Introduction
Hyaluronan (HA) is a non-sulfated linear
glycosaminoglycan present in the extracellular matrix (ECM) of most
tissues. It is synthesized and extruded at the plasma membrane by
HA synthases (HAS1, HAS2 and HAS3) and
consists of repeating d-glucuronic acid and
N-acetyl-d-glucosamine units (1,2).
Several studies have shown that HA plays important roles in matrix
assembly, cell proliferation, differentiation and migration during
development and disease (3).
Previous studies have shown that elevated HA in the tumor stroma
correlates with tumor aggressiveness and poor prognosis in patients
with breast, prostate and ovarian cancers (2,4).
Knockdown of HAS genes in cancer cells inhibits proliferation,
invasion, and motility in vitro and tumor growth and
metastasis in vivo(5). In
breast cancer cells, HAS2 expression is often strongly
correlated with malignant behavior (6–8). Thus,
abnormalities of HA synthesis and/or degradation are frequently
observed in various cancers.
4-methylumbelliferone (4-MU;
7-hydroxy-4-methyl-2H-1-benzopyran-2-one) was first found to
specifically inhibit HA synthesis in human skin fibroblasts
(9). It does so by causing
substrate inhibition of HASs due to 4-MU binding to GlcUA
via UDP-GlcUA (10). 4-MU also
inhibits HA synthesis via repression of HAS2 and/or
HAS3 mRNA in breast cancer, melanoma and ovarian cancer
cells (11). 4-MU-mediated
inhibition of HA synthesis produces anticancer effects on cell
proliferation, migration, invasion and metastasis in vitro
and in vivo in several human cancers such as breast and
prostate cancers (11–15). As it produces the anticancer effect
without causing severe side effects, 4-MU has the potential to
become a novel anticancer drug. However, it remains unclear whether
4-MU exhibits anticancer activity against canine mammary tumor
cells.
Metastasis is the primary cause of mortality in
various human and canine cancers. Metastatic cells exhibit elevated
cell motility, which mediates the epithelial to mesenchymal
transition (EMT). In general, cell motility may be categorized as
chemokinesis and chemotaxis. Chemokinesis is random cell movement,
which involves the separation of tumor cells from their primary
site and is thus important during the EMT process (16,17).
Chemotaxis is defined as a directional cell movement. Once ECM
remodeling has been activated, mesenchymal-like cancer cells have
many opportunities for interaction with components of the ECM such
as HA, collagen and laminin (18–20).
Canine mammary tumors are one of the most frequent
cutaneous tumors of female dogs. Histologically, approximately 50%
of canine mammary tumors are malignant, and metastases and/or
recurrences are common causes of mortality in these animals
(21,22). Recent studies of human and canine
gene expression in tumor and normal mammary samples suggest many
cancer-related genes that are deregulated in human breast cancer
are also found in canine mammary tumors (23). For example, in malignant mammary
tumors in dogs, the expression patterns of ECM remodeling-related
genes are very similar to those in humans (23). Canine mammary tumors are classified
based on cytological characteristics as epithelial, mesenchymal or
mixed, according to origin. Histologically complex carcinoma is
commonly observed in canine mammary tumors. In benign canine
mammary tumors, complex adenomas and benign mixed tumors are most
common. This histological type has both epithelial and mesenchymal
(myoepithelial) components (24).
However, it is not clear whether 4-MU acts as an antitumor agent
against mesenchymal cells in canine mammary tumors. The aim of this
study was, therefore, to define the antitumor effect of 4-MU on
CF41.Mg cells with properties of mesenchymal-like canine mammary
tumor cells.
Materials and methods
4-MU
4-MU was purchased from Wako Pure Chemicals (Osaka,
Japan). The 4-MU stock solution was dissolved in DMSO. The final
concentration of DMSO in the medium was adjusted to 0.1% in all
experiments.
Cell culture
Canine mammary tumor cell line CF41.Mg and CF33
cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). Both were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) (Nissui, Tokyo, Japan) supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 4 mM l-glutamine, 10
mg/ml streptomycin and 10,000 U/ml penicillin G. The cells were
maintained at 37°C in a humidified atmosphere with 5%
CO2.
Cell proliferation analysis
We used the Cell Counting Kit-8 (Dojindo
Laboratories, Kumamoto, Japan) to assess the effect of 4-MU on cell
proliferation. CF41.Mg cells were plated in 96-well plates
(4.5×103 cells/well). At each time point (days 0–4), 10
μl CCK-8 reagent was added, and the plates were incubated
for 4 h. After incubation, the absorbances were measured at 450 nm
with a Benchmark plus microplate reader (Bio-Rad, Tokyo, Japan). In
these experiments, 5 replicate wells were used for each time point;
the results are presented as means ± SD.
Cell cycle and apoptosis analysis
Cells were harvested and washed with
phosphate-buffered saline (PBS), resuspended in 70% ethanol in
distilled water, and kept at −30°C overnight. Before analysis,
cells were mixed and incubated for 30 min in PBS containing 0.05
mg/ml propidium iodide (PI) and 100 U/ml RNase A. The suspension
was filtered through a 5-ml polystyrene round-bottom tube with a
cell-strainer cap (Becton Dickinson, Franklin Lakes, NJ, USA) and
analyzed by FACSCalibur (Becton Dickinson) and Flow-Jo 7 software
(Tree Star, Ashland, OR, USA).
