Introduction
Recently, sugar chains have drawn extensive
attention from sugar biologists due to their increasing role in
tumor growth and invasion and metastasis of cancer. Each step of
the sugar chain synthesis requires a specific glycosyltransferase
to catalyze the transfer of a monosaccharide moiety from a glycosyl
donor to an acceptor substrate; therefore, the abnormal sugar chain
is controlled by the transfer of enzymes from sugar chain synthesis
catalyzed glycosylation in the final analysis. Polypeptide
N-acetylgalactosaminyltransferase (pp GalNAc-T, EC2.4.1.4.1) is an
O-sugar chain synthesis-starting glycosyltransferase, which
catalyzes the transfer of the GalNAc group of UDP-GalNAc to the
polypeptide chain Thr or Ser hydroxyl in a specific sequence in
order to synthesize GalNAcα-O-Ser/Thr peptide; the peptide chain
gradually extends under the action of other glycosyltransferases
(1). A growing number of studies
have demonstrated that pp GalNAc-Ts is closely connected with tumor
occurrence and development. Ishikawa et al(2) identified that pp GalNAc-T3 expression
is a useful indicator of tumor differentiation in early gastric
cancer and its expression is positively correlated with the depth
of tumor invasion and lymph node metastasis. Wu et
al(3) revealed that the
expression of pp GalNAc-T14 is associated with clinicopathological
characteristics of breast carcinoma. Berois et al(4) reported that pp GalNAc-T6 is expressed
in the majority of human breast carcinomas, but not in normal
breast epithelium and is sporadically found in non-malignant breast
diseases. Gu et al(5)
hypothesised that low expression of pp GalNAc-T3 may be a useful
indicator of early tumor recurrence in stage I lung adenocarcinoma.
pp GalNAc-T10 is a member of the pp GalNAc family; however, limited
study has been conducted. In this study, an immunohistochemical
method was applied to detect pp GalNAc-T10 protein expression in
different gastric mucosal lesions. Expression of pp GalNAc-T10 in
gastric cancer tissues was identified to be higher than that in
adjacent non-tumor gastric tissue. pp GalNAc-T10 protein expression
in gastric cancer was significantly positively correlated with
histological type and degree of differentiation of the gastric
cancer. Therefore, pp GalNAc-T10 may be a specific biomarker for
gastric cancer.
Materials and methods
Collection of tissue samples and
histological classification
In this study, 96 gastric adenocarcinoma surgical
samples and 88 5-cm adjacent cancer non-tumor gastric mucosa
control samples were used. All specimens were collected from the
two Affiliated Hospitals of Suzhou University. The study was
approved by the ethics committee of Southern Medical University,
Guangzhou, China. The patients, ranging in age from 38 to 83 years
(median age, 62 years; mean age, 63.4 years), had received no other
treatment prior to surgery. All patients provided informed consent
to participate in this study. The clinicopathological parameters
included gender, age, TNM stage, Laurén’s type, histological grade,
depth of tumor invasion and lymph node metastasis (Table II) in which TNM staging system is
according to the 2004 AJCC (American Joint Committee on
Cancer)/UICC (Union Internationale Contre le Cancer) gastric cancer
staging.
 | Table IIpp GalNAc-T10 expression and
clinicopathological parameters in 96 patients with gastric
carcinoma. |
Table II
pp GalNAc-T10 expression and
clinicopathological parameters in 96 patients with gastric
carcinoma.
| | pp GalNAc-T10
expression
| |
|---|
| Clinicopathological
parameters | n | Strong positive | Weakly positive | Negative | P-value |
|---|
| Age (years) | | | | | 0.753 |
| ≥60 | 62 | 39 | 13 | 10 | |
| <60 | 34 | 23 | 5 | 6 | |
| Gender | | | | | 0.699 |
| Female | 29 | 17 | 6 | 6 | |
| Male | 67 | 45 | 12 | 10 | |
| TNM stage (AJCC) | | | | | 0.588 |
| I to II | 32 | 20 | 5 | 7 | |
| III to IV | 64 | 42 | 13 | 9 | |
| Depth of tumor
invasion | | | | | 0.416 |
| T1 to T2 | 22 | 16 | 2 | 4 | |
| T3 to T4 | 74 | 46 | 16 | 12 | |
| Lymph node
metastasis | | | | | 0.564 |
| Yes | 70 | 47 | 13 | 10 | |
| No | 26 | 15 | 5 | 6 | |
| Histological
grade | | | | | 0.016 |
| High, mid | 28 | 12 | 8 | 8 | |
| Low | 68 | 50 | 10 | 8 | |
| Laurén’s type | | | | | 0.022 |
| Intestinal | 62 | 34 | 14 | 14 | |
| Diffuse | 34 | 28 | 4 | 2 | |
Tissue microarray production
Cores (diameter 1 mm) were taken from the marked
region of individual formalin-fixed paraffin-embedded gastric
adenocarcinoma specimens and the 5-cm adjacent cancer non-tumor
gastric mucosa control samples. In 8 cases, a non-tumor gastric
mucosa control sample was missing. Two cores from each tissue were
assembled adjacently into a single recipient TMA block (Outdo
Biotech, Shanghai, China).
