Introduction
Lung cancer represents 28% of all cancer
mortalities, second only to breast cancer. Each year, ∼180,000 new
cases of lung cancer are diagnosed in the United States (1,2).
Patients with stage IV non-small cell lung cancer
(NSCLC) have among the lowest survival rates of patients with any
type of cancer, but the overall survival time in this group is very
heterogeneous. The median overall survival time ranges from eight
to ten months, but 25–30% of patients succumb within six months,
and ∼20% survive more than 18 months after the spread of the
neoplasm (3). Therefore, accurate
prognostic factors are required to improve decision-making
regarding standard treatment options, to stratify patients for
inclusion in innovative therapeutic trials and to identify patients
that would be better served by palliative care than by systemic
chemotherapy. Several prognostic factors appear to influence
survival time, some more concretely than others (3,4).
Malnutrition is a common co-morbidity in patients
with advanced NSCLC and is a major contributor to morbidity and
mortality. Several studies have reported a decrease in overall
survival, of from 30–50%, in malnourished patients with advanced
lung cancer (4). A less common tool
to assess nutritional status is mid-arm muscle circumference
(MAMC), which provides an estimate of somatic protein reserve. This
measurement is an early indicator of nutritional depletion, and it
is simple, non-invasive, objective and inexpensive (5).
Decisions regarding choice of treatment modality and
intensity are made more complex due to the heterogeneous survival
in this population. Prognostic assessment may allow a better
individualization of treatments for each patient. Thus, we
investigated whether MAMC may be a useful prognostic factor in
patients with stage IV NSCLC.
Patients and methods
Study design
Fifty-nine consecutive patients were identified as
potential participants in this prospective study. Of these
patients, only three declined to participate. The study was
approved by the local ethics committee of Santa Casa de
Misericórdia Hospital of Porto Alegre, Brazil. The inclusion
criteria were: a) metastatic NSCLC confirmed by imaging, with more
than one lesion identified; b) a pathological diagnosis of NSCLC
made no more than one month before the signing of the informed
consent form, and no prior specific anti-cancer treatment; c)
patients must have signed the informed consent form. We performed
nutritional and Karnofsky performance status (KPS) analysis
immediately after the patients provided informed consent. The
variables analyzed were MAMC, KPS, gender and age. Survival status
was determined by telephone call. Data regarding chemotherapy
treatment was retrieved by subsequent analysis of patient
records.
Nutritional and performance
assessment
To evaluate MAMC, the mid-arm circumference (MAC) of
the right arm was measured to the nearest centimeter with a
measuring tape. Then, triceps skinfold thickness (TST), an
established measure of fat stores, was measured to the nearest
millimeter in the right arm using a skinfold caliper (Cescorf,
Porto Alegre, Brazil) in a standard manner. Three measurements were
taken for both TST and MAC, and the average values were calculated
and recorded. MAMC, an established measure of muscle protein mass,
was calculated from MAC and TST using a standard formula: MAMC =
MAC - (3.1415 × TSF). The MAMC results were expressed as a
percentage of the expected reference values, adjusted for gender
and age. Patients were categorized as normal (MAMC ≥90%) or
depleted (MAMC <90%) (6). KPS
was used to classify the patients’ functional impairment.
Statistical analysis
The statistical analysis was performed using SPSS
software (version 17.0). Continuous variables were expressed as the
means and standard deviation. Survival analyses were performed by
Kaplan-Meier and log-rank tests. For inferential purposes, the MAMC
was dichotomized using a threshold of 90%. Multifactorial analysis
was performed using the Cox proportional hazards estimation.
Results
A slight majority of the 56 NSCLC patients included
in this study were female (52%), and the mean age was 63 years
(range, 47–80).
Most patients had adenocarcinoma (76%), and the
remaining patients had squamous cell carcinoma. Further descriptive
characteristics of this sample are presented in Table I.
 | Table IBaseline characteristics. |
Table I
Baseline characteristics.