Real-time RT-PCR
Total RNA was extracted from cells using the TRIzol
reagent (Invitrogen, Carlsbad, CA, USA), and cDNAs were synthesized
with a PrimeScript™ RT Master Mix (Takara Bio, Shiga, Japan)
according to the manufacturer’s protocols. Real-time PCR was
performed with SYBR Premix Ex Taq™ (Takara Bio) and the ABI
Prism 7500 Real-Time PCR System (Applied Biosystems, Foster City,
CA, USA) under the following conditions: 95°C for 30 sec; 40 cycles
of 95 kC for 5 sec and 60°C for 34 sec. Specific primer sets for
BAX (forward, 5′-CGCATCGGAGATGAACTGGA-3′; reverse,
5′-ACCAGTTTGCTGGCAAAGTAGAAG-3′) and N-cadherin (forward,
5′-AGGAATCCGACGATTGGA TGAG-3′; reverse, 5′-GTGGGATCATTGTCAGCAGCT
TTA-3′) were purchased from Takara Bio. HAS1 (forward,
5′-GGACTACGTGCAGGTGTGTG-3′; reverse, 5′-CTCAC CTAGGGGACCACTGA-3′),
HAS2 (forward, 5′-CTTAGA GCACTGGGA-3′; reverse, 5′-TCTAAAACT
TTCACCA-3′), HAS3 (forward, 5′-AAGTAGGGGGAG TTGG-3′;
reverse, 5′-CCCAGAGGCCCACTAA-3′), vimentin (forward,
5′-ATTGCTCTGCCTCTTC-3′; reverse, 5′-GGCAAG CTT CACTCAA-3′),
E-cadherin (forward, 5′-CCCTCATTATAG CCAT-3′; reverse,
5′-AGTCCATATTTCGAGG-3′), and GAPDH (forward,
5′-AAGGCTGAGAACGGGA-3′; reverse, 5′-GGAGGCATTGCTGACA-3′) were
obtained from Operon Biotechnology (Tokyo, Japan). The specificity
of each amplification was confirmed by a dissociation curve
consisting of a single peak. All samples were amplified in
triplicate in each experiment. The values were normalized to GAPDH.
Relative levels of mRNA were calculated using the ΔΔCt method.
Motility assay
To investigate the effect of 4-MU on chemokinesis
and chemotaxis, the Boyden chamber migration assay was employed
(25,26). Before the motility assay, cells were
starved overnight in DMEM supplemented with 1% FBS. CF41.Mg cells
(1.5×104 cells/well) treated with 4-MU for 24 h were
loaded in the upper chambers of polycarbonate membrane transwell
inserts (Corning Inc., Corning, NY, USA). The Boyden chamber
contained two medium-filled compartments. Each chamber
(upper/lower) contained a different concentration (1%/1%, and
1%/10%) of FBS. Each set of lower and upper chambers was separated
by an 8-μm pore size polycarbonate membrane. The cells were
allowed to migrate for 10 h. The membranes were then fixed with 4%
paraformaldehyde phosphate buffer solution (Wako) and stained with
Meyer’s hematoxylin (Wako). The cells on the upper side of each
membrane were removed with cotton swabs. The cells on the lower
side were counted under a light microscope at ×200 magnification.
Four random microscopic fields were counted. Results are presented
as means ± SD.
Statistical analysis
The statistical significance of differences in
chemokinesis and chemotaxis were determined by Student’s t-test.
P<0.05 was considered to indicate a statistically significant
result.
Results
Canine mammary tumor CF41.Mg cells have
properties of mesenchymal-like cells
During tumor progression, advanced tumor cells
frequently exhibit a conspicuous loss of cell-cell adhesion such as
downregulation of E-cadherin. The loss of epithelial features is
accompanied by increased motility, resistance to anti-cancer drugs,
and expression of mesenchymal genes such as vimentin and N-cadherin
(16,27). These processes are known as EMT, and
are thought to be critical to cancer cell invasion and metastasis.
To examine the effect of 4-MU on mesenchymal-like cells of canine
mammary tumors, we first determined whether canine mammary tumor
CF41.Mg cells possess features characteristic of epithelial or
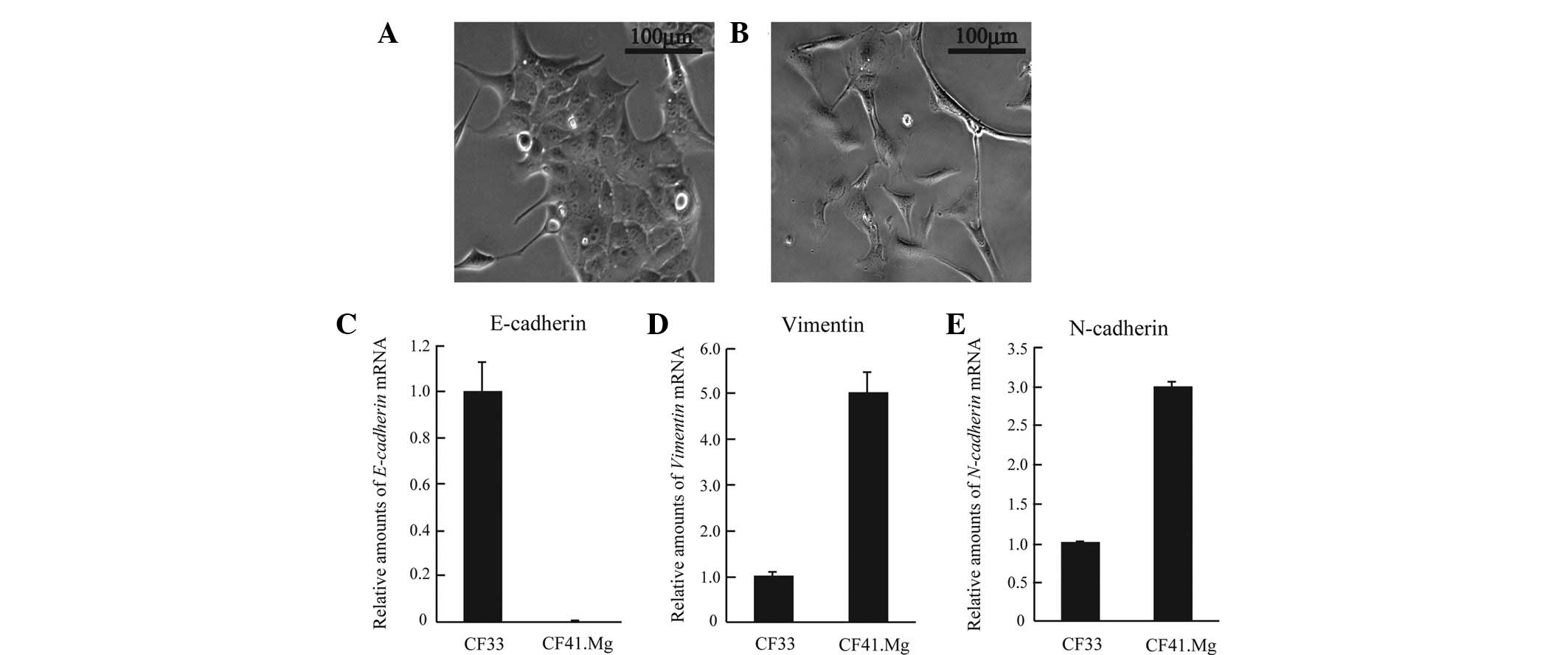
mesenchymal cells. First, cell morphology was examined by
microscopy. CF41.Mg displayed highly elongated mesenchymal
morphology, whereas canine mammary tumor CF33 cells showed
epithelial morphology and formed cell-cell attachments (Fig. 1A and B). Next, molecular markers of
cell origin such as E-cadherin, vimentin and N-cadherin were
investigated. CF41.Mg cells expressed markedly lower levels of
E-cadherin (an epithelial marker) than did CF33 (Fig. 1C). Furthermore, CF41.Mg exhibited
higher levels of vimentin and N-cadherin (mesenchymal markers;
Fig. 1D and E). Thus, CF41.Mg cells
have a mesenchymal-like phenotype in canine mammary tumor cell
lines. To evaluate the antitumor activity of 4-MU (via cell
proliferation, apoptosis and motility), we used CF41.Mg canine
mammary tumor cells as a model of the morphology and
characteristics of mesenchymal-like cells.
