Contents
Pregnane X receptor
Correlation between the expression of PXR in various
types of cancer and drug resistance
PXR and tumor cell apoptosis
Preventing drug resistance by regulating PXR
Conclusions and prospects
Pregnane X receptor
Nomenclature
Pregnane X receptor (PXR; NR1I2) belongs to the NR1I
subfamily of nuclear receptors (NRs). PXR was discovered by several
groups in 1998 (1–4) and is alternatively referred to as the
steroid and xenobiotic receptor (PXR) and pregnane-activated
receptor (PAR), also known as SXR or hPXR in humans.
Structure of PXR
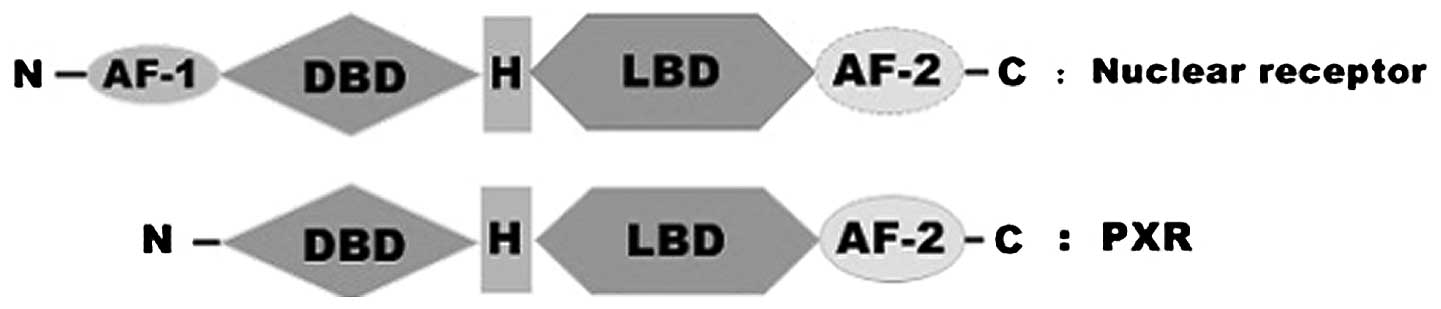
PXR contains the highly conserved DNA-binding domain
(DBD), a characteristic structure of NRs. The far N-terminus is a
short activation function-1 (AF-1) region which permits the
regulation of receptor action in a ligand-independent manner. The
structure of the PXR DBD is highly similar to that of the retinoid
X receptor α (RXRa) DBD, which is a double zinc-finger motif that
contacts DNA in a sequence-specific fashion. The response elements
include direct repeats with 3 to 5 bases separating the DBD binding
sites (DR-3, DR-4 and DR-5) and inverted repeats (with the
beginning of each sequence in proximity) separated by 6 or 8 bases
(ER-6 and ER-8, respectively) (1,4,5). The
two most important PXR target genes, multidrug resistance protein 1
(MDR1) and cytochrome P450 3A4 (CYP3A4), contain DR-4/ER-6
(6) and DR-3/ER-6 (4,7) in
their promoter regions, respectively. The PXR DBD is also reported
to contain a bipartite nuclear localization sequence (8). The DBD is linked to the ligand-binding
domain (LBD) in PXR by a hinge region which is considerably shorter
than that observed in the majority of NRs. The LBD contains the
ligand-dependent activation function 2 region and the
ligand-binding pocket. It is possible for the LBD of PXR to
heterodimerize with the LBD of RXRa, similar to the known
structures of other NR LBDs with the RXRa LBDs (9). Conformational changes upon ligand
binding in AF-2, which are responsible for the recruitment of
coregulator proteins, lead to changes in the transcription of
target genes (Fig. 1) (10–12).