Immunohistochemistry
For pp GalNAc-T10 immunostaining, quenching of
endogenous peroxidase activity was performed with 3%
H2O2 in PBS for 20 min, which was then
blocked with normal goat serum (Gibco, Carlsbad, CA, USA) for 20
min. Anti-pp GalNAc-T10 (Abcam, Cambridge, MA, USA) was incubated
overnight at 4°C and peroxidase-conjugated goat anti-mouse
polyvalent antibody (Maixin-Bio, Jinan, China) was incubated for 60
min at room temperature. Reactions were revealed with
diaminobenzidine, washed in water, counterstained in Mayer’s
hematoxylin and dehydrated in ethanol and xylene and were then
mounted. Between each step, sections were washed in PBS. For every
assay, negative controls using PBS without primary antibody were
included.
Immunocytochemical analysis
The immunostaining frequency for each tumor was
scored as follows: 0 for negative samples or ≤5% stained tumor
tissue; 1 for staining between 6 and 25% of the tumor tissue; 2 for
staining between 26 and 50% of the tumor tissue; 3 for staining
between 51 and 75% of the tumor tissue; and 4 for staining >75%
of the tumor tissue. Signal intensity was scored as strong
(3), moderate (2), weak (1) and null (0). Total immunostaining score
resulted from the addition of both parameters. Scores were
established jointly by four observers under a multi-head
microscope. Clinicopathological information was masked to the
observers.
Statistical analysis
