Introduction
Metanephric adenoma (MA) is a rare benign renal
tumor which is related to the developing proximal tubule of the
fetal kidney or nephrogenic rests. The tumor generally occurs in
female adults and occasionally in children (1). Wilms’ tumor (WT), also known as
nephroblastoma, is the most common malignant tumor which originates
from developing nephrogenic tissue, occurring in the genitourinary
tract in children (2). The nature
of metanephric adenoma is still not entirely clear, although some
investigators believed that it is related to WT because these two
tumors sometimes share morphologic similarities and
immunoreactivity for Wilms’ tumor gene1 (WT-1) (3,4).
Here, a rare composite tumor with MA and WT
histopathologic features of the kidney in a male adult is reported.
This case represents the third report of this composite tumor of MA
and WT and may present clues to elucidate the pathogenesis of MA
and WT (5,6). The study was approved by the Ethics
Committee of Tongji hospital, Tongji Medical College, Huazhong
University of Science and Technology, Wuhan, China. Written
informed consent was obtained from the patient.
Case report
A 36-year-old male visited the Department of
Urology, Tongji Hospital, Wuhan, China, for evaluation of his renal
area following approximately 4 months of illness. The medical
history and review of symptoms were noncontributory.
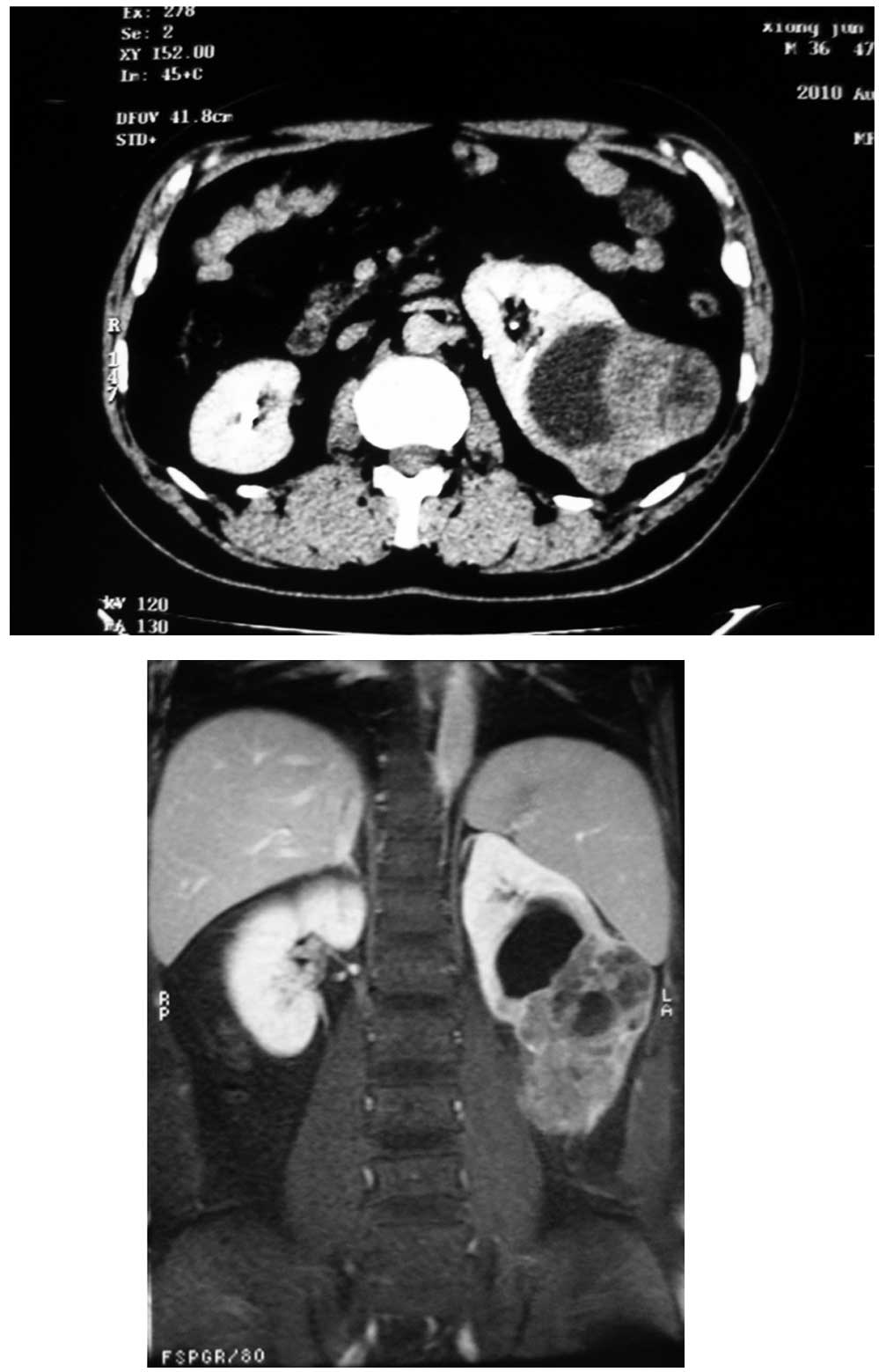
Computed tomography (CT) and magnetic resonance (MR)