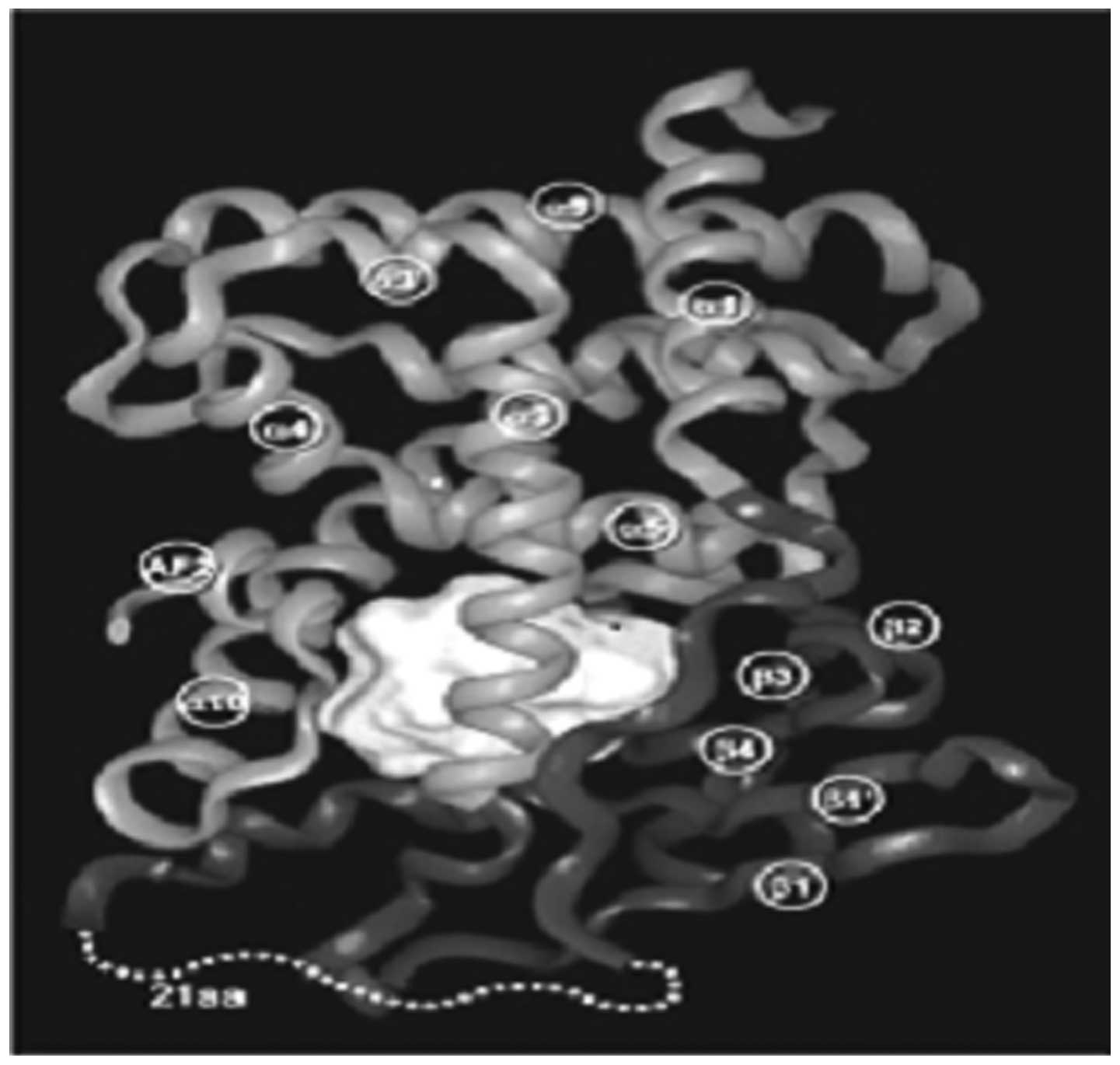
The three-dimensional structure of the LBD in PXR
reveals that it has a large, spherical ligand-binding cavity which
enables it to interact with a wide range of hydrophobic chemicals.
Structural flexibility appears to be extremely important to allow
PXR to conform to a series of ligands that differ in size and
shape. Therefore, unlike other NRs which interact selectively with
their physiological ligands, PXR serves as a generalized sensor of
hydrophobic substances (Fig.
2).
Function of PXR
Unlike the majority of NRs, the list of genes which
are regulated by PXR is growing rapidly, including not only the
systems associated with drug and xenobiotic metabolism but also
those central to cholesterol and bile acid metabolism and
excretion.
PXR has been shown to regulate the expression of
genes involved in the oxidation (phase I), conjugation (phase II)
and transport (phase III) of xenobiotics, which promote the
metabolism, elimination and detoxification of chemotherapeutic
agents. Phase I drug metabolizing enzymes (DMEs), regulated by PXR,
include a number of CYPs, carboxylesterases, alcohol and aldehyde
dehydrogenases and enzymes involved in heme production and the P450
reaction cycle (13,14). PXR is a significant regulator of the
xenobiotic-responsive expression of CYP3A genes. Accordingly, PXR
is highly expressed in the human liver and intestine, where CYP3A
is abundant and capable of metabolizing a broad range of
structurally diverse xenobiotics (15,16). A
large number of compounds that induce CYP3A expression are also PXR
activators (17). Phase II DMEs
which are regulated by PXR facilitate the excretion of phase I
biotransformed xenobiotics, including glucuronyl transferases
(UGTs), sulfotransferases (SULTs) and glutathione transfer-ases
(GSTs) (18). UGT-catalyzed
glucuronidation reactions are vital for the clearance of bilirubin
and xenobiotics. A number of UGTs, including UGT1A1, UGT1A3,
UGT1A4, UGT1A6 and UGT1A9, have been identified as PXR targets
(19–24). Numerous efflux transporters,
including ABC drug efflux transporters, breast cancer resistance
protein (BCRP), multi-drug resistance-associated proteins (MRPs)
and P-glycoprotein (P-gp) are also regulated by PXR (6,15,16,25,26).
PXR coordinately regulates a large proportion of
genes and proteins in the liver, intestine and other organs that
are involved in all aspects of the detoxification and elimination
of xenobiotics and drugs.
PXR is unique in the NR superfamily since it
responds promiscuously to a wide range of chemically-distinct
ligands from 232 to >800 Da (1–3,25,27).
The induction of CYP3A4 expression represents the basis for an
important class of drug-drug interactions in which one drug
accelerates the metabolism of other drugs. It is evident that the
majority of the prescription drugs which induce the expression of
CYP3A4 do so by activating PXR. Furthermore, PXR has also been
associated with the interaction between the herbal remedy St.