SPSS 13.0 statistical software was used for
statistical analysis. Gastric cancer tissues and adjacent non-tumor
gastric tissue protein expression differing in intensity were
tested with the two sample rate of the matched pair, designed to
compare the Chi-square. The Chi-square test was applied to analyze
the correlation between intensity and clinicopathological
parameters of the protein expression. P<0.05 was considered to
indicate a statistically significant difference.
Results
pp GalNAcT10 expression in gastric cancer
tissues and adjacent non-tumor gastric tissues
To determine whether there are differences between
the expression of pp GalNAc-T10 in cancerous gastric cancer tissues
and adjacent non-cancerous gastric mucosa, we designed paired
experiments of gastric cancer tissues and adjacent non-tumor
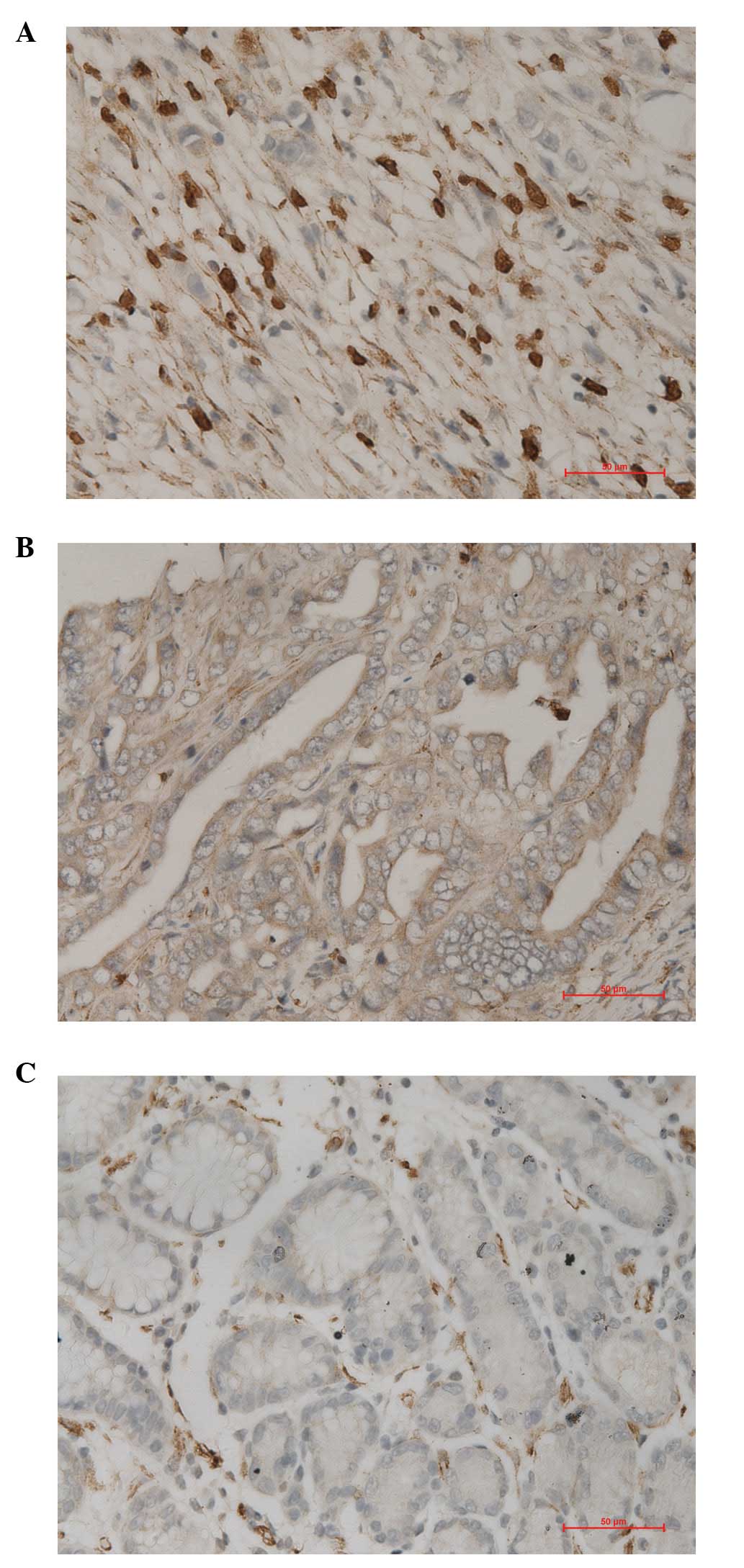
gastric mucosa. Under observation using an optical microscope, we
identified that pp GalNAc-T10 protein is expressed in gastric
cancer cells and normal gastric epithelial cell cytoplasm and cell
membrane, which was observed as brown granules (Fig. 1). Statistical analysis results
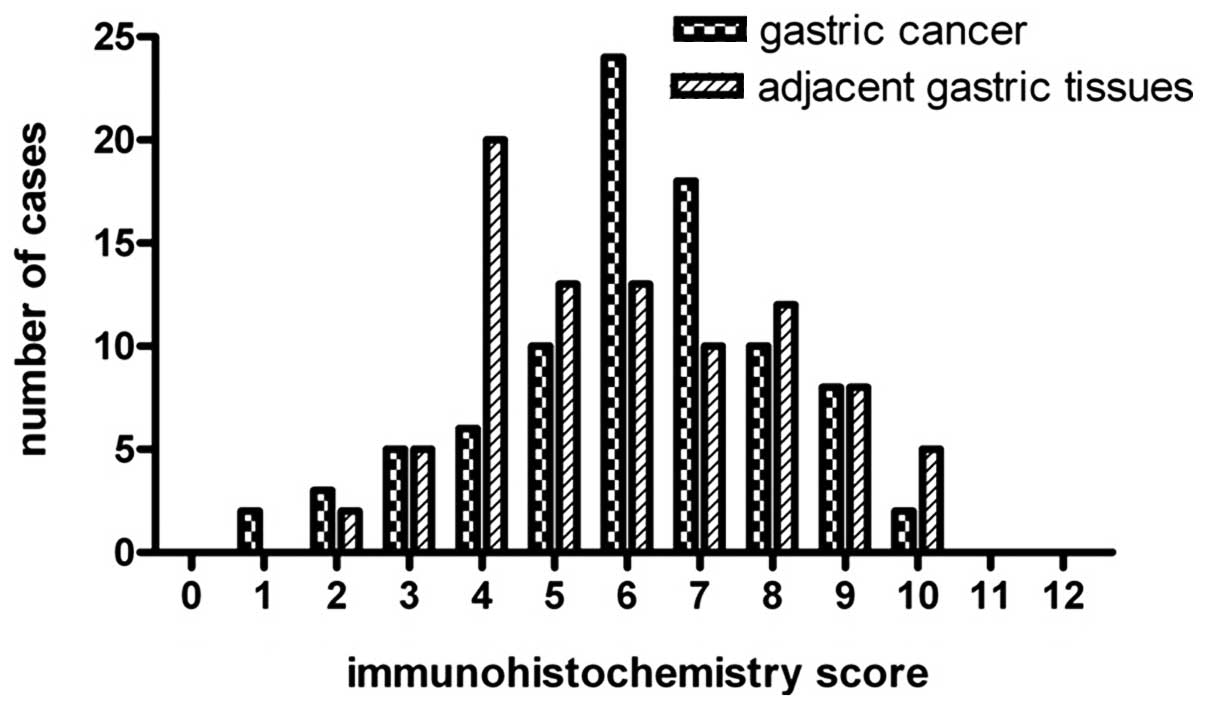
demonstrated (Fig. 2) that the
expression of pp GalNAc-T10 in gastric cancer tissues was higher
than that in adjacent non-tumor gastric cancer organizations, and
that the difference was significant (P<0.01; Table I).