scan revealed an expansile, solid mass measuring ∼10 cm in the
middle lower pole of the left kidney (Fig. 1). The mass was relatively
ill-marginated. The patient was admitted for surgical intervention
and the lesion was excised under general anesthesia.
Pathological examination
Specimens from the mass were fixed in 10% formalin,
embedded in paraffin, sectioned, and stained with hematoxylin and
eosin (H&E) by means of routine procedures. Immunostaining was
performed on 4-μm-thick sections using the standard
avidin-biotin complex technique. A panel of antibodies (Table I) was used.
 | Table IAntibodies and dilutions used in the
evaluation of composite metanephric adenoma and Wilms’ tumor of the
kidney. |
Table I
Antibodies and dilutions used in the
evaluation of composite metanephric adenoma and Wilms’ tumor of the
kidney.
| Antibody | Dilution | Source | Antigen
retrieval |
|---|
| Vimentin | 1:20 | Dako | Heat |
| AE1/AE3 | 1:20 | Dako | Heat |
| CD57 | 1:50 | Dako | Heat |
| WT-1 | 1:200 | Dako | Heat |
| EMA | 1:100 | Dako | Heat |
| CK7 | 1:200 | Dako | Heat |
| CK8/18 | 1:100 | Dako | Heat |
| Ki67 | 1:40 | Dako | Heat |
Immunostaining was performed by an enhancement
method based on repetitive microwave heating of slides that were
placed into 0.01 M citrate buffer at pH 6.0. Binding of primary
antibodies was visualized with an Envision two-step method.
Diaminobenzidine was used as the chromogen. Nuclei were stained
with Mayer’s hematoxylin. Appropriate positive and negative
controls were included.