John’s wort (SJW) and prescription drugs (28). The knowledge that PXR is the
molecular basis for common drug-drug interactions should contribute
to the development of safer medicines.
Numerous studies have demonstrated new and mostly
unexpected roles for PXR in regulating inflammation, bone
homeostasis, energy homeostasis, lipid homeostasis and cancer.
Distribution of PXR
The expression of PXR is widespread in the tissues
of humans and rodents. PXR is highly expressed in the liver, small
intestine and colon in humans, rabbits, rats and mice (1–4,27–30).
Notably, these are also the same tissues where the CYP3A
genes are most highly induced and expressed. In rodents, PXR mRNA
has been detected in the kidney (31), brain (32), lung (33) stomach, ovary, placenta (34,35),
immune cells (36), peripheral
mononuclear blood cells (37,38),
heart, bone marrow and spinal cord (39). PXR is expressed not only in normal
tissues, but also in numerous types of human cancer, including
breast (40,41), osteosarcoma (42), colon (43), endometrial (44,45),
ovarian (46), prostate (47) and esophageal (48) cancers. Most significantly, the
expression levels of PXR in these cancer tissues are usually higher
than in non-neoplastic tissues.
Correlation between the expression of PXR in
various types of cancer and drug resistance
PXR and colon cancer
Of all types of cancer, studies of the correlation
between PXR and colon cancer are the most common.
Pfrunder et al(49) noted that hPXR mRNA was highly
expressed in three colon cancer cell lines, Caco-2 parental, Caco-2
TC-7 (TC-7) and LS180. Jiang et al(50) showed that the mRNA and protein
levels of PXR and MRP3 were markedly higher in colon cancer tissues
than in non-neoplastic tissues. MRP3 mRNA was significantly
correlated with PXR mRNA in cancerous and non-neoplastic colon
tissues. Furthermore, the protein level of MRP3 decreased following
stable RNA interference against PXR. The authors also observed that
PXR was able to enhance or reduce cell resistance to
chemotherapeutic agents when activated by rifampicin (RIF) or
knocked down via short hairpin RNAs (shRNA), respectively. The
results suggest that PXR may be important in human colon cancer
resistance to chemotherapeutics.
Harmsen et al(51) showed that tamoxifen, vincristine,
vinblastine, flutamide, ifosfamide, docetaxel and paclitaxel were
able to activate PXR-mediated P-gp induction and were also shown to
affect the intracellular accumulation of the P-gp probe rhodamine
123. Moreover, PXR activation was also shown to reduce the
cytotoxic activity of the P-gp substrate doxorubicin in colon
cancer cells. The results indicated that several anticancer drugs
are able to activate PXR-mediated induction of P-gp and affect the
accumulation of P-gp substrates. Habano et al(52) demonstrated that PXR promoter
methylation was involved in the regulation of intestinal PXR and
CYP3A4 mRNA expression, which may be associated with the
inter-individual variability in the drug responses of colon cancer
cells.
Raynal et al(53) investigated whether PXR was markedly
expressed in colon tumor samples and showed a great variability of
expression. The expression of hPXR in human colorectal cancer cells
led to a marked chemoresistance to SN38, the active metabolite of
the anticancer drug irinotecan. This result demonstrated that PXR
affected the tumoral metabolism of SN38 and suggested the potential
therapeutic importance of PXR quantification in the prediction of
the response to irinotecan. Basseville et al(54) reported that endogenous PXR is
activated in response to SN38 in human colon cancer cell lines. The
authors observed that endogenous PXR translocates into the nucleus
and associates with RXR upon SN38 treatment. Using ChIP, the
authors demonstrated that activated endogenous PXR binds to the
native promoter of the CYP3A4 gene to induce expression. RNA
interference experiments supported PXR’s involvement in CYP3A4
overexpression and allowed the identification of CYP3A5 and MRP2
transporter as PXR target genes. As a result, the authors observed
that cells overexpressing PXR are less sensitive to irinotecan
treatment. These results suggest that the PXR pathway is implicated
in irinotecan resistance in a colon cancer cell line via the
upregulation of specific detoxification genes.
Zheng et al(55) observed that the induction of CYP3A4
in human colon adenocarcinoma LS180 cells by hPXR agonist RIF led
to a greater metabolic clearance of 1α,25-dihydroxyvitamin D
(3) [1α, 25 (OH) (2)D (3)]
and reduced the effects of the hormone on intestinal calcium
absorption, which may contribute to an increased risk of
drug-induced osteoporosis in patients receiving long-term therapy
with hPXR agonists.
In addition, PXR has also been regarded as a
regulator of the growth and apoptosis of colon tumors. Wang et
al(56) used a xenograft model
of colon cancer to define a molecular mechanism which may underlie
PXR-driven colon tumor growth and malignancy. The activation of PXR
was able to sufficiently enhance the neoplastic characteristics of
human colon tumor cell lines and primary human colon cancer tissues
xenografted into immunodeficient mice, including cell growth,
invasion and metastasis. The authors also revealed that the
PXR-mediated phenotype required FGF19 signaling. PXR bound to the
FGF19 promoter in ‘normal’ intestinal crypt cells and human colon
tumor cells. However, while the two cell types proliferated in
response to PXR ligands, the FGF19 promoter was activated by PXR
only in cancer cells. These data may lead to improved therapeutic
regimens for colon cancer.