 | Table Ipp GalNAc-T10 expression in 88 cases
of gastric cancer and adjacent non-tumor gastric tissue. |
Table I
pp GalNAc-T10 expression in 88 cases
of gastric cancer and adjacent non-tumor gastric tissue.
| Tissue | Strong positive | Weakly positive | Negative | P-value |
|---|
| Gastric cancer | 56 | 16 | 16 | 0.007 |
| Adjacent non-tumor
gastric | 48 | 33 | 7 | |
Correlation between pp GalNAc-T10
expression in gastric cancer tissues and clinicopathological
parameters
In order to further determine the correlation
between pp GalNAc-T10 expression and gastric cancer
clinicopathological parameters, correlation analysis between the pp
GalNAc-T10 expression strength and gastric cancer
clinicopathological parameters was conducted. The results revealed
that pp GalNAc-T10 protein expression had a significant positive
correlation with gastric cancer histological type (differentiation
degree; P<0.05) and expression in diffuse-type gastric cancer
was significantly higher than that in the intestinal-type gastric
cancer. The intensity of expression in poorly differentiated
gastric carcinoma was significantly higher than that in moderate
and highly differentiated cases. There was no significant positive
correlation with age, gender, clinical stage, depth of invasion or
lymph node metastasis (P<0.05; Table
II).
Discussion
It is widely acknowledged that glycosyltransferases
affect the development of tumors by altering the structure of sugar
chains. The changes in cell surface sugar chains have an impact on
the interaction between cancer cells, and cancer cells and the
extracellular matrix. This in turn has a major impact on the
behavior of cancer cells, including tumor growth, invasion and
metastasis. The T-antigen O-glycosylation originates in the
peptide: the enzyme family of pp GalNAc-Ts. Certain members of the
pp GalNAc-T family have reported abnormal expression in squamous,
colon, stomach and lung cancer, leukemia and other types of cancer.
pp GalNAc-T10 is a member of the pp GalNAc-T family, which is
highly expressed in human gastrointestinal tissues (6), but at present there is no study
relating pp GalNAc-T10 expression to cancer. In this study, pp
GalNac-T10 protein expression was immunohistochemically analyzed,
in 96 gastric adenocarcinoma samples and 88 5-cm adjacent cancer
non-tumor gastric mucosa control samples. Results demonstrated that
pp GalNAc-T10 protein expression in gastric cancer tissues was
higher than that in adjacent non-tumor gastric tissues, and the
expression had a significant positive correlation with the degree
of tumor differentiation (P<0.05). Expression in poorly
differentiated gastric carcinoma was significantly higher than that
in cases of high and mid degree in differentiation, and the
expression intensity in the diffuse-type gastric cancer was
significantly higher than in the intestinal-type gastric cancer. pp
GalNAc-T10 protein expression was not significantly positively
correlated with clinical stage, depth of invasion or lymph node
metastasis (P>0.05). Therefore, abnormal expression of pp
GalNAc-T10 may be a significant event which determines whether
normal cells tranform into malignant poorly differentiated gastric
cancer cells. This may also be used as a diagnostic indicator of
malignant gastric cancer cells. Through analysis of the protein
expression in gastric cancer cells, we may be able to provide
suggestions for the diagnosis of gastric cancer development and
treatment.
