Introduction
Nasopharyngeal carcinoma (NPC) is a non-lymphomatous
squamous cell carcinoma that originates from the epithelial lining
of the nasopharynx (1,2). Pathologically, NPC presents in varying
degrees of differentiation and is frequently located on the
pharyngeal recess posteromedial to the medial crura of the
eustachian tube (3). NPC is not
common in most countries; its age-adjusted incidence for both
genders is <1 per 100,000 (4).
According to the International Agency for Research on Cancer, there
were ∼84,400 cases of NPC and 51,600 related mortalities in 2009
(4).
The histological types of NPC are defined as either
squamous cell carcinoma or non-keratinizing carcinoma. They are
further classified into either differentiated or undifferentiated
carcinoma (5). The three
well-established etiological factors for NPC include
genetically-determined susceptibility, early-age exposure to
chemical carcinogens (e.g., salted fish in southern China), and
Epstein-Barr virus (EBV) infection (6).
Irradiation is the primary therapeutic modality for
NPC due to its anatomical location and high radiosensitivity.
Treatment of early-stage disease with radiotherapy alone usually
results in successful control (7)
but the response of locoregionally-advanced NPC with radiotherapy
alone is unsatisfactory, with a 5-year overall survival (OS) rate
of 87–96% for stages I–II and 67–77% for stages III–IV (1). This is significant because according
to the 6th American Joint Commission on Cancer (AJCC) staging
system, 60–70% of patients present with stage III–IV disease at the
time of diagnosis (8).
Concurrent chemoradiotherapy with adjuvant
chemotherapy has been deemed the standard of care for advanced NPC
since 1998 (9). This was initally
due to the Intergroup 0099 study, which found a 31% increase in
3-year OS. Recently, seven randomized-control phase III clinical
trials (comparing chemo-radiation with radiotherapy alone)
confirmed the effects of additional chemotherapy on the survival of
patients with advanced NPC. Three of these trials compared
concurrent chemoradiotherapy with radiotherapy alone (10–13),
while the other four trials adopted the concurrent
chemoradiotherapy plus adjuvant chemotherapy regimen (8,9,14,15),
analogous to that of the Intergroup 0099 study. The latter four
trials were, however, unable to rule out the contribution of
adjuvant chemotherapy since radiotherapy alone was regarded as the
control. Furthermore, three phase III trials, in which adjuvant
chemotherapy was applied alone (16) did not show a positive effect on the
OS of patients with advanced NPC. It is, therefore, unclear whether
adjuvant chemotherapy contributes to an additional survival benefit
over concurrent chemoradiotherapy in advanced NPC.
Currently, radiotherapy is the standard treatment
for NPC. Unfortunately, it leads to the development of undesirable
complications, primarily due to the anatomical location of the
tumors at the base of the skull where they are in close proximity
to radiation dose-limiting organs, such as the brain stem and
spinal cord. With the advent of intensity-modulated radiotherapy
(IMRT), radiation beams can now be modulated so that a high dose
can be efficiently delivered to the tumor while limiting the dose
to the surrounding normal tissue (17). Except for the conformal distribution
of radiation, IMRT further exploits accelerated forms of
radiotherapy, such as simultaneous modulated accelerated radiation
therapy (SMART), in which different doses are simultaneously
delivered to different target lesions in an overall shorter
treatment time (18,19).
With the advantage of IMRT, the control of
locoregional NPC has been substantially improved and the
development of distant metastases is now the main cause of
treatment failure (20). Further
improvements in systemic control of advanced NPC using concurrent
chemotherapy are likely, despite the drug-related toxic effects.
Meanwhile, it is pivotal to address the issue of neo-adjuvant and
adjuvant chemotherapy. In the present study, a retrospective trial
was undertaken to determine the efficacy of concurrent
chemoradiotherapy plus adjuvant and neo-adjuvant chemotherapy to
appraise the contribution of chemotherapy and SMART-IMRT-based
radiotherapy in locoregionally-advanced NPC.
Materials and methods
Participants and pre-treatment
evaluation
From January 2005 to December 2007, 45 NPC patients
between stages IIA and IVa (AJCC, 7th edition, 2005) were treated
with SMART-IMRT, combined with concurrent chemotherapy and adjuvant
chemotherapy following an initial induction chemotherapy regimen.
Eligibility criteria for this study were: histologically-confirmed,
locoregionally-advanced stage IIA to IVB NPC [World Health
Organization (WHO) histopathological type I–III (2)], no previous history of chemotherapy or
radiotherapy, no evidence of distant metastases, age 17 to 75
years, an Eastern Cooperative Oncology Group (ECOG) performance
status of 0 to 1 or Karnofsky performance score (KPS) ≥70, white
blood cells ≥4,000/ml, platelets ≥100,000/ml, serum creatinine ≤1.6
mg/dl or 24-h calculated creatinine clearance ≤60 ml/min, ALT
and/or AST ≤40 U/l and no limitation of co-morbidities for the
therapeutic protocol. The patients with locoregionally-advanced NPC
were excluded if they refuted the entire therapeutic protocol or
reported a previous history of other human cancers.