| Variables | Mean ± standard
deviation |
|---|
| Age (years) | 63±8 |
| MAMC (mm) | 22.3±2.8 |
| MAMC adequacy
(%) | 89±13 |
| KPS (%) | 56±14 |
| Overall survival
(days) | 187±17 |
| Overall survival
(months) | 6.23± 0.56 |
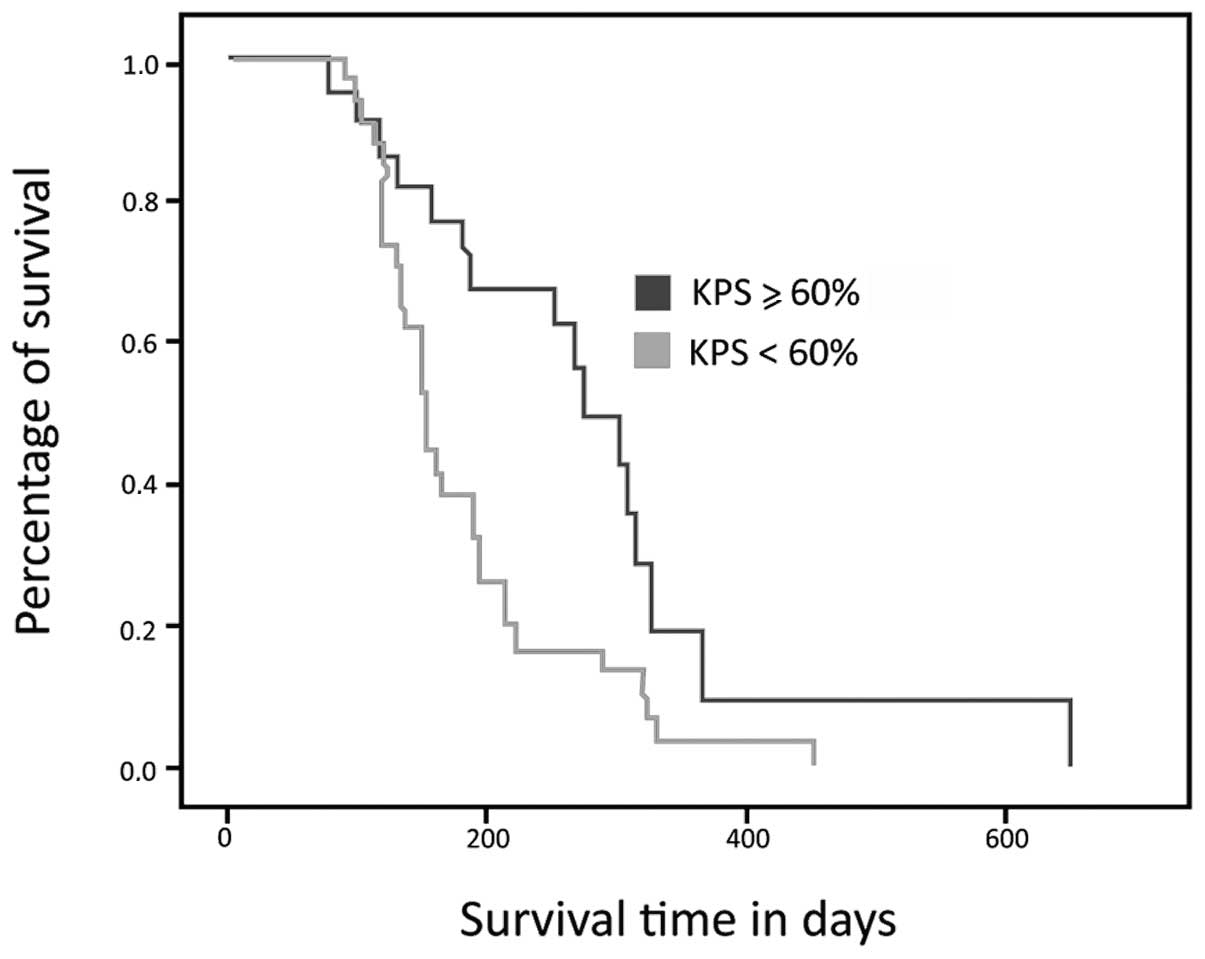
Survival curve analysis showed that the survival
time of the group of patients with a KPS <60% was lower than
that of the group with a KPS ≥60% (Fig.
1). The patients with a KPS <60% had a median survival of
187 days (95% CI, 153–221), while the other group had a median
survival of 273 days (95% CI, 215–331); this difference was
statistically significant (p= 0.006). The mean KPS in this sample
was 52.5%. No difference in KPS between genders was observed (p=
0.36).