Results
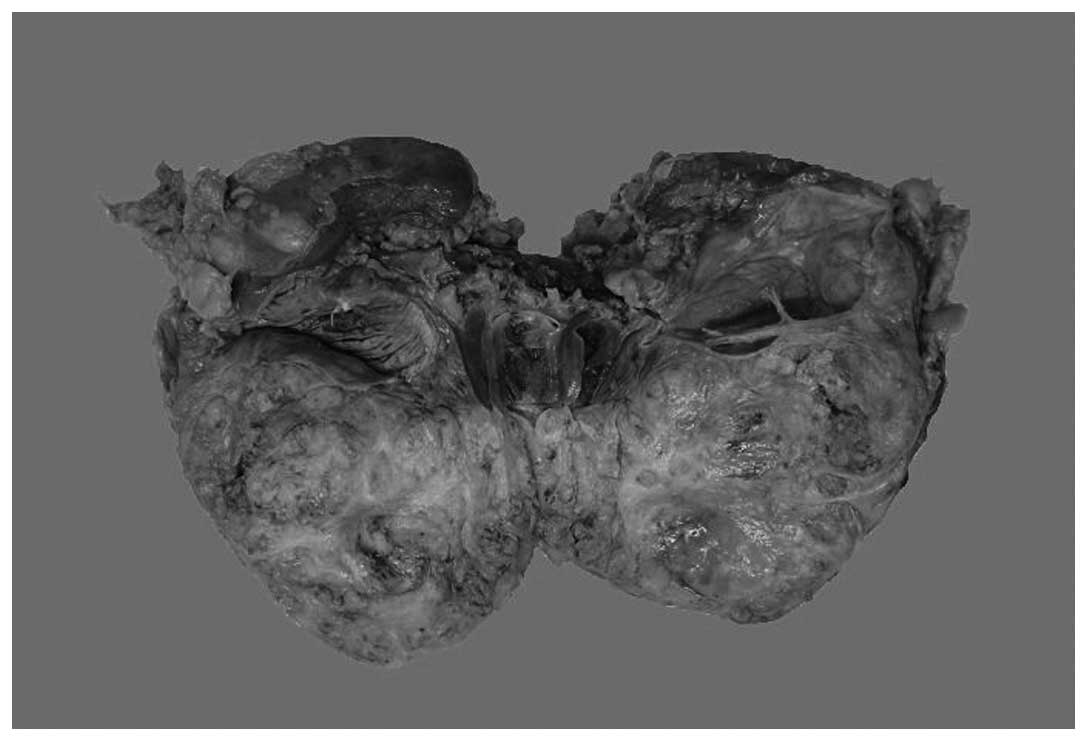
Macroscopically, the specimen was ill-circumscribed
and measured 10 cm at its greatest dimension, and hydronephrectasia
could be seen in the left kidney due to the occupation of the
tumor; the cut surfaces were yellowish gray and solid (Fig. 2). There was no obvious necrosis or