The pre-treatment evaluation consisted of a complete
medical history and physical examination, fiber optic endoscopic
examination, routine blood counts and serum biochemistry. In all
patients, a head and neck CT and/or magnetic resonance imaging
(MRI) were used to accurately evaluate the extent of the primary
tumor and regional lymph nodes. In addition, chest radiography and
abdominal ultra-sonograph were routinely performed. In the clinical
trial, positron emission tomography (PET)/CT was suboptimal for the
assessment and bone scintigraphy was involved as indicated. All
patients in this study explored the potential risks and benefits of
SMART-IMRT concurrent with cisplatin-based chemotherapy. The
protocol was approved by the institutional ethics committees of the
individual participating centers (Shandong Province Oncology
Hospital, Jinan, Shandong, China). The trial was monitored by an
independent Data Monitoring Committee composed of radiation
oncologists, medical oncologists and statistical consultants. All
patients provided written informed consent.
Radiotherapy treatment
Patients were immobilized from the head to shoulders
in the supine position using a thermoplastic mask. Pre-treatment
planning CT with serial 3 mm slices from the head down through the
top of the aortic arch was obtained and the images were transmitted
to Pinnacle 8.0 for further analysis. Target volumes were
delineated on the treatment planning CT images; lesion location and
body position by CT scan was re-evaluated at four weeks after
radiation. In certain cases, the planned volume was modified, due
to variations in target volume and body contour.
The gross tumor volume (GTV) was defined by a
combination of imaging studies (such as CT, MRI or PET), clinical
information, endoscopic findings and the initial physical
examination. GTV included the nasopharyngeal primary,
retropharyngeal lymphadenopathy and all gross nodal disease. Gross
nodal disease was defined as any lymph nodes that were
histopathologically confirmed as metastasis at the routine drainage
regions.
The clinical target volume (CTV) was characterized
as all potentially gross and microscopic involvements of NPC, which
was divided into high-risk CTV (CTV1) and low-risk CTV (CTV2). CTV1
was contoured as the GTV plus margin for potential microscopic
spread, including the entire nasopharynx, skull base, clivus,
inferior sphenoid, posterior ethmoid, posterior maxillary antrum
and nasal cavity, pterygopalatine fossa, retropharyngeal nodal
space and parapharyngeal space. The CTV1 for primary and nodal
disease was a concentric volume entirely encompassing the GTV with
an additional 5.0–10.0 mm margin. CTV1 for lymphonode disease
included the first echelon nodal areas. When level II lymphonodes
were grossly involved, ipsilateral levels I and III were considered
as CTV1. Furthermore, CTV2 was characterized as the volume
involving low-risk subclinical disease. In some cases, grossly
negative lymphonode, retropharyngeal, and level II lymph node areas
were regarded as CTV1 and the remaining nodal regions (III, IV and
V) were regarded as CTV2.
Tumor volumes were contoured with an extra margin of
at least 5.0 mm to accommodate variations with the patients’
set-up, and were defined as the planning target volume (PTV). The
PTV was practical for the clinical experience of radiologists, but
presented a dilemma: an inadequate irradiation treatment dose vs.
impairments of fatal organs or tissues. The various nodal levels in
the neck were delineated according to the recommendation by an
experienced radiologist. In addition, the critical normal tissues,
including the brain stem, spinal cord, eye globes, optic nerves,
chiasm, parotid glands and lens, were contoured as ‘organs at risk’
(OARs). Whenever possible, MRI images were fused with CT images to
delineate the target volumes and the surrounding critical normal
structures.
In our institution, IMRT guidelines using SMART
techniques were developed for the treatment of head and neck
cancers. According to the guidelines, daily fractions of 2.2 and
2.0 Gy were prescribed to the PTV1 and PTV2 with a total dose of 72
and 60 Gy, respectively, in 30 fractions over six weeks. The
planning goal was as follows: the prescription dose was to
encompass at least 95% of the PTV, no more than 20% of the PTV was
to receive more than 110% of the prescribed dose, no more than 1%
of the target volume was to receive less than 93% of the prescribed
dose, and no more than 1% of the tissue outside the PTV was to
receive more than 110% of the prescribed dose. For the OARs, the
maximum-tolerated dose was as follows: 54 Gy to the brain stem,
optic nerve and chiasm; 45 Gy to spinal cord; and 8 Gy to the lens.
As a parallel structure, up to 50% of the parotid glands could
receive no more than 30–35 Gy. The total parotid volume was the sum
of right and left parotid volumes. SMART was delivered using 6 MV
photons generated by a linear accelerator with Millennium 120 MLC
(Varian Medical Systems, Palo Alto, CA, USA).