PXR and breast cancer
Breast cancer is the most common cancer in women
worldwide (57). Studies suggest
that PXR has a potential clinically relevant role in breast cancer.
However, the relevant pathway or target genes of PXR in breast
cancer biology and progression have not yet been fully clarified.
Dotzlaw et al(40) first
detected the expression of PXR mRNA in normal and human breast
tumor tissues. The expression of PXR mRNA did not differ between
the tumor tissues and adjacent matched normal breast tissues,
although the level of PXR mRNA did vary among the breast tumors.
The authors also observed a statistically significant inverse
correlation between the level of PXR mRNA and estrogen receptor
(ER) status, but no correlation with progesterone receptor (PR)
status. These data indicate the possibility that PXR has a
potential role in human breast tissues. Conde et al(58) processed breast tissue samples from
99 patients, including in situ, infiltrative carcinomas and
benign breast diseases, by immunohistochemistry and western blot
analysis. The results showed that cancer cells from patients who
developed recurrent tumors exhibited high cytoplasmic levels of
hPXR isoform 1 and isoform 2, while infiltrative carcinomas that
recurred showed a nuclear localization for hPXR and RXR-α.
Therefore, the overexpression and the subcellular location changes
of hPXR may be regarded as a potential new prognostic
indicator.
Miki et al(41) detected PXR in carcinoma tissues but
not in the non-neoplastic and stromal cells of breast cancers. A
marked positive correlation was detected between the PXR labeling
index and the histological grade and lymph node status of the
carcinoma cases. PXR expression was also positively correlated with
the expression of the cell proliferation marker Ki-67 in
ER-positive cases. Microarray analysis showed that organic anion
transporting polypeptide-A (OATP-A) was closely correlated with PXR
gene expression and OATP-A mRNA and protein levels were
significantly associated with PXR in breast carcinoma tissues and
derived cell lines. Meyer zu Schwabedissen et al(59) also observed that the mRNA expression
of OATP-1A2, a transporter capable of mediating the cellular uptake
of estrogen metabolites, was ∼10-fold higher in breast cancer
relative to adjacent healthy breast tissues. Of note, treatment of
breast cancer cells in vitro with the PXR agonist
RIF-induced OATP1A2 expression in a concentration-dependent and
time-dependent manner. The RIF response was abrogated following
small interfering RNA (siRNA) targeting of PXR. The authors used a
novel potent and specific antagonist of PXR (A-792611) to show the
reversal of the RIF effect on the cellular uptake of estrone
1-sulfate (E1S), an estrogen metabolite. The data indicate that PXR
and its target gene may be key to the biology of human breast
cancers and may also prove to be previously unrecognized targets
for breast cancer treatment.
Sandanaraj et al(60) showed that PXR*1B was
associated with reduced hepatic mRNA expression of PXR and its
target genes, CYP3A4 and ABCB1. Genotype-phenotype correlations in
breast cancer patients showed PXR*1B to be significantly
associated with lower doxorubicin clearance, suggesting that PXR
haplotype constitution may be important in influencing
interindividual and interethnic variations in the effects of its
putative drug substrates.
Choi et al(61) noted that TAM-resistant MCF-7
(TAMR-MCF-7) cells expressed higher levels of MRP2 than control
MCF-7 cells. Molecular analyses using MRP2 gene promoters supported
the involvement of PXR in MRP2 overexpression in TAMR-MCF-7 cells.
Chen et al(62) also
demonstrated hPXR expression in breast cancer cell lines and normal
and cancerous human breast tissue specimens. Preactivation of hPXR
by SR12813 in MCF-7 cells led to an increased resistance to
tamoxifen. A significant increase in resistance to Taxol was also
observed in MDA-MB-231 cells with hPXR preactivation. Following
activation of hPXR, the expression of MDR1 and CYP3A4, two possible
mediators of hPXR-mediated drug resistance in breast cancers was
increased. Furthermore, a knock-down of hPXR through shRNA
sensitized MCF-7 and MDA-MB-231 cells to treatment with tamoxifen,
Taxol or vinblastine. Together, the data suggest a potential role
for hPXR in breast cancer resistance to drug treatment.
PXR and gynecological oncology
Masuyama et al(44) revealed various levels of PXR
expression in endometrial cancer tissues but not normal tissues.
Tissues showing high PXR expression exhibited markedly high
expression of CYP3A4/7 and low expression of ER. HEC-1 cells, an
endometrial cancer cell line, which express high PXR and low ER and
PR, showed stronger transcriptional activity of the PXR-CYP3A
pathway to the PXR ligands than Ishikawa cells. These results
suggest that the steroid/xenobiotic metabolism may be important in
the tumor tissue through PXR-CYP3A pathway, particularly in the
alternative pathway for sex hormone and endocrine-disrupting
chemical effects on endometrial cancer expressing low ER-α. The
authors also examined whether endocrine-disrupting chemicals (EDCs)
and anticancer agents were PXR ligands. PXR-mediated transcription
was markedly activated by certain steroids/EDCs through the
CYP3A4-responsive element compared with the MDR1-responsive
element, whereas these steroids/EDCs also enhanced the expression
of CYP3A4 compared with the expression of MDR1. However, anticancer
agents, including cisplatin (CDDP) and paclitaxel, were able to
markedly activate PXR-mediated transcription through the
MDR1-responsive element compared with the CYP3A4-responsive
element, whereas these drugs were also able to enhance the MDR1
expression compared with the CYP3A4 expression (63).