hemorrhage. The adjacent renal parenchyma appeared normal. The
renal capsule was intact.
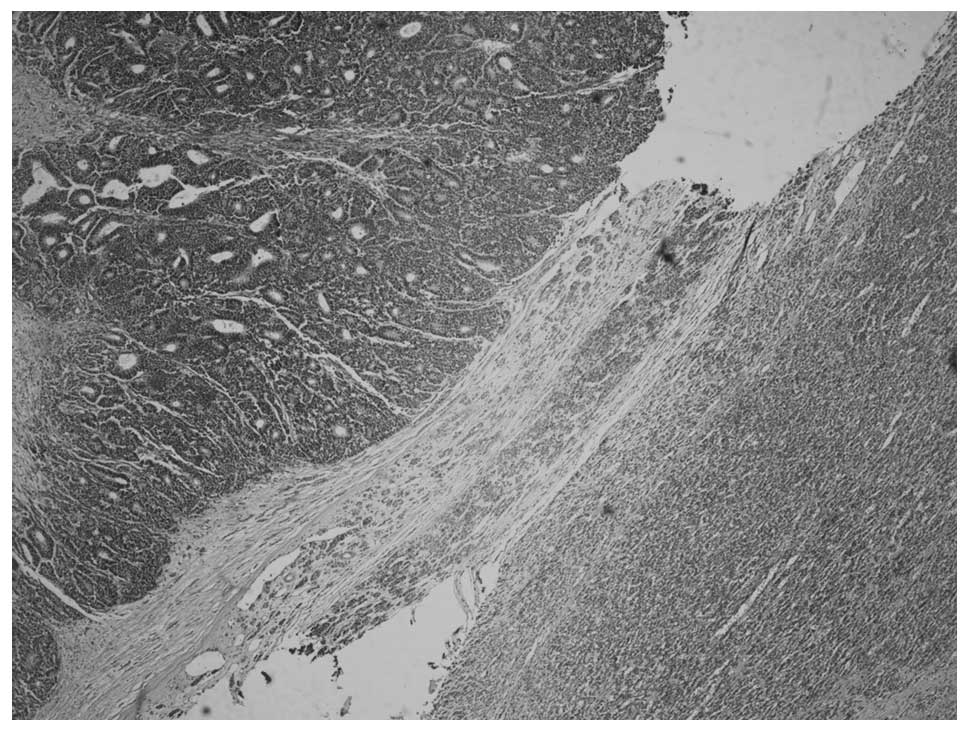
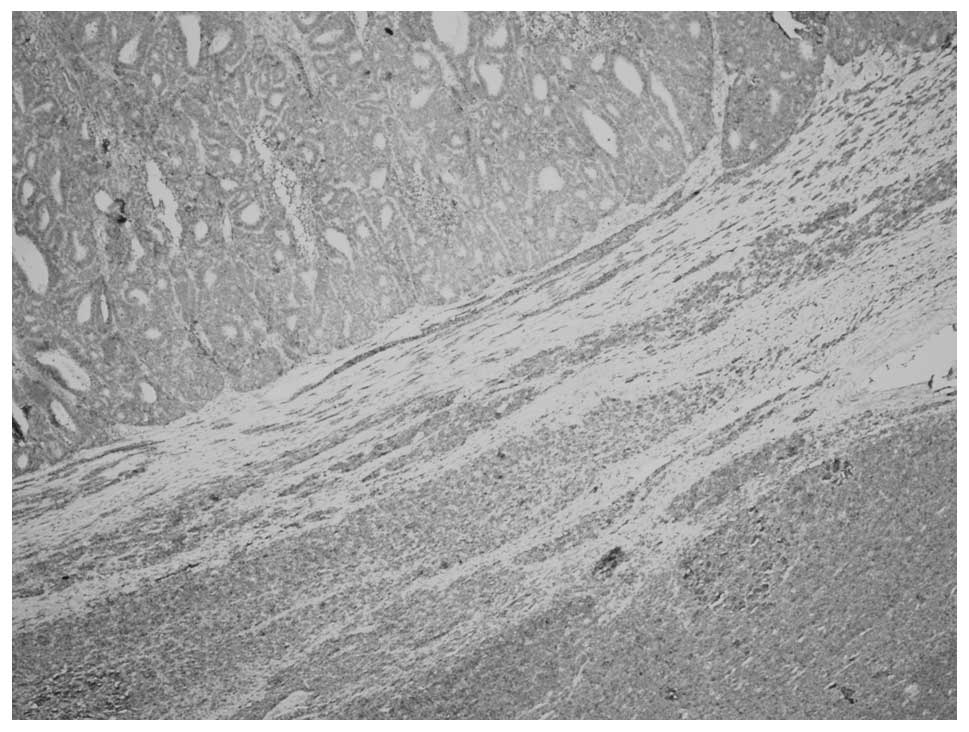
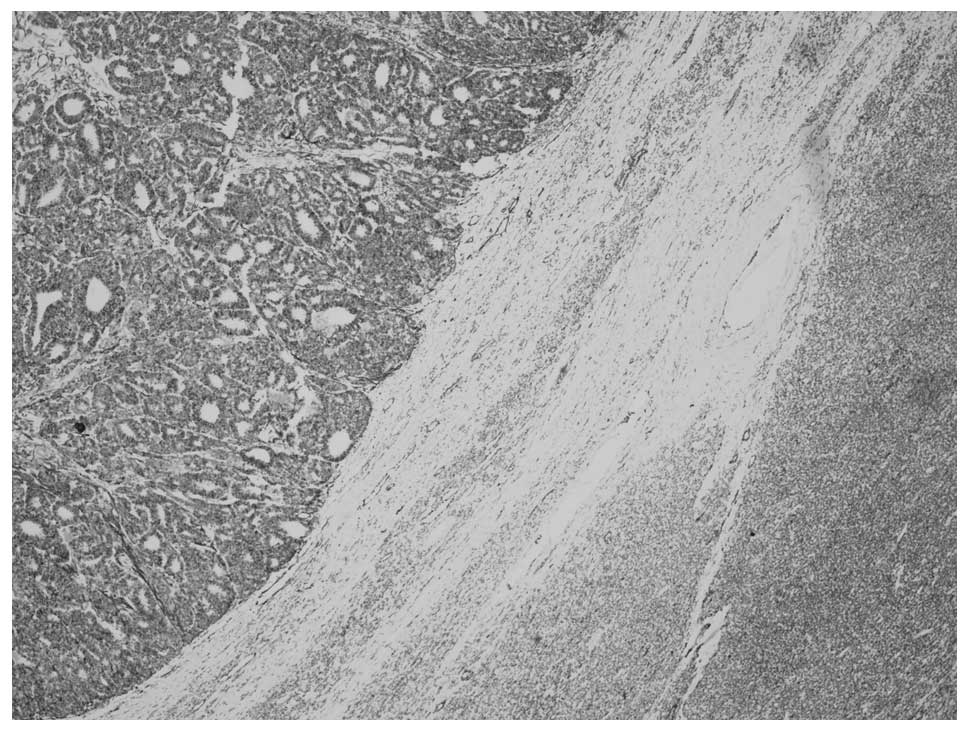
Microscopically, the tumor consisted of two distinct
areas of MA and WT that were separated by a band of fibrous stroma
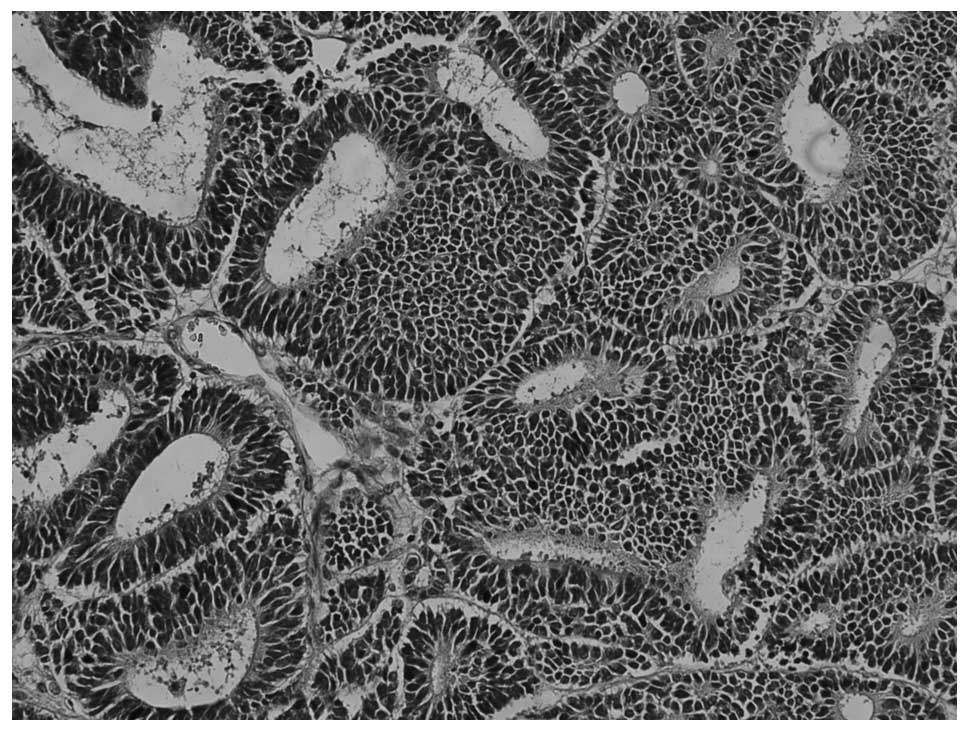
(Fig. 3). MA showed a pushing
border with no capsule and an ordered array of small, tightly
packed acini and tubules separated by acellular stroma (Fig. 4), but no papillary structure. The
tumor cells were small and uniform with round to oval nuclei and
scant cytoplasm. Nuclei showed delicate chromatin and inconspicuous
nucleoli; mitotic activity was rare to absent.