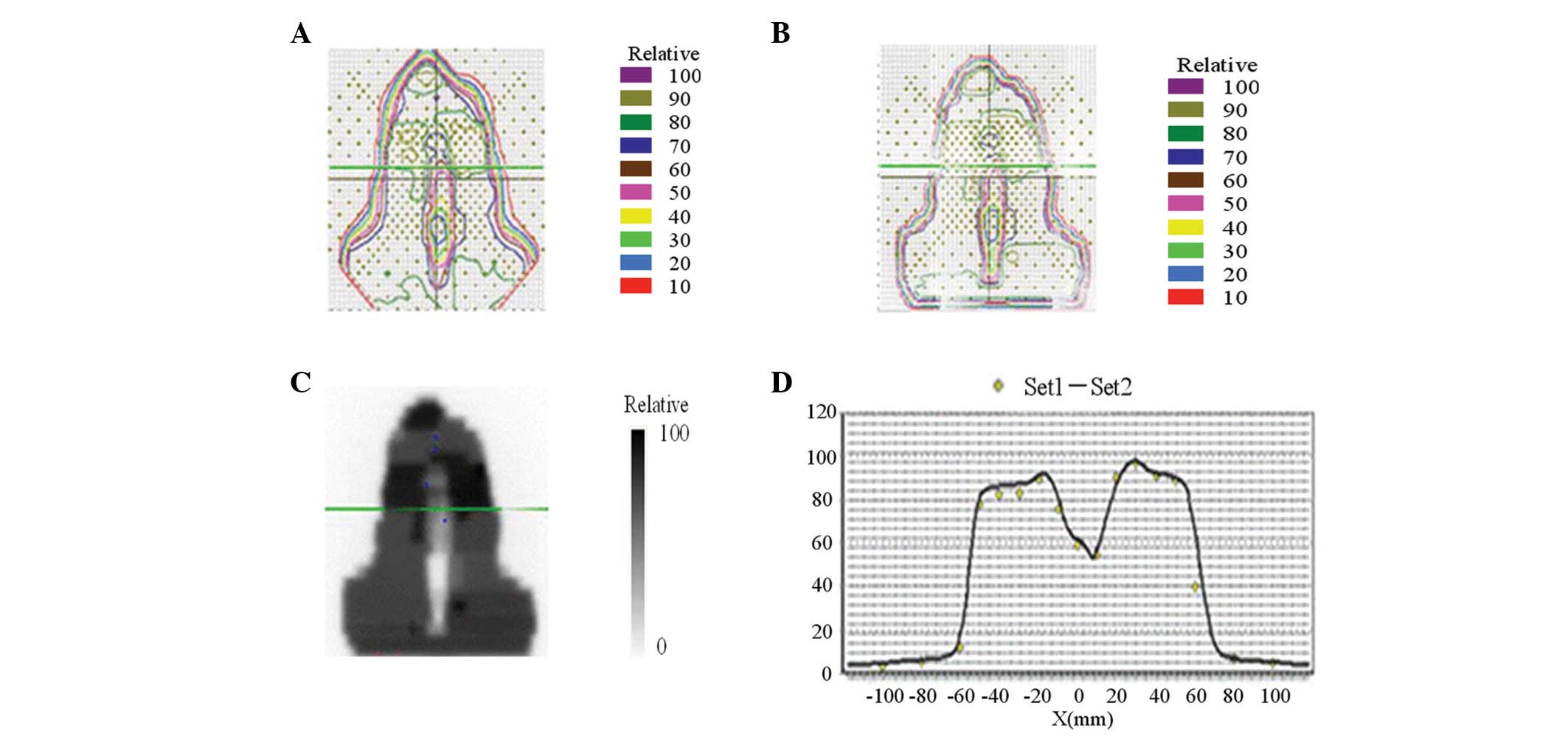
As a pre-treatment dose-verification method, the
Pin-Point Ionization Chamber (for absolute point dose) and 2D film
dosimetry (for dose distribution) were performed. The set-up
verification was performed using portal vision and confirmed by the
physician every day before treatment. Examples of dose distribution
curves of target volume are illustrated in Fig. 1.
Chemotherapy treatment
All patients recruited into this study were assigned
to receive the entire therapeutic protocol consisting of six cycles
of a step-by-step radio-chemotherapy. A total cycle of chemotherapy
consisted of two cycles of inductive, two cycles of concurrent and
two cycles of adjuvant chemotherapy. Inductive and adjuvant
chemotherapeutic regimens consisted of cisplatin (30
mg/m2/d, i.v. infusion, days 1–3) and 5-FU (400
mg/m2/d, i.v. infusion, days 1–5) every three weeks for
two cycles. Concurrent chemotherapy was performed alongside
SMART-IMRT and consisted of cisplatin alone (30 mg/m2/d,
i.v. infusion, days 1–3), given every three weeks for two cycles.
In this study, a patient’s concurrent chemotherapeutic regimen was
chosen by the medical oncologists. Side effects that were ascribed
to chemotherapy did not limit the compliance of treatment and all
patients fulfilled the therapeutic regimen as planned.