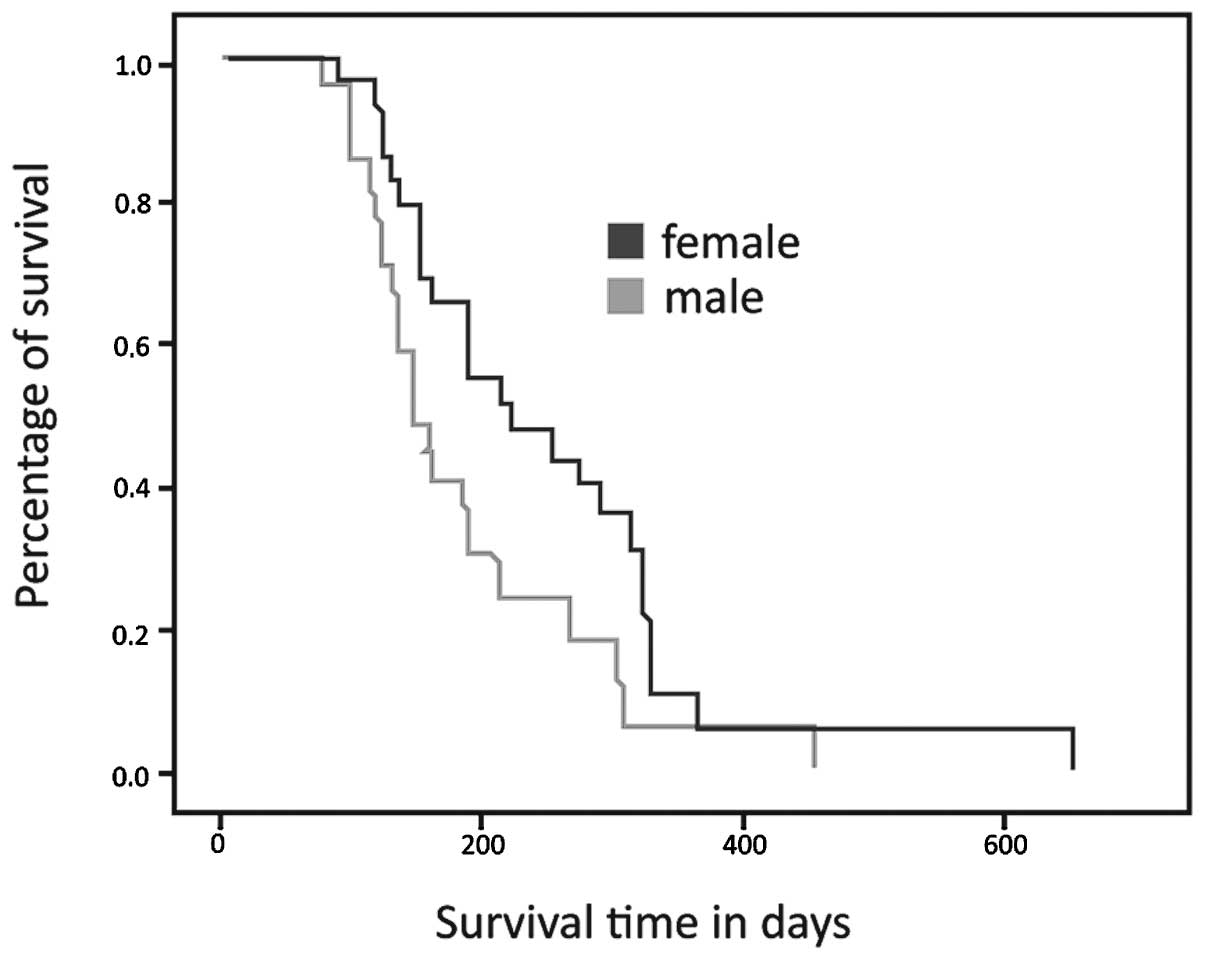
Fig. 2 shows the
survival curves according to gender. Female patients had a median
survival of 221 days (95% CI, 139–302), while the males had a
median survival of 149 days (95% CI, 153–220). This difference was
statistically significant (p= 0.029).
The majority (55%) of patients had subnormal MAMC
measurements. Malnourishment was more common in male patients (74%)
than in female (p=0.014).