Gupta et al(46) studied the presence of PXR and its
effects on ovarian cancer cells following activation by its cognate
ligand. In SKOV-3 cells, an ovarian carcinoma cell line, the
activation of PXR by cognate ligands induces target genes (CYP2B6,
CYP3A4 and UGT1A1) but not MDR1 and MRP2. PXR activation also
induced SKOV-3 cell proliferation and drug resistance. In mice with
SKOV-3 xenografts, RIF, a PXR agonist, induced cancer cell
proliferation and tumor growth. These data served as the basis for
identifying novel inhibitors of PXR activation as an approach for
controlling tumor growth and preventing induction of drug
resistance.
Takami et al(64) used the CDDP-sensitive Ishikawa cell
line and its CDDP-resistant sub-clone (ISIW+).
ISIW+ cells showed higher PXR expression. When Ishikawa
cells were cultured with PXR anti-sense oligonucleotides (AS), the
cells did not gain CDDP resistance. In SCID mice, the authors
observed that all AS-treated mice survived, whereas the controls
had 50% survival at 35 days. The data indicated that PXR is a key
factor for inducing, maintaining and reversing a CDDP-resistant
phenotype in endometrial cancer cells. Yue et al(65) observed the expression of PXR and
evaluated its clinical significance in human epithelial ovarian
carcinoma. PXR was detected in 35 of 141 (24.8%) tumor tissues and
showed significant differences with age, histology, grade, ER-α and
PR. There was a statistically significant negative correlation
between the PXR expression status and disease-free survival and
overall survival. The results indicate an association of PXR with
ER-α and PR in epithelial ovarian cancers. The data support PXR as
a potential prognostic factor in epithelial ovarian cancer and PXR
may serve as a useful marker for identifying patients at risk of
recurrence or mortality.
PXR and prostate cancer
Chen et al(47) first detected the expression of PXR
in normal and cancerous prostate tissues. Pretreatment with SR12813
enhanced the resistance of PC-3 cells to Taxol and vinblastine.
Futhermore, the PXR gene was knocked down, with PXR-targeting
shRNA. The activity of PXR towards the promoter of CYP3A4 in
PXR-ablated clones decreased compared with wild-type PC-3 cells.
The cells’ sensitivities to Taxol and vinblastine were increased by
PXR ablation. The data indicated that PXR may be important in
prostate cancer resistance to chemotherapeutic agents.
Zhang et al(66) reported a novel PXR-mediated and
metabolism-based mechanism for reducing androgenic tone. The study
showed that genetic or pharmacological activation of PXR reduced
the androgenic activity and inhibited androgen-dependent prostate
regeneration in castrated male mice receiving daily injections of
testosterone propionate by inducing the expression of
hydroxysteroid SULT2A1 and CYP3As, which are enzymes significant
for the metabolic deactivation of androgens. In human prostate
cancer cells, treatment with the PXR agonist RIF inhibited
androgen-dependent proliferation of LAPC-4 cells but had little
effect on androgen-independent isogenic LA99 cells. Downregulation
of PXR or SULT2A1 in LAPC-4 cells by siRNA abrogated the RIF
effect, indicating that the inhibitory effect of RIF on androgens
was PXR- and SULT2A1-dependent. In summary, PXR may be a potential
therapeutic target for lowering androgen activity and may
contribute to the treatment and prevention of hormone-dependent
prostate cancer.
Fujimura et al(67) investigated PXR expression in human
prostate tissues. The authors identified PXR immunore-activity
using an anti-PXR antibody in benign (n=78) and cancerous (n=106)
tissues obtained through radical prostatectomy. The authors
analyzed the associations between the clinicopathological features
of the patients, PXR status and CYP3A4 immunoreactivity. The
experimental results showed differential PXR expression in human
prostate tissues. High expression of PXR and CYP3A4 was a
significant prognostic indicator of favorable outcomes in prostate
cancer and may serve as a therapeutic target.
PXR and liver cancer
Maruyama et al(68) showed that PXR mRNA was expressed in
HepG2 cells, but not human fetal liver (HFL) cells.
To examine the role of PXR in hepatocellular
carcinoma (HCC) as a receptor activated by vitamin K2, Azuma et
al(69) established the cells
stably overexpressing PXR using an HCC cell line, HuH7.
Overexpression of PXR led to reduced proliferation and motility of
the cells. More marked inhibition of cellular proliferation and
motility was observed when PXR overexpressing clones were treated
with vitamin K2. The data indicate that the activation of PXR may
contribute to the tumor suppressing effects of vitamin K2 on HCC
cells. The GADD45β gene is a direct target of PXR and stimulates
cell signals to regulate various cellular functions. Kodama and
Negishi (70) demonstrated that PXR
activated the GADD45β gene, increased p38 MAPK phosphorylation and
caused HepG2 cells to change morphology and migrate.