Dose modifications during chemotherapy were based on
the nadir blood counts and interim toxic effects. Cisplatin was
decreased to 20 mg/m2 if the neutro-phil count was
1,000–1,500 cells i.v., the platelet count was
50,000–75,000/μl or the creatinine clearance was 40–60
ml/min. Cisplatin was decreased to 10 mg/m2 if the
neutro phil count was <1,000 cells/μl or the platelet
count was <50,000/μl. Chemotherapy was stopped completely
if the creatinine clearance was <40 ml/min or if grade 3 or
higher neuro toxicity or ototoxicity developed. 5-FU was decreased
to 300 mg/m2 if grade 2 (or 200 mg/m2 if
grade 3) mucositis or diarrhea occurred. Chemotherapy was paused
indefinitely if any grade 4 toxic effects developed.
Toxicity evaluation and follow-up
Throughout the therapeutic process, toxic effects
were assessed weekly, both during and after the completion of
adjuvant chemotherapy and at subsequent predefined intervals. One
month after radiation, all patients underwent a cranial CT scan.
After completion of treatment, patients were checked every three
months for two years, and every six months thereafter. The
patients’ therapeutic responses to radiotherapy were categorized in
accordance with the Response Evaluation Criteria in Solid Tumors
(RECIST). Chemotherapy-related toxic effects were graded in
accordance with WHO criteria. Radiotherapy-related toxic effects
were graded in accordance with the Radiation Therapy Oncology Group
(RTOG) criteria.
All local recurrences were diagnosed with fiber
optic endoscopy and biopsy and/or MRI scan of the nasopharynx and
skull base to determine the degree of bone erosion and soft tissue
swelling. Regional recurrences were diagnosed by clinical
examination of the neck and, in the rare cases, by fine needle
aspiration or an MRI scan of the neck. Distant metastasis was
diagnosed by clinical symptoms, physical examinations and imaging
methods that included chest radiography, bone scan, MRI or CT. PET
was also recommended when locoregional recurrence was suspected on
CT or MRI. Whenever possible, salvage treatments (including
re-irradiation, chemotherapy, and surgery) were given to patients
after documented relapse or after persistent disease, in accordance
with the standard practice of each center.
Statistical analysis
A complete response (CR) was defined as the complete
regression of all gross or microscopic tumors. A partial response
(PR) was defined as >50% regression of all measurable tumors.
Treatment failure was recorded as a local failure, regional failure
or distant metastasis. OS was defined from the date of
randomization initiation to death from any causes. Progression-free
survival (PFS) was defined from the date of randomization
initiation to treatment failure or death from any cause, whichever
occurred first. The toxicity variation in the patients treated with
radio-chemotherapy were assessed using the Chi-square or Fisher’s
exact test. An independent t-test was used to evaluate the
correlation between dose-volumetric parameters and the
toxicity.
Results
Immediate and long-term treatment
outcomes
Forty-five patients with locoregionally-advanced NPC
were selected for this study. All cases were histopathologically
confirmed as nasopharyngeal squamous cell carcinoma (NPSCC),
including 43 poorly-differentiated, 1 moderately-differentiated and
1 well-differentiated NPSCC. All patients were categorized
according to the AJCC staging system of NPC (2 for stage IIa, 24
for stage IIb, 16 for stage III, and 3 for stage IV) and displayed
no other abnormalities on radiographic and serological examinations
with a KPS of at least 70. All patients in this clinical study were
able to complete the planned treatment without unexpected
interruption.
After two cycles of inductive chemotherapy and a
median duration of 40 days (range, 37–43 days) of radiation, tumor
shrinkage presented in 24/45 (53.3%) of cases. Ten cases were
defined as PR, with no cases of CR. After one month of adjuvant
chemotherapy, 30/45 patients (66.7%) achieved CR, while 14/45
(31.1%) achieved PR, and 1/45 (2.2%) achieved SD. Although four
patients with PR still had persistent regional disease, no
hypermetabolic lesions were detectable by PET/CT. The response rate
to the therapy regimen in our study was 97.8%.
All patients completed the follow-up regimen as
planned, after 51 months of follow-up (range, 36–60 months).
Follow-up data were collected and analyzed by a multidisciplinary
team of radiologists and medical oncologists. The rates of OS and
PFS were 95.5 and 93.3%, respectively, although three cases of
treatment failure were documented. One of these cases, initially a
stage III, developed multiple lung metastases at five months after
adjuvant chemotherapy. The other two patients presented with
progressive disease for multiple bone metastases and locoregional
recurrence, respectively. These three patients subsequently
received salvage radiotherapy, chemotherapy or surgery to attain an
expectedly prolonged survival and higher quality of life.