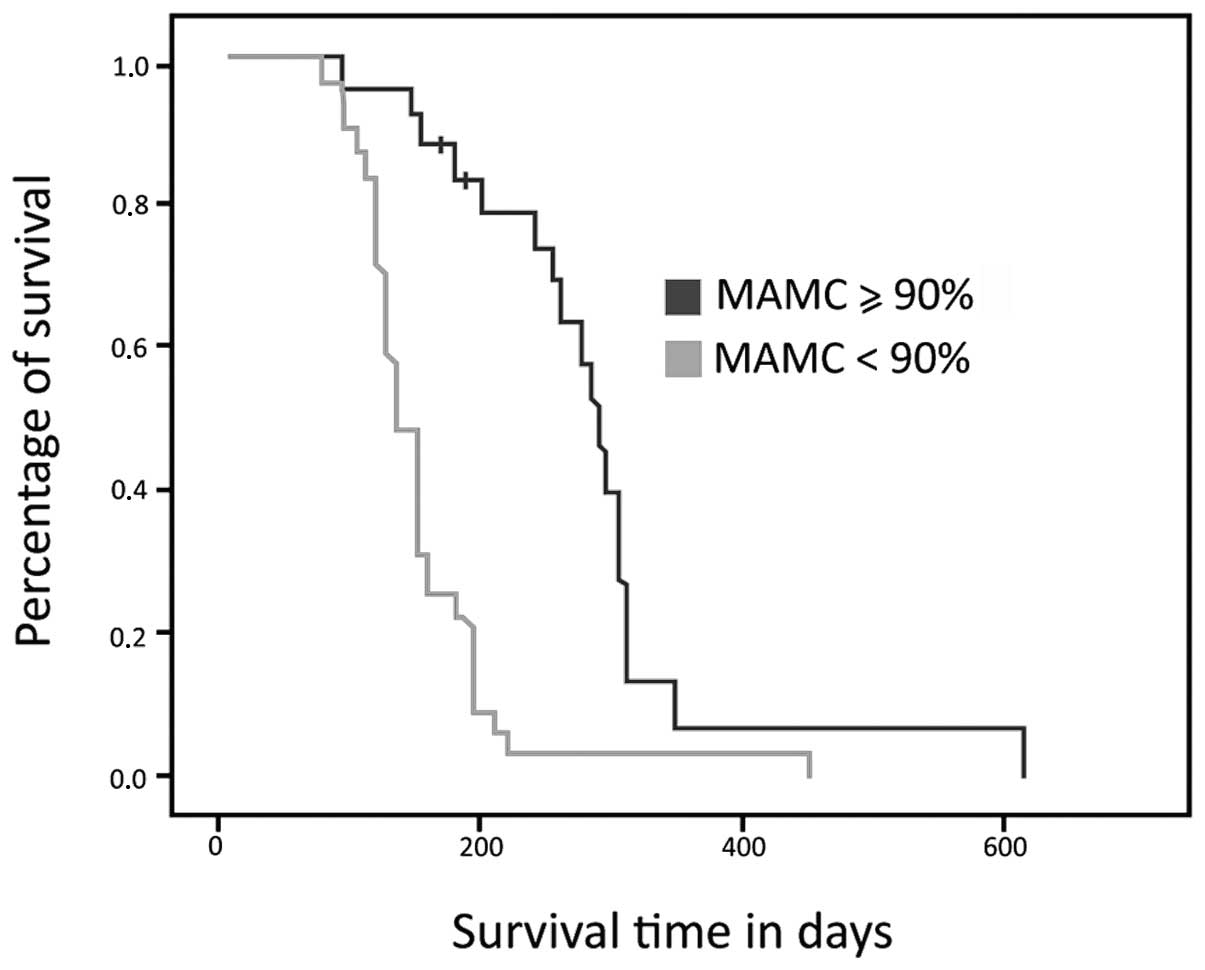
Fig. 3 demonstrates
that the patients identified as malnourished based on MAMC had
shorter overall survival times compared to the non-malnourished
group. The median survival time was 137 days in the malnourished
group, (95% CI, 119–155) and 306 days in the non-malnourished group
(95% CI, 278–333; p=0.001).