PXR and esophageal cancer
Takeyama et al(48) performed immunohistochemical and
quantitative RT-PCR evaluations in human esophageal squamous cell
carcinoma (ESCC) in order to clarify the biological and clinical
significance of PXR. The authors first immunolocalized PXR in 73
human ESCC cases. PXR immunoreactivity was detected in the
cytoplasm and nuclei of carcinoma cells (20 and 98% of cases,
respectively). The level of nuclear PXR immunoreactivity was
inversely correlated with the histological grade, lymph node
metastasis status, Ki-67/MIB1 labeling index and positively
correlated with the RXR-α status. Furthermore, multivariate
analysis further demonstrated that the PXR status in carcinoma
cells was able to serve as an independent favorable prognostic
indicator of the patients. The results of quantitative RT-PCR
showed that PXR mRNA expression was detected in 60% of cases and
was notably higher in the cancerous tissues compared with the
non-neoplastic tissues of the patients. This was the first study to
detect the status of PXR in human ESCC and the data indicate that
PXR is a significant favorable prognostic factor of human ESCC.
van de Winkel et al(71) demonstrated PXR expression in
Barrett’s esophagus (BE) and esophageal adenocarcinoma tissue and
showed its nuclear localization in adenocarcinoma tissue. Upon
activation with lithocholic acid, PXR translocated to the nuclei of
OE19 adenocarcinoma cells. Together with the observed association
of a PXR polymorphism and BE, the data suggest that PXR may have a
potential role in the prediction and treatment of esophageal
disease. The authors also revealed that PXR was able to separate
high-grade dysplasia (HGD) from low-grade dysplasia (LGD) and no
dysplasia (ND). PXR also appears to have diagnostic and prognostic
value, but future prospective studies are required to investigate
its predictive ability for neoplastic progression in BE (72).
PXR and leukemia
Kawai et al(73) first identified the expression of PXR
in HL-60 human promyelocytic leukemic cells in 2003.
All-trans-retinoic acid (ATRA) is an effective
treatment for acute promyelocytic leukemia and several solid
tumors, but its function is limited by resistance caused by
increased metabolism. Wang et al(74) designed a study to demonstrate the
role of PXR in ATRA metabolism. The study indicated that the
coadministration of PXR ligands was able to increase the ATRA
metabolism through the activation of the PXR-CYP3A pathway, which
may be a mechanism for the ATRA resistance. Other PXR target
transporters may also be implicated.
PXR and osteosarcoma
Mensah-Osman et al(42) observed differences in the molecular
size of the PXR protein expressed in sarcoma cell lines and the
wild-type PXR expressed in the normal liver, small intestine or
kidney. A polyclonal PXR antibody raised against the N-terminus of
the wild-type PXR did not detect PXR expressed in the OS187, WOL
and COL osteosarcoma cell lines. In these osteosarcoma cell lines,
etoposide and doxorubicin were better inducers of P450 3A4 and MDR1
compared with RIF. siRNA against PXR down-regulated P450 3A4
expression levels only in the osteosarcoma cell line. Cytotoxicity
assays indicated that the resistance of the osteosarcoma cell lines
to etoposide correlated with the PXR protein expression levels and
activation of P450 3A4 and was suppressed by ketoconazole. The
results suggest that PXR is important in the regulation of P450 3A4
expression in osteosarcoma and its expression and activation may
influence the effects of chemotherapeutic agents which induce PXR
target genes implicated in drug resistance.
PXR expression is low or nonexistent in the lung,
stomach, pancreas, kidneys, brain and other organs and there have
been few studies of the correlation between PXR and these organic
tumors at present.
PXR and tumor cell apoptosis
Studies have demonstrated that PXR is implicated in
the apoptosis of tumor cells and may be an important factor in
MDR.
In 2005, Zucchini et al(75) demonstrated that PXR expression was
required for Bcl-2 and Bcl-xL upregulation upon PXR activator
treatment in human and rat hepatocytes. PXR may protect the liver
against harmful chemicals by simultaneously regulating
detoxification and the cell apoptotic pathway.
Wang et al(76) examined the role of PXR in
lipopolysaccharide (LPS)/D-galactosamine (GalN)-induced acute liver
injury using PXR-null and wild-type mice. LPS/GalN-treated PXR-null
mice had more marked increases in alanine transaminase (ALT),
hepatocyte apoptosis, necrosis and hemorrhagic liver injury
compared with wild-type mice. LPS/GalN-mediated phosphorylation of
JNK1/2 and ERK1/2 was differentially regulated in PXR-null and
wild-type mice. In addition, LPS/GalN-induced hepatic Stat3
survival signaling was impaired and early activation of Jak2 was
delayed in PXR-null mice. After LPS/GalN treatment, the expression
levels of the pro-survival proteins heme oxygenase-1 (HO-1) and
Bcl-xL, which are downstream of Stat3, were markedly lower in
PXR-null compared with wild-type mouse livers. The lack of PXR
resulted in a significant reduction of LC3B-I and -II as well as
Beclin-1 protein levels following LPS/GalN treatment. PXR is also
implicated in hepatocyte homeostasis. Taken together, the results
indicate that PXR is a key hepato-protective factor.
Masuyama et al(45) demonstrated that PXR overexpression
led to a marked decrease in endometrial cancer cell growth
inhibition and inhibited apoptosis in the presence of CDDP or
paclitaxel. The data implied that PXR downregulation may be a novel
therapeutic approach for the augmentation of sensitivity to
anticancer agents or even to overcome resistance to them, in the
treatment of endometrial cancer.