4-MU inhibits HA synthesis by
downregulating HAS2 mRNA expression
In mammalian cells, HA is produced at the plasma
membrane by three HASs (HAS1-3). Recently, Kultti
et al reported that 4-MU inhibits HA synthesis by
transcriptional repression of HAS2, HAS3 or both in
human breast cancer cell lines (11). To determine the effect of 4-MU on HA
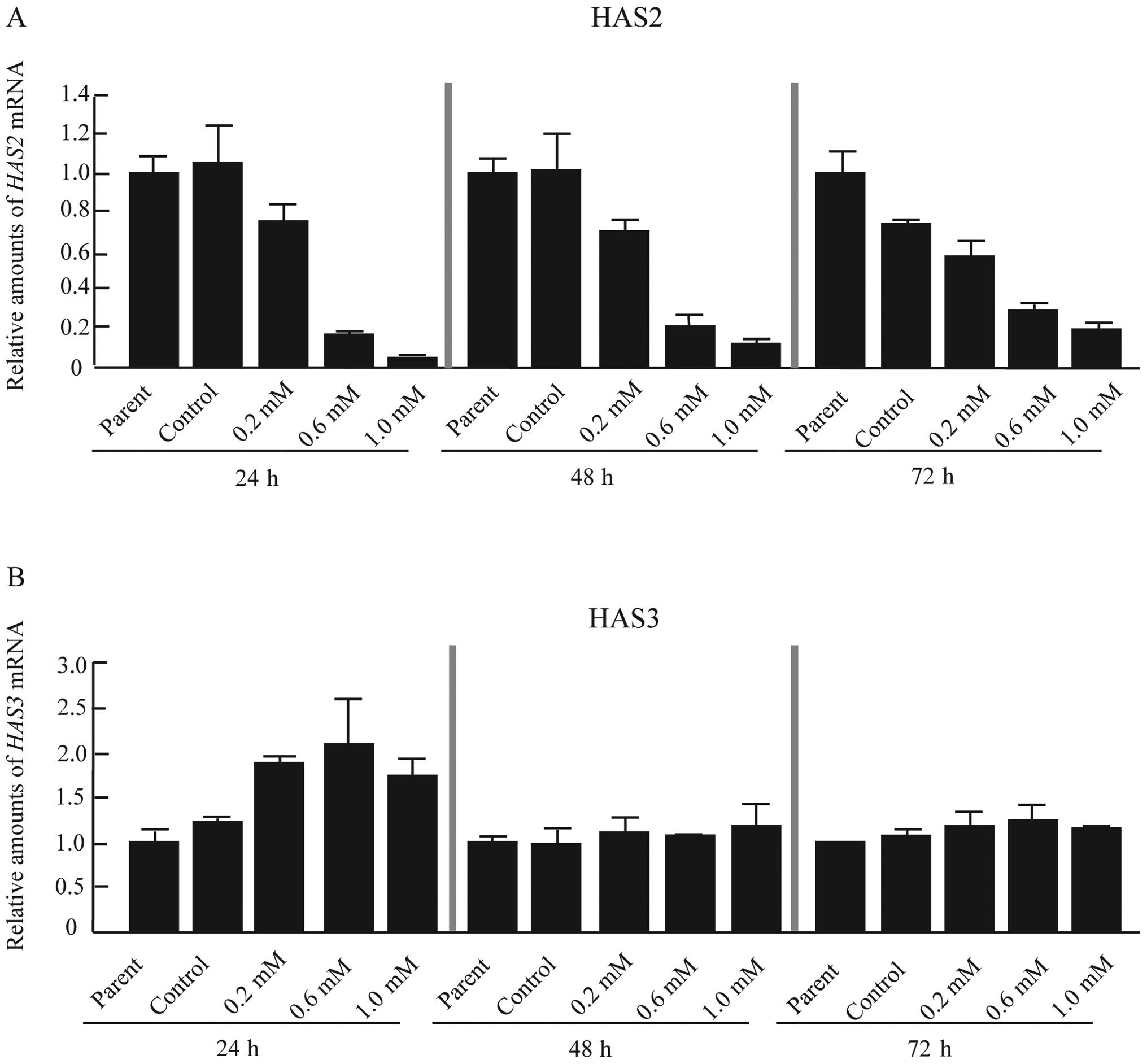
synthesis in CF41.Mg cells, the expression of HAS1-3 mRNA
was analyzed. HAS1 mRNA was undetectable by real-time RT-PCR
(data not shown). The data therefore indicated that CF41.Mg cells
principally synthesized HA by HAS2 and HAS3 (Fig. 2A and B). CF41.Mg cells treated with
4-MU showed a dose-dependent reduction in HAS2 mRNA
expression (Fig. 2A). In contrast,
HAS3 mRNA was induced 24 h after treatment with 4-MU
(Fig. 2B); this effect disappeared
by 48 and 72 h (Fig. 2B).