Table II shows the
results of multivariate Cox regression analysis. Multivariate Cox
modeling, after adjusting for gender, use of chemotherapy, KPS and
MAMC showed that only MAMC and the use of chemotherapy were
statistically significant (p<0.001; HR=0.2; 95% CI,
0.082–0.48).
 | Table IIMultivariate Cox proportional hazard
model. |
Table II
Multivariate Cox proportional hazard
model.
| P-value | HR | 95% CI |
|---|
| KPS | 0.800 | 1.102 | 0.519–2.341 |
| MAMC adequacy | <0.001 | 0.200 | 0.082–0.487 |
| Gender | 0.803 | 1.090 | 0.553–2.148 |
| Use of
chemotherapy | <0.001 | 16.309 | 5.19–51.23 |
Discussion
The incidence of lung cancer has increased since the
early twentieth century, when the disease was uncommon. The onset
of lung cancer typically occurs in patients aged 50–70, but due to
cigarette smoking, lung cancer may also be diagnosed in younger
people (7). The patients in our
study had an average age of 63, and the youngest patient was 47
years old. Several studies have shown an increase in the incidence
of younger patients with lung cancer (3,7).
We observed a greater prevalence of lung cancer
among females. The literature has shown a gradual increase in the
incidence of this disease in women; however, a greater proportion
of the patients with this malignancy are male. This increased
incidence in women may be attributed to smoking, which is becoming
increasingly common among women. Tobacco consumption was not
evaluated in this study. However, the increased incidence among
females is likely reflected by the predominance of adenocarcinoma
(76%), the histological type more prevalent in females (8–10).
The treatment of neoplastic disease requires
professional knowledge of several variables that may be involved in
the evolutionary history of tumors. Prediction of survival is,
perhaps, the most important piece of information to offer patients
after diagnosis. However, prognostic analysis in cancer is also
considered essential for defining protocols and treatment
strategies that are appropriate for the clinical condition of the
individual patient and for monitoring and evaluating the efficacy
of the treatment (11).
KPS has always been among the main determinants of
survival in patients with advanced lung cancer (7,11–15).
In the present study, KPS was shown to be a prognostic factor only
in univariate analysis, and it did not act as an independent
determinant of overall survival in our multivariate analyses. This
may be explained by the sample size, which did not allow the effect
of this variable to reach statistical significance.
The data with respect to differential survival in
men and women are controversial. Several papers have demonstrated
better overall survival in women, while others show no difference
(16–18). Certain authors consider female
gender to be a positive factor for survival due to the presence of
steroid receptors in patients with lung cancer (18).
Weight loss is common in cancer patients and is
often a symptom already present at diagnosis. A high prevalence of
weight loss is found in individuals with lung and gastrointestinal
tumors. Several studies have shown that weight loss is an
independent predictor of survival in patients with cancer, and it
has been associated with poorer physical function, increased
psychological distress and reduced quality of life (19–21).
Moreover, patients with advanced lung cancer are
distinct in that a marked loss of lean body mass does not typically
occur in patients with weight loss due to other causes. A
significant loss of lean body mass is one diagnostic factor for
anorexia cachexia syndrome (ACS), which occurs in ∼50–70% of
patients in this population (22).
High rates of total organic protein synthesis and
turnover and metabolic abnormalities in muscle protein catabolism
are commonly observed in patients with advanced cancer (23). One of the manifestations of these
phenomena is the atrophy of skeletal muscle and visceral organs and
hypoalbuminemia (24). Decreased
physical activity in cachectic patients contributes to the
suppression of protein synthesis, further favoring muscle
catabolism (25).
Studies have shown that protein depletion
predisposes patients to inadequate wound repair, increases their
susceptibility to infections and leads to weakness and reduced
functional capacity. Biochemically, a loss of body protein is
associated with increased serum levels of proteolysis-inducing
factor (PIF), which is able to induce degradation and inhibit
protein synthesis in skeletal muscle.
PIF is present in the urine of cachectic cancer
patients with marked weight loss but not in patients with little
weight loss. PIF is not observed in malnourished patients without
tumors, in whom the mechanisms that protect muscle tissue from
catabolism are still functional (23,26).
Furthermore, the function of the respiratory muscles may also be
specifically affected in lung cancer (as well as in other lung
diseases) due to the deficit in muscle mass, worsening the quality
of life for patients (26).
In the present study, we observed that the majority
(55%) of patients had abnormally low MAMC measurements. By
contrast, another study that evaluated the MAMC in patients with
advanced lung cancer demonstrated a lower prevalence of
malnutrition (∼20%). It is important to note that this study was
conducted in the Canary Islands, and each population has a specific
classification for MAMC (27).
A higher rate of protein malnutrition was observed
in males, whereas >60% of female patients were well-nourished.