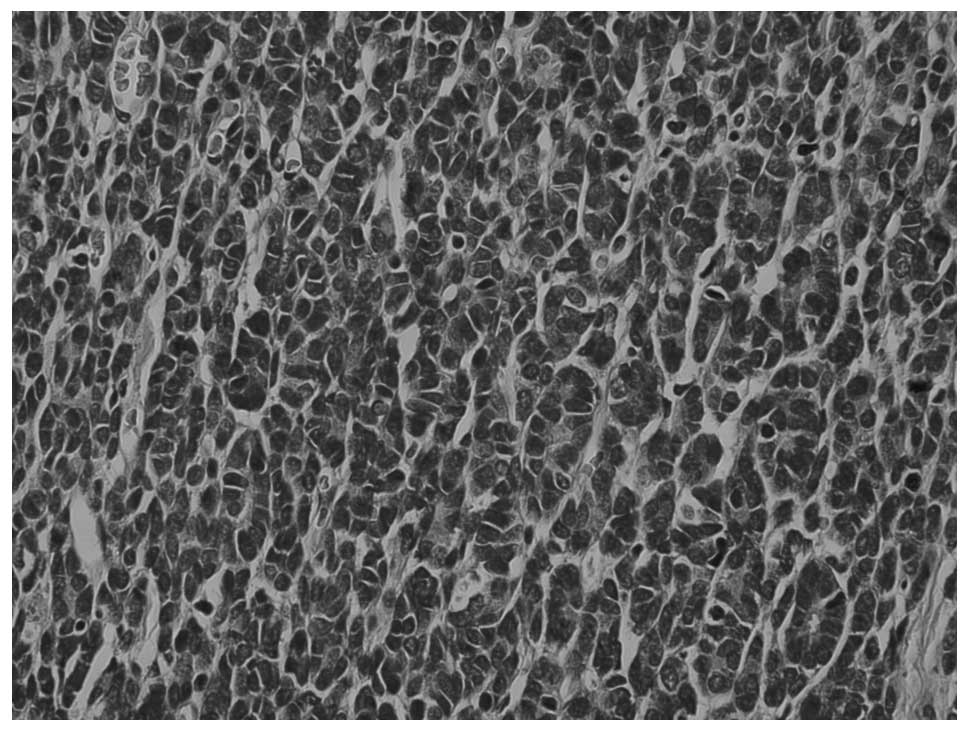
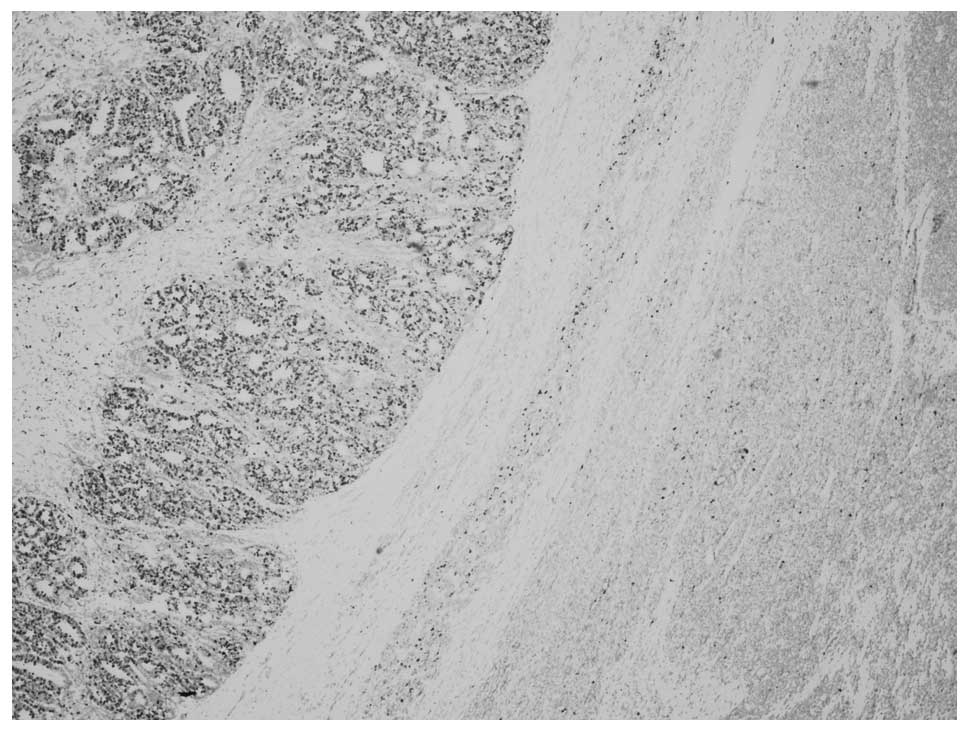
The WT area showed a predominantly epithelial
component which was in the form of either poorly formed or
well-developed tubules (Fig. 5) The
typical blastemal and stromal components were rarely observed.