Therefore, 4-MU inhibited HA synthesis through repression of
HAS2 mRNA in CF41.Mg cells.
4-MU markedly inhibited growth arrest and
apoptosis of CF41.Mg cells
In human breast cancer cells, the rate of cell
proliferation often correlates with HA synthesis and HAS2
expression (8). Furthermore, 4-MU
inhibits cell proliferation in various cancer cells (11,12).
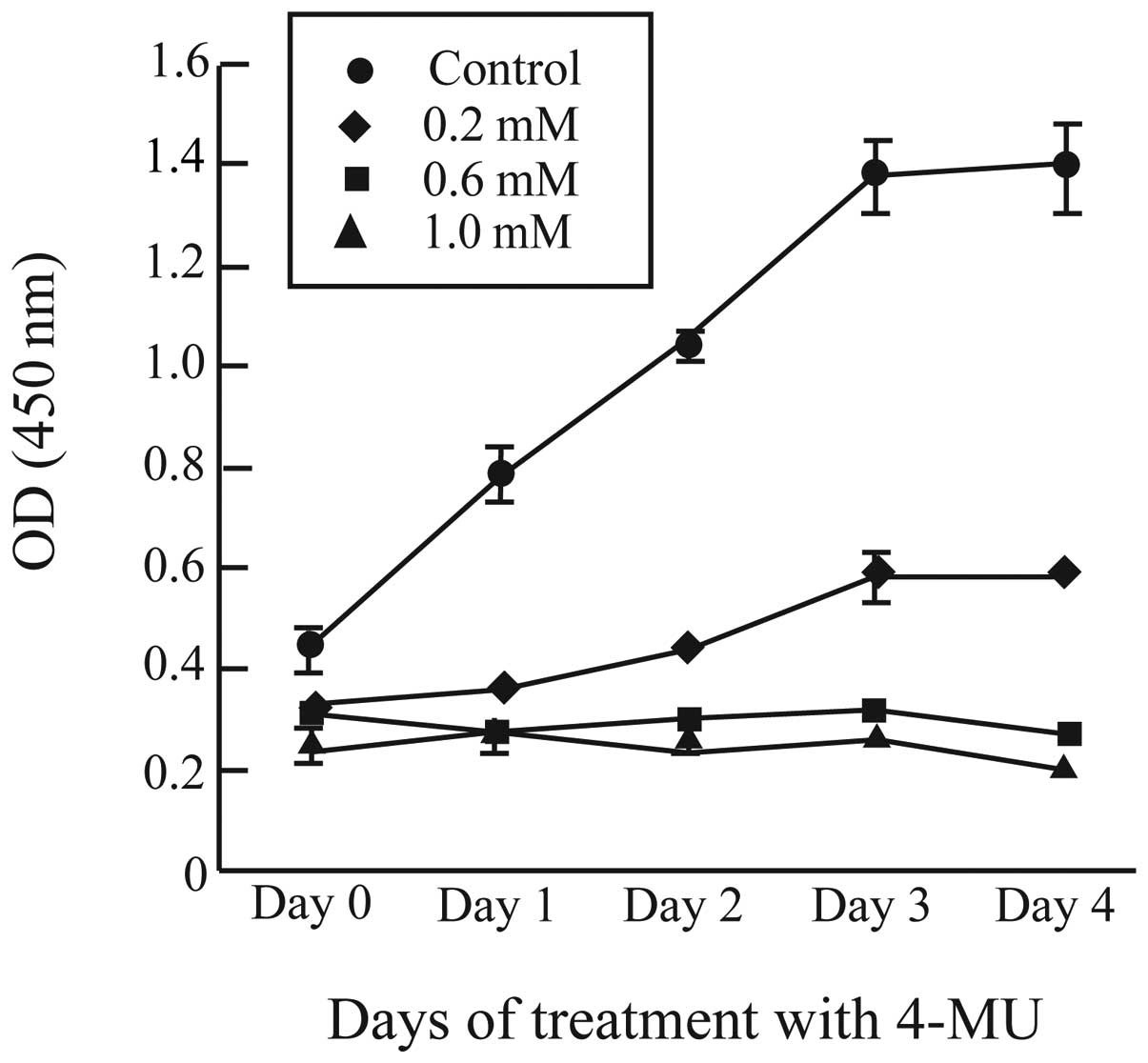
To analyze the effect of 4-MU on cell proliferation in CF41.Mg
cells, we used a quantitative WST-8 assay upon addition of 4-MU and
at 0, 1, 2, 3 and 4 days (Fig. 3).
The number of cells in control cultures increased steadily during
the days after plating, while proliferation was markedly suppressed
by 0.2, 0.6 and 1.0 mM 4-MU (Fig.
3). Proliferation of CF41.Mg cells was completely blocked by
0.6 and 1.0 mM 4-MU (Fig. 3).
Recently, Lokeshwar et al reported that human prostate
cancer PC3-ML cells exhibited a change in cell morphology within 2
days after 4-MU treatment (12).
However, CF41.Mg cells showed no changes even 4 days after
treatment with 0.2, 0.6 and 1.0 mM 4-MU (data not shown). Thus,
4-MU inhibited growth of CF41.Mg cells, as it does for several
human cancer cells. 4-MU markedly inhibited proliferation of
CF41.Mg cells in the experiments; to determine the effect of 4-MU
on cell cycle distribution and apoptosis, we used flow cytometry in
cultures treated with 4-MU for 24, 48 and 72 h. Within 48 h of
treatment with each concentration of 4-MU, CF41.Mg showed no marked
changes in cell cycle distribution and apoptosis (data not shown).