This phenomenon has also been demonstrated in other studies, but
there is no clear explanation in the literature (10,28,29). A
hypothesis given in one of these studies is that females are
generally heavier for their height than males and therefore take
longer to reach the levels of malnutrition and depletion of lean
body mass (30). However, weight
loss was not assessed in our study.
We also observed a between-gender difference in the
MAMC, but not in KPS. This suggests that the longer survival in
women may be explained by the MAMC and not by KPS, as women had a
smaller muscle deficit.
The studies in the literature that objectively
evaluate nutritional status as a prognostic factor used measures of
nutritional status other than MAMC (21,22,27–29).
However, one of these studies evaluated the association of overall
survival with nutritional status as assessed by the TST and MAC
(31). In this particular study,
the author noted that patients with lung cancer who succumbed
within six months of diagnosis had lower nutritional parameter
values compared to those who survived for more than six months
after diagnosis. However, the univariate analysis in that study
showed an association between nutritional status and poor
prognosis, whereas multivariate analysis did not confirm the
prognostic utility of these parameters. Furthermore, the authors
did not stratify the group by disease stage.
In the current study, there was a difference of
almost six months in the survival time between the eutrophic and
depleted population. MAMC may thus be considered a strong
prognostic factor. Moreover, MAMC measurement is non-invasive,
simple, painless, inexpensive and requires little to no preparation
or patient discomfort. In addition, MAMC remained a statistically
significant prognostic factor when analyzing overall survival in a
multivariate model, even after adjusting for gender, use of
chemotherapy, KPS and MAMC.
In conclusion, when evaluating various clinical
characteristics of patients with advanced NSCLC, we observed that
MAMC may be a valuable auxiliary tool for prognosis as an indicator
of proteolysis and cachexia.
Due to the heterogeneous survival of patients with
advanced NSCLC, methods to predict survival are particularly
important to allow proper selection of treatment. No diagnostic or
therapeutic method for predicting outcomes in this population is
yet considered the gold standard, but may be related to multiple
patient characteristics such as age, clinical performance and
gender, among others.
The importance of prognosis extends beyond answering
the question of survival time. Accurate prognosis is likely to
provide better matching of anticancer therapies, generating
improved quality of life and even promoting the more appropriate
selection of patients for research in developing new treatments.
Further development of this framework should provide more relevant
and appropriate clinical management. The identification of the
patient profile is undoubtedly the optimum strategy with which to
address the challenge of the individualization of treatment.
References
|
1
|
World Health Organization: International
Agency for Research on Cancer. World Cancer Report 2008 Lyon:
2008
|
|
2
|
National Cancer Institute (Brazil):
Estimative 2011: Cancer Incidence in Brazil. Rio de Janeiro:
2011
|
|
3
|
National Comprehensive Cancer Network:
Non-small cell lung cancer. National Comprehensive Cancer Network:
Clinical practice guidelines in Oncology. 2008, 1:http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf.
Accessed September 25, 2009.
|
|
4
|
Chlebowski RT, Palomares MD, Lillington L
and Grosvenor M: Recent implications of weight loss in lung cancer
management. Nutrition. 12:S43–S47. 2006.
|
|
5
|
Wannamethee SG, Shaper AG, Lennon L and
Whincup PH: Decreased muscle mass and increased central adiposity
are independently related to mortality in older men. Am J Clin
Nutr. 86:1339–1346. 2007.PubMed/NCBI
|
|
6
|
National Center for Health Statistics
Analytic and Reporting Guidelines: The Third National Health and
Nutrition Examination Survey, NHANES III. Hyattsville (MD):
1988–1994
|
|
7
|
Jamnik S, Santoro IL and Uehara C:
Comparative study of prognostic factors among longer and shorter
survival patients with bronchogenic carcinoma. J Pneumol.
28:245–250. 2002.
|
|
8
|
Alberg AJ and Samet JM: Epidemiology of
lung cancer. Chest. 23:S21–S49. 2003. View Article : Google Scholar
|
|
9
|
Blot WJ and McLaughlin JK: Are women more
susceptible to lung cancer? J Natl Cancer Inst. 96:812–813. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andrews S, Bloom K and Balogh JF: Lung
cancer in women. Lahey Clinic experience 1957–1980. Cancer.
55:2894–2898. 1985.PubMed/NCBI
|
|
11
|
Clark GM: Prognostic and predictive
factors. Diseases of breast. Harris JR, Lippman ME, Morrow M and
Hellman S: 1st edition. Lippincott-Raven; Philadelphia: pp.