In a previous study, SuperArray analysis showed that
PXR-mediated deoxycholic acid resistance was associated with the
upregulation of multiple anti-apoptotic genes, including BIRC2,
BAG3 and MCL-1 and downregulation of proapoptotic genes, such as
TP53/p53 and BAK1 in human colon cancer cells (43).
However, a number of studies produced the opposite
results. Ouyang et al(77)
showed that PXR suppressed the proliferation and tumorigenicity of
colon cancer cells by controlling the cell cycle at the
G0/G1 phase by regulating the E2F/Rb and p21
(WAF1/CIP1) pathways. Verma et al(78) also observed that the activation of
PXR was antiproliferative in p53 wild-type breast cancer cells and
this effect was mechanistically dependent upon the local production
of NO and NO-dependent upregulation of p53. This finding revealed a
novel biological function of PXR and suggested that a subset of PXR
activators may serve as effective therapeutic and
chemo-preventative agents for certain types of breast cancers. Liu
et al(79) also investigated
whether Tanshinone IIA (Tan IIA) has significant growth inhibition
effects on U-937 cells (a human leukemic monocyte lymphoma cell
line) through the induction of apoptosis. Tan IIA-induced apoptosis
may result from the activation of PXR, which inhibits the activity
of NF-κB and leads to the downregulation of monocyte
chemoattractant protein (MCP)-1 (MCP-1/CCL2) expression.
Preventing drug resistance by regulating
PXR
The hypothesis that downregulating PXR in
PXR-positive cancers increases the sensitivity of cancer cells to
chemotherapeutic agents has been proposed and investigated in
several studies. As previously mentioned, Masuyama et
al(44) showed that the
downregulation of PXR by siRNA in the endometrial cancer cell line
HEC-1 decreased the expression of MDR1 and sensitized cells to the
anticancer agents paclitaxel and CDDP. Chen et al(61) showed that treatment with the PXR
agonist SR12813 activated PXR in breast cancer cell lines and
increased the expression of MDR1 and CYP3A4 and the resistance of
cells to paclitaxel, vinblastine and tamoxifen. By contrast, the
targeted knockdown of PXR using shRNA enhanced the sensitivity of
the cells to the anticancer drugs.
One approach to overcoming PXR activation is to
chemically modify the lead compound and remove its PXR-activating
function without compromising the target activity. Several studies
have shown this concept to be possible in principle. For example,
docetaxel and paclitaxel, two inhibitors of microtubule
disassembly, have minor structural differences and equal potencies
in inhibiting microtubule depolymerization and cancer cell
proliferation. However, paclitaxel, but not docetaxel,
significantly activates PXR and regulates MDR1 expression (25). Zimmermann et al(80) reported that chemical modifications
to the first generation IGF-1R inhibitors reduce PXR
transactivation while maintaining potency against IGF-1R. However,
as a result of the agonist promiscuity of PXR, an extremely large
amount of effort is required in drug development programs to remove
the PXR activity while maintaining the target activity for the lead
compounds.
PXR antagonists that cause PXR inhibition have been
demonstrated to competitively bind to PXR using in vitro
binding assays. Ecteinascidin-743 (ET-743), an antineoplastic
agent, has been demonstrated to suppress PXR transactivation
(25). A-792611, a HIV protease
inhibitor, inhibits PXR-mediated CYP3A4 expression (81). Ketoconazole, an inhibitor of CYP3A4
enzyme activity, is able to inhibit a number of NRs, including PXR,
by disrupting the NR-coactivator interaction (82). Sulforaphane (SFN), an inhibitor of
histone deacetylases and an inducer of phase II DMEs, shows PXR
antagonist activity (83). SFN
down-regulates CYP3A4 expression by directly binding to PXR and
inhibiting coactivator recruitment. Raynal et al showed that
the activation of PXR reduced the chemosensitivity of colorectal
cancer cells to irinotecan. Notably, the reduction in
chemosensitivity could be reversed by SFN (53).
Conclusions and prospects
Overall, the expression of PXR is high in numerous
types of tumors. Studies have demonstrated that PXR has a
significant role in the drug resistance, proliferation, apoptosis
and invasion of PXR-positive tumor cells. Therefore, PXR may be
treated as a potentially key target in comprehensive cancer
treatment and the prevention of drug resistance by regulating PXR
expression is a novel and effective approach for oncotherapy.
PXR was discovered relatively recently and its
structure and function have not yet been fully clarified. The
correlation between PXR and multidrug resistance of tumors requires
further study.
References
|
1
|
Lehmann JM, McKee DD, Watson MA, et al:
The human orphan nuclear receptor PXR is activated by compounds
that regulate CYP3A4 gene expression and cause drug interactions. J
Clin Invest. 102:1016–1023. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertilsson G, Heidrich J, Svensson K, et
al: Identification of a human nuclear receptor defines a new
signaling pathway for CYP3A induction. Proc Natl Acad Sci USA.
95:12208–12213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kliewer SA, Moore JT, Wade L, et al: An
orphan nuclear receptor activated by pregnanes defines a novel
steroid signaling pathway. Cell. 92:73–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blumberg B, Sabbagh W Jr, Juguilon H, et
al: SXR, a novel steroid and xenobiotic-sensing nuclear receptor.