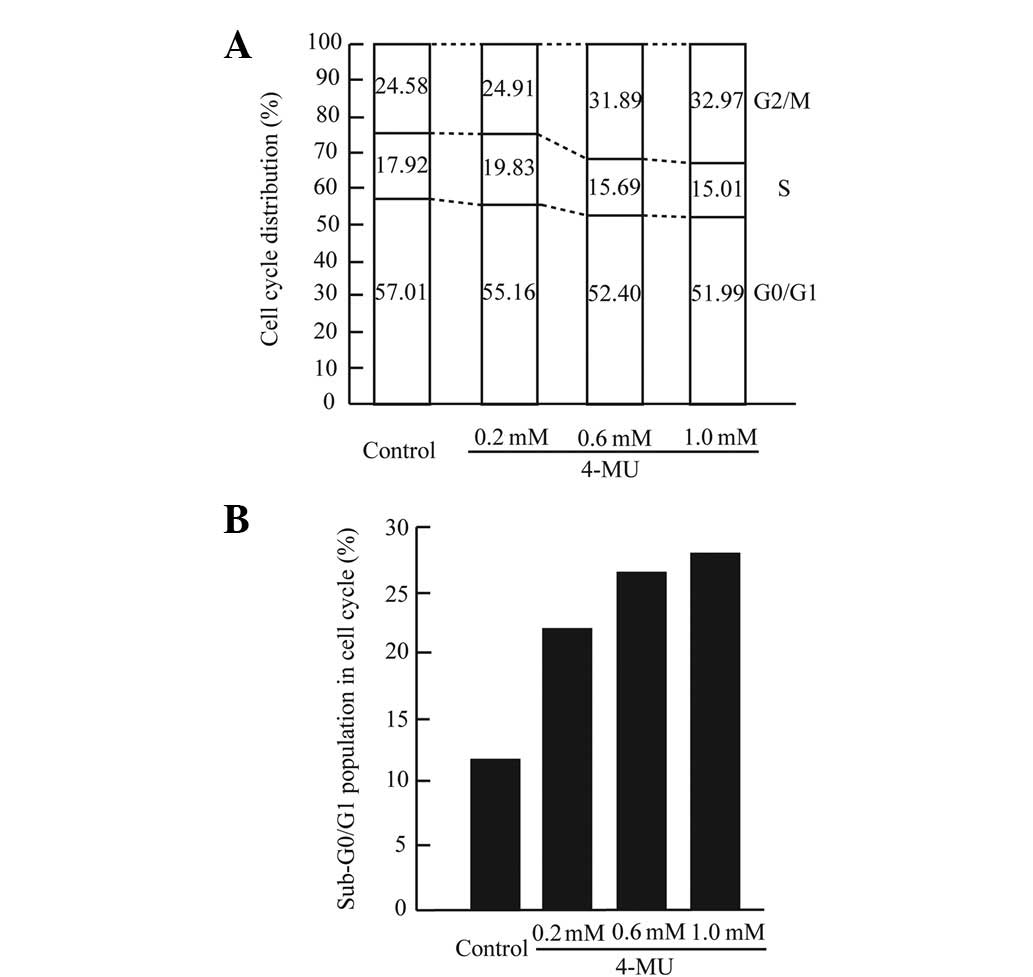
After 72 h exposure to 4-MU (0.6 and 1.0 mM), cell numbers in G2/M
phase were slightly increased, and the number of S-phase cells
decreased in a dose-dependent manner (Fig. 4A). After G2/M arrest, many cancer
cell lines, notably certain breast cancer cell lines, exhibit
morphological changes consistent with apoptosis (28). To determine the effect of 4-MU on
apoptosis in CF41.Mg cells, the percentage of apoptotic cells in
our specimens was quantified with PI staining and flow cytometry,
with the sub-G0/G1 peak representing apoptotic cells. Cells treated
with 4-MU (0.2, 0.6 and 1.0 mM) showed percentages of apoptotic
cells that were approximately 2 times higher than control cells
(Fig. 4B). To clarify the effect of
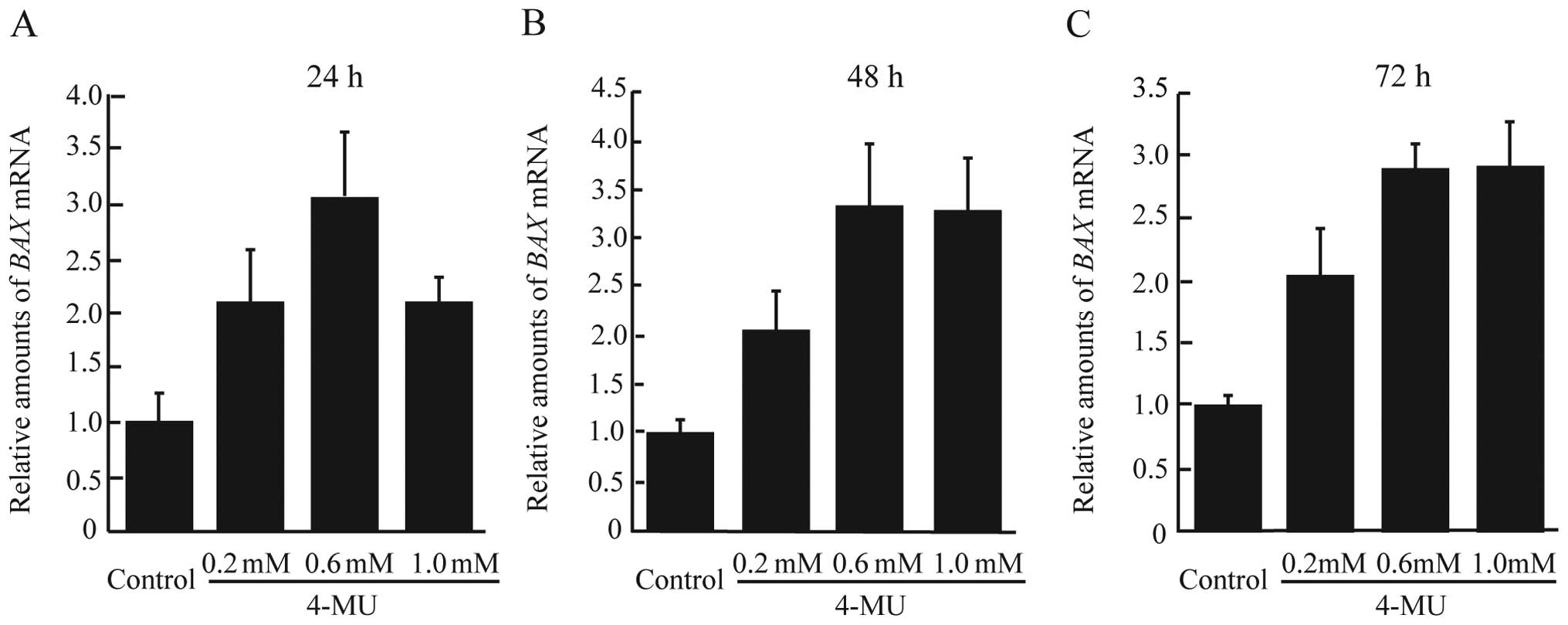
4-MU on apoptosis-related genes, the expression of BAX mRNA
was measured using real-time RT-PCR. As shown in Fig. 5A–C, 4-MU-treated cells demonstrated
higher levels of BAX mRNA expression after 24–72 h.