Treatment-related toxicities
The treatment-related toxic effects from the time of
irradiation commencement to one month after irradiation are given
in Table I. The toxicities,
according to RTOG criteria, were listed. No patients had
life-threatening or fatal toxicities related to chemotherapy or
radiotherapy. Furthermore, no grade 4 toxicities were detected in
our patient cohort. Although all the patients complained of dry
mouth, this symptom was rapidly treated and relieved. At the
completion of adjuvant chemotherapy, 8/45 cases (17.8%) had grade 1
toxicity, 27/45 (60.0%) had grade 2 toxicity, and 10/45 (22.2%) had
grade 3 toxicity. Nevertheless, compared to conventional
radio-chemotherapy regimens, the incidence of grade 3 toxicities
was significantly decreased (P<0.05, 22.2 vs. 6.7%) and
concomitant with an increase in grade 1 or 2 toxicity (P<0.05,
77.8 vs. 95.6%). Notably, conventional radio-chemotherapy-related
side effects, including hepatotoxicity, nephrotoxicity, digestive
disorders and weight loss, were severe at six months after
irradiation (Table I). In this
study, it was discovered that locoregional toxicities, such as
pharyngitis, laryngitis, stomatitis, xerostomia and skin
desquamation, were more frequent than other toxicities. Notably,
six complete cycles of cisplatin-involving chemotherapy did not
lead to serious hematological disorders (Table I).
 | Table IToxicities according to treatment. |
Table I
Toxicities according to treatment.
| Grade of toxic
effects
|
|---|
| I | II | III | IV |
|---|
| Skin | 12 | 28 | 5 | 0 |
| Mucosa | 6 | 35 | 4 | 0 |
| Salivary glands | 8 | 27 | 10 | 0 |
| Pharynx | 35 | 10 | 0 | 0 |
| Larynx | 45 | 0 | 0 | 0 |
| Digestive
disorders | 14 | 28 | 3 | 0 |
| Vomiting | 8 | 15 | 2 | 0 |
| Nausea | 14 | 20 | 2 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 |
| Hematological | 23 | 9 | 6 | 0 |
| Anemia | 15 | 5 | 0 | 0 |
| Leucocytopenia | 23 | 9 | 6 | 0 |
|
Thrombocytopenia | 1 | 0 | 0 | 0 |
| Hepatotoxicity | 2 | 0 | 0 | 0 |
| Nephrotoxicity | 0 | 0 | 0 | 0 |
| Weight loss | 10 | 30 | 5 | 0 |
Discussion
The combination of chemotherapy with radiotherapy is
a critical strategy for improving tumor control of
locoregionally-advanced NPC, due to the potential enhancement of
radiotherapy-mediated locoregional control and deracinating
micro-metastasis. A meta-analysis of 1,753 patients from eight
randomized trials has previously confirmed the added value of
additional chemotherapy (16).
Furthermore, the added benefit of cisplatin-based chemotherapy in
combination with conventional-fractionation radiotherapy was
demonstrated by the Intergroup 0099 Study (9). However, as the magnitude of long-term
efficacy and safety is essential, the chemotherapeutic regimen of
cisplatin plus 5-FU was subsequently recommended for advanced
NPC.
A recent study on recurrent or metastatic head and
neck cancer showed that, although the response rate to the
combination of cisplatin and 5-FU was superior to single drugs, OS
or PFS did not improve (21). It is
possible, therefore, that the combination of cisplatin with 5-FU is
not an effective combination for the eradication of
micro-metastasis and affecting survival in head and neck cancers.
Newer drugs, such as taxanes and gemcitabine, have exhibited
promising results in NPC despite no improvement of OS (22,23).
One strategy for improvement of NPC is, therefore, to add inductive
or/and adjuvant chemotherapy before or/and after concurrent
radio-chemotherapy. Neither of the two randomized studies of
adjuvant chemotherapy given after radio-chemotherapy demonstrated
an OS advantage over radiotherapy alone, however (6,15,21,24).
Both of these studies had a major limitation, though, which was a
significant rate of patient refusal to complete the planned
adjuvant chemotherapy. Theoretically, the poor compliance with
adjuvant chemotherapy after concurrent radio-chemotherapy can be
overcome by the addition of inductive chemotherapy. A recent
meta-analysis revealed a significant improvement of OS and PFS with
cisplatin and 5-FU induction chemotherapy in patients with squamous
cell carcinoma of the head and neck (20,25).
Thus, new chemotherapy sequences that might improve the efficacy of
chemotherapy as an adjunct should be further investigated.
In this study, all patients were assigned to receive
two cycles of inductive chemotherapy (cisplatin plus 5-FU), two
cycles of concurrent chemotherapy (cisplatin alone), and two cycles
of adjuvant chemotherapy (cisplatin plus 5-FU). The patient
compliance with all six cycles of chemotherapy and the full-course
SMART-IMRT were of great significance. All patients recruited into
this study accomplished the treatment as planned and no delays or
dose reductions were demonstrated. This indicated that this
treatment strategy had an acceptable compliance, so further
improvements in tumor control were taken into account.
This regimen resulted in a notable response rate
(97.8%) and nadir PD (CR of 66.7%, PR of 31.1%, and SD of 2.2%),
which were similar to the response rate of early-stage NPC to
radio-chemotherapy (7,26). Furthermore, OS and PFS in our study
were significantly superior to those in previous reports on
locoregionally-advanced NPC (5,27,28).