461–470. 1996
|
|
12
|
Spiegelman D, Mauser LH, Varw JH, Perry MC
and Chahinian AP: Prognostic factor in small cell carcinoma of the
lung: an analysis of 1521 patients. J Clin Oncol. 7:344–354.
1989.PubMed/NCBI
|
|
13
|
Pater JL and Loeb M: Nonanatomic
prognostic factors in carcinoma of the lung. A multivariate
analysis Cancer. 50:326–331. 1982.PubMed/NCBI
|
|
14
|
Lai SL and Perng RP: Impact of nutritional
status on the survival of lung cancer patients. Zhonghua Yi Xue Za
Zhi (Taipei). 61:134–140. 1998.PubMed/NCBI
|
|
15
|
Tadokoro H: Lung cancer: consideration of
300 cases. (unpublished PhD thesis) University of Sao Paulo: School
of Medicine. 1992
|
|
16
|
Lam PT, Leung MW and Tse CY: Identifying
prognostic factors for survival in advanced cancer patients: a
prospective study. Hong Kong Med J. 6:453–459. 2007.PubMed/NCBI
|
|
17
|
Batevik R, Grong K, Segadal L and
Stangeland L: The female gender has a positive effect on survival
independent of background life expectancy following surgical
resection of primary non-small cell lung cancer: a study of
absolute and relative survival over 15 years. Lung Cancer.
47:173–181. 2005.PubMed/NCBI
|
|
18
|
Bain C, Feskanich D, Speizer FE, Thun M,
Hertzmark E, Rosner BA, et al: Lung cancer rates in men and women
with comparable histories of smoking. J Natl Cancer Inst.
96:826–834. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas P, Piraux M, Jacques LF and Grégore
J: Clinical patterns and trends of outcome of elderly patients with
bronchogenic carcinoma. Eur J Cardiothorac Surg. 13:266–274. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andreyev HJN, Norman AR, Oates J and
Cunningham D: Why do patients with weight loss have a worse outcome
when undergoing chemotherapy for gastrointestinal malignancies? Eur
J Cancer. 34:503–509. 1998. View Article : Google Scholar
|
|
21
|
Ovesen L, Hannibal J and Mortensen E: The
interrelationship of weight loss, dietary intake, and quality of
life in ambulatory patients with cancer of the lung, breast, and
ovary. Nutr Cancer. 19:159–167. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kyle UG, Pirlich M, Schuetz T, Lochs H and
Pichard C: Is nutritional depletion by nutritional risk index
associated with increased length of hospital stay? A
population-based study JPEN. 28:99–104. 2004.PubMed/NCBI
|
|
23
|
Inui A: Cancer anorexia-cachexia syndrome:
current issues in research and management. CA Cancer J Clin.
52:72–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inadera H, Nagai S, Dong HY and Matsushima
K: Molecular analysis of lipid-depleting factor in a
colon-26-inoculated cancer cachexia model. Int J Cancer. 101:37–45.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tisdale MJ: Cachexia in cancer patients.
Nat Rev Cancer. 2:862–871. 2002. View
Article : Google Scholar
|
|
26
|
Van Halteren HK, Bongaerts GPA and Wagener
DJTH: Cancer cachexia: what is known about its etiology and what
should be the current treatment approach? Anticancer Res.
23:511–516. 2003.PubMed/NCBI
|
|
27
|
Ischaki E, Papatheodoru G, Gaki E, Papa I,
Kolouris N and Loukides S: Body mass and fat-free mass indices in
COPD: relation with variables expressing disease severity. Chest.
132:164–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin L, Watanabe S, Fainsinger R, Lau F,
Ghosh S, Quan H, et al: Prognostic factors in patients with
advanced cancer: use of the patient-generated subjective global
assessment in survival prediction. J Clin Oncol. 28:4376–4383.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jamnik S, Santoro IL and Lopes I: Lung
cancer evolution in accordance with the weight loss. Folha Med.
119:23–26. 2000.
|
|
30
|
Uehara C, Santoro IL and Jamnik S: Lung
cancer: comparison between sexes. J Pneumol. 24:347–353. 2000.
|
|
31
|
Buccheri G and Ferrigno D: Importance of
weight loss definition in the prognostic evaluation of
non-small-cell lung cancer. Lung Cancer. 34:433–440. 2001.
View Article : Google Scholar : PubMed/NCBI
|