We speculate that the impact of pp GalNAc-T10 on the
proliferation and differentiation of gastric cancer results from
its action on certain cell signaling pathways, particularly the
transforming growth factor-β (TGF-β) signal-regulated pathway. The
TGF-β superfamily is a newly discovered family of proteins that
regulate cell growth and differentiation. Studies have reported
that disruption of TGF-β signaling in diffuse-type gastric
carcinoma models may accelerate tumor growth (7) and TGF-β decreases cancer-initiating
cell proliferation within diffuse-type gastric carcinoma cells
(8). Otherwise, N-acetyl
aminotransferase is likely to play an important role in
diffuse-type gastric cancer cells (9) in a TGF-β signal-regulated pathway.
TGF-β1 mRNA level is significantly decreased in the overexpression
of pp GalNAc-T2 (10). Therefore,
we hypothesize that the pp GalNAc-T family may be correlated with
the TGF-β family. Further studies are required to study the impact
of pp GalNAc-T10 on the proliferation and differentiation of
gastric cancer, and its correlation with TGF-β signaling
pathway.
In the occurrence and development of gastric cancer,
changes in sugar chains originate from the transferring of the
glycosyltransferase. Through in-depth study of the pp GalNAc-T
family we may be able to obtain a deeper knowledge of the
pathogenesis of gastric cancer and provide a more effective
reference for existing diagnosis and treatment options.
References
|
1
|
Brockhausen I: Pathways of O-glycan
biosynthesis in cancer cells. Biochim Biophys Acta. 1473:67–95.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishikawa M, Kitayama J, Nariko H, Kohno K
and Nagawa H: The expression pattern of
UDP-N-acetyl-a-D-galactosamine: polypeptide N-acetylgalactosaminyl
transferase-3 in early gastric carcinoma. J Surg Oncol. 86:28–33.
2004. View Article : Google Scholar
|
|
3
|
Wu C, Guo X, Wang W, Wang Y, Shan Y, Zhang
B, Song W, Ma S, Ge J, Deng H and Zhu M:
N-Acetylgalactosaminyltransferase-14 as a potential biomarker for
breast cancer by immunohistochemistry. BMC Cancer. 10:2–8.
2010.PubMed/NCBI
|
|
4
|
Berois N, Mazal D, Ubillos L, Trajtenberg
F, Nicolas A, Sastre-Garau X, Magdelenat H and Osinaga E:
UDP-N-Acetyl-D-Galactosamine: polypeptide
N-Acetylgalactosaminyltransferase-6 as a new immunohistochemical
breast cancer marker. J Histochem Cytochem. 54:317–328. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu C, Oyama T, Osaki T, Li J, Takenoyama
M, Izumi H, Sugio K, Kohno K and Yasumoto K: Low expression of
polypeptide GalNAc N-acetylgalactosaminyl transferase-3 in lung
adenocarcinoma: impact on poor prognosis and early recurrence. Br J
Cancer. 90:436–442. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng L, Tachibana K, Zhang Y, Guo JM,
Tachibana KK, Kameyama A, Wang H, Hiruma T, Iwasaki H, Togayachi A,
Kudo T and Narimatsu H: Characterization of a novel human
UDP-GalNAc transferase, pp-GalNAc-T10. FEBS Lett. 531:115–121.
2002.PubMed/NCBI
|
|
7
|
Komuro A, Yashiro M, Iwata C, Morishita Y,
Johansson E, Matsumoto Y, Watanabe A, Aburatani H, Miyoshi H,
Kiyono K, Shirai YT, Suzuki HI, Hirakawa K, Kano MR and Miyazono K:
Diffuse-type gastric carcinoma: progression, angiogenesis, and
transforming growth factor beta signaling. J Natl Cancer Inst.
101:592–604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ehata S, Johansson E, Katayama R, Koike S,
Watanabe A, Hoshino Y, Katsuno Y, Komuro A, Koinuma D, Kano MR,
Yashiro M, Hirakawa K, Aburatani H, Fujita N and Miyazono K:
Transforming growth factor-beta decreases the cancer-initiating
cell population within diffuse-type gastric carcinoma cells.
Oncogene. 30:1693–1705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herr P, Korniychuk G, Yamamoto Y, Grubisic
K and Oelgeschlager M: Regulation of TGF-(beta) signalling by
N-acetylgalactosaminyltransferase-like 1. Development.
135:1813–1822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Yang L, Jin M, Xu L and Wu S:
Regulation of the invasion and metastasis of human glioma cells by
polypeptide N-acetylgalactosaminyltransferase 2. Mol Med Report.
4:1299–1305. 2011.PubMed/NCBI
|