Collectively, it implied that this regimen had potent tumor control
of locoregionally-advanced NPC and an acceptable level of
compliance. Our favorable results indicated that the use of the
SMART-IMRT technique might lower the risk of toxicity of the organ
and increase the curative effect.
Currently, IMRT is commonly chosen to treat NPC.
IMRT is preferable to conventional 3D conformal planning as it
further improves tumor coverage and dose distribution between the
tumor and dose-limiting organs (27–29).
Furthermore, IMRT also resolves the problem of dose uncertainty at
the junction between the primary tumor and lymphatic regions, as it
enables the primary tumor and the neck lymph nodes to be treated in
one volume throughout (17,28). Recent studies supporting the
superiority of IMRT over 2D-RT have reported a related 97% PFS and
88% OS at four years after IMRT, with a total of 65–70 Gy delivered
to the GTV (30). Kam et al
also reported that 3-year PFS and OS were 92 and 90%, respectively,
with a total dose of 66 Gy. These studies testify to the efficacy
of IMRT.
In addition to conformal dose distribution, IMRT can
also be applied to exploit the therapeutic advantages of
accelerated forms of radiotherapy. The acceleration scheme
(involving multiple daily fractions, concomitant boosts and weekly
six-daily treatments) improves tumor control and survival with
increased but acceptable toxicities, irrespective of the
acceleration schemes applied (7,28). The
underpinning mechanism for the improvement of outcome is primarily
due to a shortened overall treatment time and a reduction in the
rate of tumor cell repopulation.
SMART acceleration techniques deliver different
doses to different target volumes, simultaneously, through a
fraction (29). Lauve et al
reported the results of a phase I radiation dose-escalation trial
to determine the maximal tolerable dose (MTD) of an accelerated
fractionation with a simultaneous integrated boost for the
treatment of locally advanced head and neck carcinoma (31). A total dose of 70.8 Gy by 30
fractions of 2.36 Gy was determined as the MTD deliverable to the
GTV with adequate parotid sparing. The actuarial two-year
locoregional control and distant control rates were 76.3% and
71.8%, respectively. It was concluded that tumor control and
survival rates compared favorably with the outcomes of other
accelerated regimens.
Despite reducing the amount of radiation received by
non-target normal tissue, the application of accelerated RT by
SMART also delivers a higher biologically effective dose to the
normal mucosa within the target volume, which results in a higher
prevalence of locoregional radiation-related diseases (such as
orolarygopharyngeal mucositis and xerostoma) compared to
conventionally fractionated RT (18,19,27,32–34).
In this trial, all 45 patients recruited into the study were
treated with SMART-IMRT and, as reported previously (18,35),
orolaryngopharyngeal mucositis was more frequently observed than
other toxic effects. While reported by almost all of the patients,
grade 3 toxicity was only observed in 2/45 (4%) of patients and was
quickly treated in all cases. With the parotid glands spared, grade
3 xerostomia was detected in 10/45 (22.2%) patients. Lee et
al documented no chronic xerostomia (17), whereas Kam et al reported 23%
of grade 2 or 3 xerostomia (7).
However, the correlation between the salivary flow and subjective
symptoms of xerostomia was relatively weak (7).
The interaction between SMART-IMRT and chemotherapy
was analyzed. The addition of SMART-IMRT to chemotherapy in this
study might have narrowed the potential gain in local control by
the chemotherapy. Numerous phase III trials found that severe
(grade 3) mucositis was more frequently associated with CRT than RT
alone, with 37 to 62% vs. 28 to 48%, respectively (P<0.05)
(35–37). Nevertheless, the toxic effects of
concurrent radio-chemotherapy was well-tolerated, supported by the
observation that all patients in our cohort accomplished six full
cycles of inductive, concurrent and adjuvant chemotherapy as
planned. Parallel with these outcomes, hematological and
non-hematological toxic effects were ascribed to an acceptable and
short-term modality and were not life-threatening. The outcomes of
our trial therefore supported a regimen of six cycles of
cisplatin-based chemotherapy concurrent with SMART-IMRT as an
acceptable and feasible strategy for locoregionally-advanced NPC.
It is possible that administering combinations of newer drugs
before, rather than after, concurrent radio-chemotherapy to improve
compliance might result in further improvements in systemic
control. These encouraging results are currently being confirmed in
several randomized trials assessing new combinations of inductive
chemotherapy with subsequent concurrent radio-chemotherapy
(14,23).
There are several potential bias factors of this
study, including a small sample size and the relatively short
follow-up period. Effort was made to make up for these limitations.