In the MA area, tumor cells showed positive diffuse
staining for CD57 (Fig. 6), WT-1
(Fig. 7), focal staining for
vimentin, pan CK, CK7 and CK8/18, but negative staining for
epithelial membrane antigen (EMA). The Ki67 labeling index
(Fig. 8) was 2%. In the WT area,
tumor cells stained diffusely for WT-1 (Fig. 7), CK8/18, focally for vimentin, pan
CK, EMA, CD57 (Fig. 6), but were
negative for CK7. The Ki67 labeling index (Fig. 8) was 50–60%.
Discussion
Composite renal tumors are rarely reported. The most
commonly described association is of Wilms’ tumor and renal cell
carcinoma (7). Composite tumors of
MA and WT of the kidney are extremely rare (5, 6).
MA is a well-described rare benign renal tumor,
predominantly occurring in adult females, and seldom observed in
children. WT is the most common malignant renal tumor in children
but it is rare in adults. Histologically, MA is composed of tightly
packed uniform small epithelial cells in acinar, solid and tubular
configurations with small regular nuclei, a high nucleus to
cytoplasm ratio, but low mitotic figures.
Wilms’ tumors are typically composed of a mixture of
primitive blastemal cells, epithelial cells and mesenchymal
elements. In this case, the epithelial component in the WT area was
predominant; it was therefore named epithelial-predominant WT
(8). Due to overlapping
morphological features, histopathological examination of MA often
prompts an initial diagnosis of epithelial-predominant WT.
Since MA and epithelial-predominant WT could share
microscopical similarities, immunohistochemistry (IHC) played a
significant contributory role in the distinction of these two
entities. Although CD57 is not particular for diagnosis of MA, it
is helpful in the differential diagnosis between MA and
epithelial-predominant WT (9,
10). In MA, the epithelial cells
are positive for CD57, while the tumor cells in
epithelial-predominant WT are negative. The Ki67 labeling index is
significantly lower in MA compared with epithelial-predominant WT,
which also supports these two areas belonging to distinct lesions
(6). The two distinct areas had
individual histopathological features and special IHC staining,
both of which contributed to the reaffirmation of the
histomorphological diagnosis of composite MA and
epithelial-predominant WT.
Both MA and epithelial-predominant WT were positive
for WT-1, leading to the theory that the two could be linked
(9), and MA could even be a more
hyperdifferentiated, mature form of WT (11). Since there was no other supporting
evidence it was hypothesized that they were two distinct
entities.
Another diagnostic challenge is differentiating MA
from the solid variant form of papillary renal cell carcinoma
(PRCC) based on histologic features alone. IHC is helpful since
PRCC is negative for CD57 and WT-1 (12,13).
To date, the genetic basis of MA remains largely
unknown since previous reports have given conflicting results
(14–16). BRAF mutation has been reported in
90% cases in a series of MA studies but it is rarely detected in
other kidney tumors, including WT. Testing for the BRAF mutation
could, therefore, serve as a potential diagnostic tool for MA
(17).
In summary, a rare case of composite MA and
epithelial-predominant WT in an adult kidney is presented. Although
there were overlapping morphological features, it was possible to
differentiate MA from WT based on the morphologic features and IHC
staining. This case also offered additional support to the
hypothesis that these two tumors are related.
References
|
1
|
Jones EC, Pins M, Dickersin GR and Young
RH: Metanephric adenoma of the kidney. A clinicopathological,
immunohistochemical, flow cytometric, cytogenetic, and electron
microscopic study of seven cases. Am J Surg Pathol. 19:615–626.