Therefore, 4-MU inhibited cell proliferation mainly through the
induction of apoptosis. It is possible that the 4-MU-treated cells
showed no change in cell cycle distribution at 24 and 48 h due to
the lapse in time between mRNA expression and protein
synthesis.
4-MU reduces chemokinesis and chemotaxis
of CF41.Mg cells
It is well known that increased cell motility is
essential for cancer cell metastasis. Cell motility can be divided
into two types, namely random cell motility (chemokinesis) and
directional cell motility (chemotaxis). Chemokinesis and chemotaxis
play an important role in cancer invasion and metastasis (17,25,29).
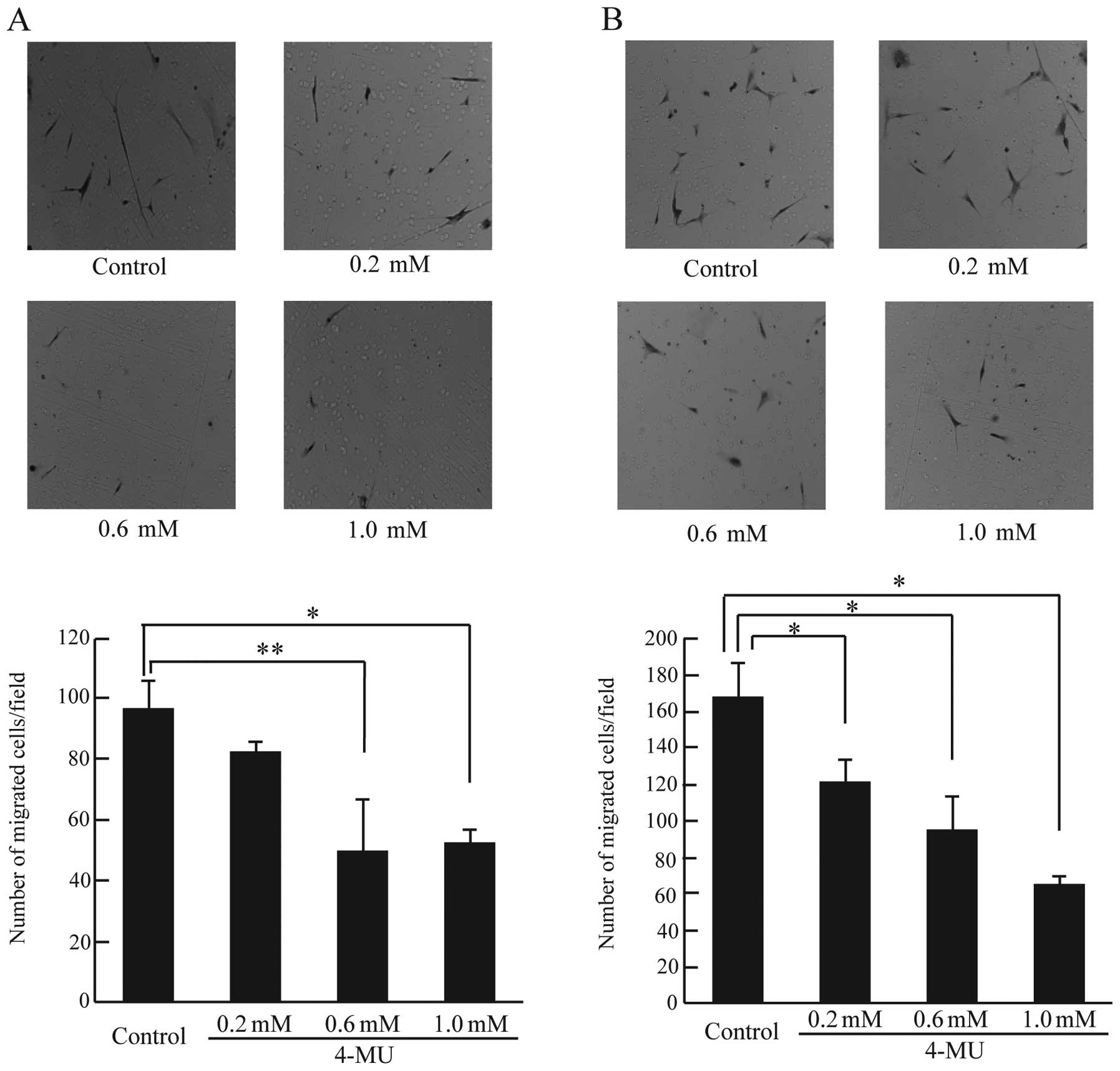
To investigate the effect of 4-MU on chemokinesis and chemotaxis in
CF41.Mg cells, a Boyden chamber assay was used. As shown in
Fig. 6A, chemokinesis in cells
treated with 0.6 and 1.0 mM 4-MU was significantly reduced compared
to control cells (Fig. 6A).
Furthermore, cells treated with 4-MU at all concentrations showed
markedly reduced chemotaxis (Fig.