The IMRT guidelines using SMART were implemented and SMART
protocols were consistent over the entire study period. In
addition, the clinical follow-up data were collected from all
patients in the cohort. In conclusion, despite a small patient
sample size and a short follow-up, the preliminary results
demonstrate an encouraging trend of locoregional control and
survival with no increase in related toxicities. Although we
reported on two-year survival results, we are still following the
patients closely and reporting five-year follow-up results when
more events become available. The findings suggest that increasing
the availability of SMART-IMRT may further increase the therapeutic
gain. Estimation of sample size for future trials should be based
on higher baseline results by SMART-IMRT and more realistic
magnitude of benefit to avoid being underpowered.
References
|
1
|
Ensley JF, Youssef E, Kim H and Yoo G:
Locally advanced nasopharyngeal cancer. Curr Treat Options Oncol.
2:15–23. 2001. View Article : Google Scholar
|
|
2
|
Chan AT, Teo PM and Johnson PJ:
Nasopharyngeal carcinoma. Ann Oncol. 13:1007–1015. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gius D and Coleman CN: Treatment of
nasopharyngeal cancer: raising the ‘Barr’. J Natl Cancer Inst.
94:1594–1595. 2002.
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
5
|
Wee J: Nasopharyngeal cancer: a promising
future. Lancet Oncol. 13:116–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwong DL, Sham JS, Au GK, et al:
Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma:
a factorial study. J Clin Oncol. 22:2643–2653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kam MK, Leung SF, Zee B, et al:
Prospective randomized study of intensity-modulated radiotherapy on
salivary gland function in early-stage nasopharyngeal carcinoma
patients. J Clin Oncol. 25:4873–4879. 2007. View Article : Google Scholar
|
|
8
|
Wee J, Tan EH, Tai BC, et al: Randomized
trial of radiotherapy versus concurrent chemoradiotherapy followed
by adjuvant chemotherapy in patients with American Joint Committee
on Cancer/International Union against cancer stage III and IV
nasopharyngeal cancer of the endemic variety. J Clin Oncol.
23:6730–6738. 2005. View Article : Google Scholar
|
|
9
|
Al-Sarraf M, LeBlanc M, Giri PG, et al:
Chemoradiotherapy versus radiotherapy in patients with advanced
nasopharyngeal cancer: phase III randomized Intergroup study 0099.
J Clin Oncol. 16:1310–1317. 1998.PubMed/NCBI
|
|
10
|
Chan AT, Leung SF, Ngan RK, et al: Overall
survival after concurrent cisplatin-radiotherapy compared with
radiotherapy alone in locoregionally advanced nasopharyngeal
carcinoma. J Natl Cancer Inst. 97:536–539. 2005. View Article : Google Scholar
|
|
11
|
Zhang L, Zhao C, Peng PJ, et al: Phase III
study comparing standard radiotherapy with or without weekly
oxaliplatin in treatment of locoregionally advanced nasopharyngeal
carcinoma: preliminary results. J Clin Oncol. 23:8461–8468. 2005.
View Article : Google Scholar
|
|
12
|
Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS
and Wang WY: Phase III study of concurrent chemoradiotherapy versus
radiotherapy alone for advanced nasopharyngeal carcinoma: positive
effect on overall and progression-free survival. J Clin Oncol.
21:631–637. 2003. View Article : Google Scholar
|
|
13
|
Chan AT, Teo PM, Ngan RK, et al:
Concurrent chemotherapyradiotherapy compared with radiotherapy
alone in locoregionally advanced nasopharyngeal carcinoma:
progression-free survival analysis of a phase III randomized trial.
J Clin Oncol. 20:2038–2044. 2002. View Article : Google Scholar
|
|
14
|
Lee NY, Zhang Q, Pfister DG, et al:
Addition of bevacizumab to standard chemoradiation for
locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a
phase 2 multi-institutional trial. Lancet Oncol. 13:172–180. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Amro A, Al-Rajhi N, Khafaga Y, et al:
Neoadjuvant chemotherapy followed by concurrent chemoradiation
therapy in locally advanced nasopharyngeal carcinoma. Int J Radiat
Oncol Biol Phys. 62:508–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Langendijk JA, Leemans CR, Buter J,
Berkhof J and Slotman BJ: The additional value of chemotherapy to
radiotherapy in locally advanced nasopharyngeal carcinoma: a
meta-analysis of the published literature. J Clin Oncol.
22:4604–4612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee N, Harris J, Garden AS, et al:
Intensity-modulated radiation therapy with or without chemotherapy
for nasopharyngeal carcinoma: radiation therapy oncology group
phase II trial 0225. J Clin Oncol. 27:3684–3690. 2009. View Article : Google Scholar
|
|
18
|
Koom WS, Kim TH, Shin KH, et al: SMART
(simultaneous modulated accelerated radiotherapy) for locally
advanced nasopharyngeal carcinomas. Head & neck. 30:159–169.
2008.PubMed/NCBI
|
|
19
|
Lee SW, Back GM, Yi BY, et al: Preliminary
results of a phase I/II study of simultaneous modulated accelerated
radiotherapy for nondisseminated nasopharyngeal carcinoma. Int J
Cancer. 65:152–160. 2006.PubMed/NCBI
|
|
20
|
Chan AT: Head and neck cancer: treatment
of nasopharyngeal cancer. Ann Oncol. 16(Suppl 2): ii265–268.
