sorafenib in hepatocellular carcinoma: where is the evidence for
phosphorylated extracellular signaling-regulated kinase (pERK)? BMC
Med. 7:422009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strumberg D, Richly H, Hilger RA, et al:
Phase I clinical and pharmacokinetic study of the Novel Raf kinase
and vascular endothelial growth factor receptor inhibitor BAY
43-9006 in patients with advanced refractory solid tumors. J Clin
Oncol. 23:965–972. 2005. View Article : Google Scholar
|
|
10
|
Petrelli A and Giordano S: From single- to
multi-target drugs in cancer therapy: when aspecificity becomes an
advantage. Curr Med Chem. 15:422–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abbadessa G, Rimassa L, Pressiani T, et
al: Optimized management of advanced hepatocellular carcinoma: four
long-lasting responses to sorafenib. World J Gastroenterol.
17:2450–2453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edeline J, Boucher E, Rolland Y, et al:
Comparison of tumor response by Response Evaluation Criteria in
Solid Tumors (RECIST) and modified RECIST in patients treated with
sorafenib for hepatocellular carcinoma. Cancer. 118:147–156. 2012.
View Article : Google Scholar
|
|
13
|
Milano A, Perri F, Ciarmiello A, et al:
Targeted-therapy and imaging response: a new paradigm for clinical
evaluation? Rev Recent Clin Trials. 6:259–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horger M, Lauer UM, Schraml C, et al:
Early MRI response monitoring of patients with advanced
hepatocellular carcinoma under treatment with the multikinase
inhibitor sorafenib. BMC Cancer. 9:208–209. 2009. View Article : Google Scholar
|