6B). 4-MU reduced cell motility (chemokinesis and chemotaxis)
in CF41.Mg cells; it is possible that 4-MU could prevent the
invasion and metastasis of canine mammary tumor cells.
Discussion
Previous studies have reported that 4-MU acts as a
tumor suppressor against various cancers (11–15).
However, it is not clear whether 4-MU shows anticancer effects
against mesenchymal-like cells derived from canine mammary tumors.
Our results revealed that 4-MU inhibited HA synthesis via reduction
of HAS2 mRNA levels, as well as conspicuous growth
inhibition, apoptosis associated with BAX mRNA, and
reduction of chemokinesis and chemotaxis. Thus, 4-MU is an
anticancer agent that inhibits cell growth and cell motility of
mesenchymal-like canine mammary tumor cells.
HA is one of the major components of ECM and is
essential for embryonic development and wound healing in normal
tissue. HA also plays an important role in cancer cell
proliferation, angiogenesis, invasion and metastasis. Increased
levels of HA in the stroma or serum are associated with malignancy
in human patients with breast and ovarian carcinomas, prostate
cancer and non-small cell lung adenocarcinomas (2,4,30–32).
Because increased levels of HA are observed in many cancer types,
we conclude that an imbalance of HA synthesis and/or degradation
may contribute to tumor progression. A previous study described
siRNA-mediated knockdown of HAS2 and the resulting reduction
of cell growth and cellular migratory and invasive potentials in
human breast cancer cells (8). Our
data also showed that HAS2 downregulation by 4-MU inhibited
cell proliferation and motility in CF41.Mg cells. These data
support the notion that repression of HA accumulation or
overproduction is a useful target for therapy against breast cancer
in humans and animals.
CD44, a major receptor for HA, is a transmembrane
glycoprotein involved in cell-cell and cell-matrix interactions.
HA-CD44 interactions activate cellular signaling pathways such as
promotion of proliferation, survival, angiogenesis, migration and
invasion of cancer cells (33,34).
CD44 has also been identified as a marker of cancer stem cells in
breast, head and neck, and colon cancer (35). CD44 has been implicated in human
breast cancer tumor progression, although little is known about the
pathological role of CD44 in canine mammary tumors. CD44 is
preferentially expressed in benign canine mammary tumors and normal
mammary tissue versus simple carcinomas and metastatic cells
(36). However, our findings
suggested that overproduction of HA induced proliferation,
anti-apoptosis and cell motility. Therefore, our results support
the notion that HA promotes tumor progression mediated by other
receptors such as the receptor for HA-mediated motility (RHAMM),
lymphatic vessel endothelial receptors (LYVE-1), and toll-like
receptors 2 (TLR2) and 4 (TLR4) in canine mammary tumor.
EMT plays a key role during embryonic development,
wound healing, tissue regeneration, organ fibrosis, and cancer
metastasis (16). EMT is
characterized by loss of cell-cell adhesion and cell polarity and
increased migration of cancer cells. Other reports have
demonstrated the correlation between drug resistance and EMT; it is
now important to evaluate the effects of anticancer agents against
mesenchymal-like cancer cells. The findings showed that 4-MU
treatment of CF41.Mg cells, with the mesenchymal-like properties of
canine mammary tumors, yielded marked growth retardation and
apoptosis. Furthermore, 4-MU inhibited chemokinesis and chemotaxis
of CF41.Mg cells. Chemokinesis plays a particularly important role
in separation from the primary tumor mass, and the correlation
between chemokinesis and EMT has been suggested (17). In addition, chemotaxis is associated
with vascular invasion of cancer cells. The results suggest the
possibility that 4-MU suppressed invasion and metastasis of canine
mammary tumor cells.
It is well known that canine mammary tumors are more
histologically complex than mammary tumors in humans. In addition,
mesenchymal components such as myoepithelial cells are observed in
many types of canine mammary tumors. Thus, it is often difficult to
distinguish benign and malignant mammary tumors by cytological
analyses in dogs (24). Our
findings revealed that 4-MU effectively inhibited the growth and
motility of CF41.Mg cells. A previous report also showed the
anticancer effect of 4-MU on CF33 cells with epithelial properties.
Therefore, the data suggest that 4-MU may be useful for
wide-spectrum therapy of canine mammary tumors.
In summary, 4-MU blocked cell proliferation and cell
migration mediated by downregulated HAS2 mRNA expression in
CF41.Mg cells. This study shows that 4-MU may be a potential agent
for improved chemotherapy against breast cancers in dogs.
Acknowledgements
The authors thank H. Shibuya for
critical discussions. This study was supported in part by a
grant-in-aid from the Life Science Research Center Nihon University
(To T.S.), a grant-in-aid from Nihon University (To T.S.), and
funds from the Laboratory of Veterinary Pharmacology, Nihon
University College of Bioresource Science.
References
|
1
|
Weismann B, Rapport MM, Linker A and Meyer
K: Isolation of the aldobionic acid of umbilical cord hyaluronic
acid. J Biol Chem. 205:205–221. 1953.PubMed/NCBI
|
|
2
|
Toole BP: Hyaluronan: from extracellular
glue to pericellular cue. Nat Rev Cancer. 4:528–539. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knudson CB and Knudson W:
Hyaluronan-binding proteins in development, tissue homeostasis, and
disease. FASEB J. 7:1233–1241. 1993.PubMed/NCBI
|
|
4
|
Auvinen P, Tammi R, Parkkinen J, et al:
Hyaluronan in peritumoral stroma and malignant cells associates
with breast cancer spreading and predicts survival. Am J Pathol.
156:529–536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Itano N and Kimata K: Altered hyaluronan
biosynthesis in cancer progression. Semin Cancer Biol. 18:268–274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Udabage L, Brownlee GR, Waltham M, et al:
Antisense-mediated suppression of hyaluronan synthase 2 inhibits
the tumorigenesis and progression of breast cancer. Cancer Res.
65:6139–6150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Udabage L, Brownlee GR, Nilsson SK and
Brown TJ: The over-expression of HAS2, Hyal-2 and CD44 is
implicated in the invasiveness of breast cancer. Exp Cell Res.