2005.PubMed/NCBI
|
|
21
|
Licitra L, Bossi P, Locati L and Zunino F:
Cisplatin and fluorouracil concurrent to radiotherapy in
nasopharyngeal cancer: is the schedule compatible? J Clin Oncol.
22:377author reply 377–378, 2004.
|
|
22
|
Chua DT, Ma J, Sham JS, et al: Long-term
survival after cisplatin-based induction chemotherapy and
radiotherapy for nasopharyngeal carcinoma: a pooled data analysis
of two phase III trials. J Clin Oncol. 23:1118–1124. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hui EP, Ma BB, Leung SF, et al: Randomized
phase II trial of concurrent cisplatin-radiotherapy with or without
neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal
carcinoma. J Clin Oncol. 27:242–249. 2009. View Article : Google Scholar
|
|
24
|
Hong RL, Ting LL, Ko JY, et al: Induction
chemotherapy with mitomycin, epirubicin, cisplatin, fluorouracil,
and leucovorin followed by radiotherapy in the treatment of
locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol.
19:4305–4313. 2001.
|
|
25
|
Chan AT, Ma BB, Lo YM, et al: Phase II
study of neoadjuvant carboplatin and paclitaxel followed by
radiotherapy and concurrent cisplatin in patients with
locoregionally advanced nasopharyngeal carcinoma: therapeutic
monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol.
22:3053–3060. 2004. View Article : Google Scholar
|
|
26
|
Cheng SH, Tsai SY, Yen KL, et al:
Concomitant radiotherapy and chemotherapy for early-stage
nasopharyngeal carcinoma. J Clin Oncol. 18:2040–2045.
2000.PubMed/NCBI
|
|
27
|
Lee TF, Chao PJ, Ting HM, et al:
Comparative analysis of SmartArc-based dual arc
volumetric-modulated arc radio-therapy (VMAT) versus
intensity-modulated radiotherapy (IMRT) for nasopharyngeal
carcinoma. J Appl Clin Med Phys. 12:35872011.PubMed/NCBI
|
|
28
|
Cheng JC, Chao KS and Low D: Comparison of
intensity modulated radiation therapy (IMRT) treatment techniques
for nasopharyngeal carcinoma. Int J Cancer. 96:126–131. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kristensen CA, Kjaer-Kristoffersen F,
Sapru W, Berthelsen AK, Loft A and Specht L: Nasopharyngeal
carcinoma. Treatment planning with IMRT and 3D conformal
radiotherapy. Acta Oncologica. 46:214–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee N, Xia P, Quivey JM, et al:
Intensity-modulated radiotherapy in the treatment of nasopharyngeal
carcinoma: an update of the UCSF experience. Int J Radiat Oncol
Biol Phys. 53:12–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lauve A, Morris M, Schmidt-Ullrich R, et
al: Simultaneous integrated boost intensity-modulated radiotherapy
for locally advanced head-and-neck squamous cell carcinomas:
II--clinical results. Int J Radiat Oncol Biol Phys. 60:374–387.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao WW, Huang SM, Han F, et al: Local
control, survival, and late toxicities of locally advanced
nasopharyngeal carcinoma treated by simultaneous modulated
accelerated radiotherapy combined with cisplatin concurrent
chemotherapy: long-term results of a phase 2 study. Cancer.
117:1874–1883. 2011. View Article : Google Scholar
|
|
33
|
Lee VH, Ng SC, Leung TW, Au GK and Kwong
DL: Dosimetric predictors of radiation-induced acute nausea and
vomiting in IMRT for nasopharyngeal cancer. Int J Cancer.
2012.PubMed/NCBI
|
|
34
|
Park SH, Park HC, Park SW, et al:
Multi-institutional comparison of intensity modulated radiation
therapy (IMRT) planning strategies and planning results for
nasopharyngeal cancer. J Korean Med Sci. 24:248–255. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen QY, Wen YF, Guo L, et al: Concurrent
chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal
carcinoma: phase III randomized trial. J Natl Cancer Inst.
103:1761–1770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen L, Hu CS, Chen XZ, et al: Concurrent
chemoradiotherapy plus adjuvant chemotherapy versus concurrent
chemoradiotherapy alone in patients with locoregionally advanced
nasopharyngeal carcinoma: a phase 3 multicentre randomised
controlled trial. Lancet Oncol. 13:163–171. 2012. View Article : Google Scholar
|
|
37
|
Lee AW, Tung SY, Chua DT, et al:
Randomized trial of radiotherapy plus concurrent-adjuvant
chemotherapy vs radiotherapy alone for regionally advanced
nasopharyngeal carcinoma. J Natl Cancer Inst. 102:1188–1198. 2010.
View Article : Google Scholar : PubMed/NCBI
|