1995. View Article : Google Scholar
|
|
2
|
Md Zin R, Murch A and Charles A:
Pathology, genetics and cytogenetics of Wilms’ tumour. Pathology.
43:302–312. 2011.
|
|
3
|
Obulareddy SJ, Xin J, Truskinovsky AM,
Anderson JK, Franklin J and Dudek AZ: Metanephric adenoma of the
kidney: an unusual diagnostic challenge. Rare Tumors. 2:e382010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Portugal R and Barroca H: Clear cell
sarcoma, cellular mesoblastic nephroma and metanephric adenoma:
cytological features and differential diagnosis with Wilms tumour.
Cytopathology. 19:80–85. 2008. View Article : Google Scholar
|
|
5
|
Davis CJ Jr, Barton JH, Sesterhenn IA and
Mostofi FK: Metanephric adenoma. Clinicopathological study of fifty
patients Am J Surg Pathol. 19:1101–1114. 1995.PubMed/NCBI
|
|
6
|
Pasricha S, Gandhi JS, Gupta G, Mehta A
and Beg S: Bilateral, multicenteric metanephric adenoma associated
with Wilms’ tumor in a child: A rare presentation with important
diagnostic and therapeutic implications. Int J Urol. 19:1114–1117.
2012.PubMed/NCBI
|
|
7
|
Galluzzo ML, Garcia de Davila MT and
Vujanic GM: A composite renal tumor: metanephric adenofibroma,
Wilms tumor, and renal cell carcinoma: a missing link? Pediatr Dev
Pathol. 15:65–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verschuur AC, Vujanic GM, Van Tinteren H,
Jones KP, de Kraker J and Sandstedt B: Stromal and epithelial
predominant Wilms tumours have an excellent outcome: the SIOP 93 01
experience. Pediatr Blood Cancer. 55:233–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muir TE, Cheville JC and Lager DJ:
Metanephric adenoma, nephrogenic rests, and Wilms’ tumor: a
histologic and immunophenotypic comparison. Am J Surg Pathol.
25:1290–1296. 2001.
|
|
10
|
Olgac S, Hutchinson B, Tickoo SK and
Reuter VE: Alpha-methylacyl-CoA racemase as a marker in the
differential diagnosis of metanephric adenoma. Mod Pathol.
19:218–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Argani P: Metanephric neoplasms: the
hyperdifferentiated, benign end of the Wilms tumor spectrum? Clin
Lab Med. 25:379–392. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown JA, Anderl KL, Borell TJ, Qian J,
Bostwick DG and Jenkins RB: Simultaneous chromosome 7 and 17 gain
and sex chromosome loss provide evidence that renal metanephric
adenoma is related to papillary renal cell carcinoma. J Urol.
158:370–374. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lugli A, Stoffe F, Terracciano L,
Dirnhofer S, Mihatsch MJ and Moch H: Differentiated papillary
kidney tumors. Differentiation between metanephric adenoma and
papillary adenoma. Pathologe. 23:303–307. 2002.(In German).
|
|
14
|
Burger M, Junker K, Denzinger S, et al:
Metanephric adenoma of the kidney: a clinicopathological and
molecular study of two cases. J Clin Pathol. 60:832–833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brunelli M, Eble JN, Zhang S, Martignoni G
and Cheng L: Metanephric adenoma lacks the gains of chromosomes 7
and 17 and loss of Y that are typical of papillary renal cell
carcinoma and papillary adenoma. Mod Pathol. 16:1060–1063. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pesti T, Sukosd F, Jones EC and Kovacs G:
Mapping a tumor suppressor gene to chromosome 2p13 in metanephric
adenoma by microsatellite allelotyping. Hum Pathol. 32:101–104.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choueiri TK, Cheville J, Palescandolo E,
et al: BRAF mutations in metanephric adenoma of the kidney. Eur
Urol. 62:917–922. 2012. View Article : Google Scholar : PubMed/NCBI
|