310:205–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Li L, Brown TJ and Heldin P:
Silencing of hyaluronan synthase 2 suppresses the malignant
phenotype of invasive breast cancer cells. Int J Cancer.
120:2557–2567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura T, Funahashi M, Takagaki K,
Munakata H, Tanaka K, Saito Y and Endo M: Effect of
4-methylumbelliferone on cell-free synthesis of hyaluronic acid.
Biochem Mol Biol Int. 43:263–268. 1997.PubMed/NCBI
|
|
10
|
Kakizaki I, Kojima K, Takagaki K, et al: A
novel mechanism for the inhibition of hyaluronan biosynthesis by
4-Methylumbelliferone. J Biol Chem. 279:33281–33289. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kultti A, Pasonen-Seppänen S, Jauhiainen
M, et al: 4-Methylumbelliferone inhibits hyaluronan synthesis by
depletion of cellular UDP-glucuronic acid and downregulation of
hyaluronan synthase 2 and 3. Exp Cell Res. 315:1914–1923. 2009.
View Article : Google Scholar
|
|
12
|
Lokeshwar VB, Lopez LE, Munoz D, et al:
Antitumor activity of hyaluronic acid synthesis inhibitor
4-methylumbelliferone in prostate cancer cells. Cancer Res.
70:2613–2623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshihara S, Kon A, Kudo D, et al: A
hyaluronan synthase suppressor, 4-methylumbelliferone, inhibits
liver metastasis of melanoma cells. FEBS Lett. 579:2722–2726. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arai E, Nishida Y, Wasa J, et al:
Inhibition of hyaluronan retention by 4-methylumbelliferone
suppresses osteosarcoma cells in vitro and lung metastasis in vivo.
Brit J Cancer. 105:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Urakawa H, Nishida Y, Wasa J, et al:
Inhibition of hyaluronan synthesis in breast cancer cells by
4-methylumbelliferone suppresses tumorigenicity in vitro and
metastatic lesions of bone in vivo. Int J Cancer. 130:454–466.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liotta LA, Mandler R, Murano G, et al:
Tumor cell autocrine motility factor. Proc Natl Acad Sci USA.
83:3302–3306. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shintani Y, Maeda M, Chaika N, Johnson KR
and Wheelock MJ: Collagen I promotes epithelial-to-mesenchymal
transition in lung cancer cells via transforming growth factor-beta
signaling. Am J Respir Cell Mol Biol. 38:95–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zoltan-Jones A, Huang L, Ghatak S and
Toole BP: Elevated hyaluronan production induces mesenchymal and
transformed properties in epithelial cells. J Biol Chem.
278:45801–45810. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metast Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Benjamin SA, Lee AC and Saunders WJ:
Classification and behavior of canine mammary epithelial neoplasms
based on life-span observations in beagles. Vet Pathol. 36:423–436.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klopfleisch R, von Euler H, Sarli G, Pinho
SS, Gärtner F and Gruber AD: Molecular carcinogenesis of canine
mammary tumors: News from an old disease. Vet Pathol. 48:98–116.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uva P, Aurisicchio L, Watters J, et al:
Comparative expression pathways analysis of human and canine
mammary tumors. BMC Genomics. 10:1352009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meuten DJ: Tumors in Domestic Animals.
Meuten DJ: Iowa State Press; pp. 575–606. 2002
|
|
25
|
Saito T, Kawana H, Azuma K, Toyoda A,
Kitagawa M and Harigaya K: Fragmented hyaluronan is an autocrine
chemo-kinetic motility factor supported by the HAS2-HYAL2/CD44
system on the plasma membrane. Int J Oncol. 39:1311–1320.
2011.PubMed/NCBI
|
|
26
|
Toyoda A, Yokota A, Saito T, et al:
Overexpression of human ortholog of mammalian enabled (hMena) is
associated with the expression of mutant p53 protein in human
breast cancers. Int J Oncol. 38:89–96. 2011.PubMed/NCBI
|
|
27
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia W, Spector S, Hardly L, Zhao S, Saluk
A, Alemane L and Spector NL: Tumor selective G2/M cell cycle arrest
and apoptosis of epithelial and hematological malignancies by
BBL22, a benzazepine. Proc Natl Acad Sci USA. 97:7494–7499. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu M, Pollock RE and Nicolson GL:
Purification and characterization of human lung fibroblast
motility-stimulating factor for human soft tissue sarcoma cells:
identification as an NH2-terminal fragments of human fibronectin.
Cancer Res. 57:3577–3584. 1997.
|
|
30
|
Anttila MA, Tammi RH, Tammi MI, Syrjänen
KJ, Saarikoski SV and Kosma VM: High levels of stromal hyaluronan
predict poor disease outcome in epithelial ovarian cancer. Cancer
Res. 60:150–155. 2000.PubMed/NCBI
|
|
31
|
Lipponen P, Aaltomaa S, Tammi R, Tammi M,
Agren U and Kosma VM: High stromal hyaluronan level is associated
with poor differentiation and metastasis in prostate cancer. Eur J
Cancer. 37:849–856. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Posey JT, Soloway MS, Ekici S, Sofer M,
Civantos F, Duncan RC and Lokeshwar VB: Evaluation of prognostic
potential of hyaluronic acid and hyaluronidase (HYAL1) for prostate
cancer. Cancer Res. 63:2638–2644. 2003.PubMed/NCBI
|
|
33
|
Bourguignon LY, Zhu H, Chu A, Iida N,
Zhang L and Hung MC: Interaction between the adhesion receptor,
CD44, and the oncogene product, p185HER2, promotes human ovarian
tumor cell activation. J Biol Chem. 272:27913–27918. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ouhit A, Abd Elmageed ZY, Abdraboh ME,
Lioe TF and Raj MH: In vivo evidence for the role of CD44s in
promoting breast cancer metastasis to the liver. Am J Pathol.
171:2033–2039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paltian V, Alldinger S, Baumgärtner W and
Wohlsein P: Expression of CD44 in canine mammary tumours. J Comp
Path. 141:237–247. 2009. View Article : Google Scholar : PubMed/NCBI
|