Genes Dev. 12:3195–3205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kast HR, Goodwin B, Tarr PT, et al:
Regulation of multidrug resistance-associated protein 2 (ABCC2) by
the nuclear receptors pregnane X receptor, farnesoid X-activated
receptor, and constitutive androstane receptor. J Biol Chem.
277:2908–2915. 2002. View Article : Google Scholar
|
|
6
|
Geick A, Eichelbaum M and Burk O: Nuclear
receptor response elements mediate induction of intestinal MDR1 by
rifampin. J Biol Chem. 276:14581–14587. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goodwin B, Hodgson E and Liddle C: The
orphan human pregnane X receptor mediates the transcriptional
activation of CYP3A4 by rifampicin through a distal enhancer
module. Mol Pharmacol. 56:1329–1339. 1999.PubMed/NCBI
|
|
8
|
Kawana K, Ikuta T, Kobayashi Y, Gotoh O,
Takeda K and Kawajiri K: Molecular mechanism of nuclear
translocation of an orphan nuclear receptor, SXR. Mol Pharmacol.
63:524–531. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Kuehl P, Green ED, Touchman JW,
et al: The human pregnane X receptor: genomic structure and
identification and functional characterization of natural allelic
variants. Pharmacogenetics. 11:555–572. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Renaud JP, Rochel N, Ruff M, Vivat V,
Chambon P, Gronemeyer H and Moras D: Crystal structure of the
RAR-gamma ligand-binding domain bound to all-trans retinoic acid.
Nature. 378:681–689. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu HE, Stanley TB, Montana VG, et al:
Structural basis for antagonist-mediated recruitment of nuclear
co-repressors by PPARalpha. Nature. 415:813–817. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nolte RT, Wisely GB, Westin S, et al:
Ligand binding and co-activator assembly of the peroxisome
proliferator-activated receptor-gamma. Nature. 395:137–143. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watkins RE, Maglich JM, Moore LB, et al:
2.1 A crystal structure of human PXR in complex with the St. John’s
wort compound hyperforin. Biochemistry. 42:1430–1438. 2003.
|
|
14
|
Rosenfeld JM, Vargas R Jr, Xie W and Evans
RM: Genetic profiling defines the xenobiotic gene network
controlled by the nuclear receptor pregnane X receptor. Mol
Endocrinol. 17:1268–1282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guengerich FP: Cytochrome P-450 3A4:
regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol.
39:1–17. 1999. View Article : Google Scholar
|
|
16
|
Wrighton SA, Schuetz EG, Thummel KE, Shen
DD, Korzekwa KR and Watkins PB: The human CYP3A subfamily:
practical considerations. Drug Metab Rev. 32:339–361. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moore LB, Parks DJ, Jones SA, et al:
Orphan nuclear receptors constitutive androstane receptor and
pregnane X receptor share xenobiotic and steroid ligands. J Biol
Chem. 275:15122–15127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Runge-Morris M, Wu W and Kocarek TA:
Regulation of rat hepatic hydroxysteroid sulfotransferase
(SULT2-40/41) gene expression by glucocorticoids: evidence for a
dual mechanism of transcriptional control. Mol Pharmacol.
56:1198–1206. 1999.
|
|
19
|
Xie W, Yeuh MF, Radominska-Pandya A, et
al: Control of steroid, heme, and carcinogen metabolism by nuclear
pregnane X receptor and constitutive androstane receptor. Proc Natl
Acad Sci USA. 100:4150–4155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen C, Staudinger JL and Klaassen CD:
Nuclear receptor, pregname X receptor, is required for induction of
UDP-glucuronosyltranferases in mouse liver by pregnenolone-16
alpha-carbonitrile. Drug Metab Dispos. 31:908–915. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yueh MF, Huang YH, Hiller A, Chen S,
Nguyen N and Tukey RH: Involvement of the xenobiotic response
element (XRE) in Ah receptor-mediated induction of human
UDP-glucuronosyltransferase 1A1. J Biol Chem. 278:15001–15006.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shelby MK and Klaassen CD: Induction of
rat UDP-glucuronosyltransferases in liver and duodenum by
microsomal enzyme inducers that activate various transcriptional
pathways. Drug Metab Dispos. 34:1772–1778. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vyhlidal CA, Rogan PK and Leeder JS:
Development and refinement of pregnane X receptor (PXR) DNA binding
site model using information theory: insights into PXR-mediated
gene regulation. J Biol Chem. 279:46779–46786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buckley DB and Klaassen CD: Induction of
mouse UDP-glucuronosyltransferase mRNA expression in liver and
intestine by activators of aryl-hydrocarbon receptor, constitutive
androstane receptor, pregnane X receptor, peroxisome
proliferator-activated receptor alpha, and nuclear factor erythroid
2-related factor 2. Drug Metab Dispos. 37:847–856. 2009.
|
|
25
|
Synold TW, Dussault I and Forman BM: The
orphan nuclear receptor SXR coordinately regulates drug metabolism
and efflux. Nat Med. 7:584–590. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kast HR, Goodwin B, Tarr PT, et al:
Regulation of multidrug resistance-associated protein 2 (ABCC2) by
the nuclear receptors pregnane X receptor, farnesoid X-activated
receptor, and constitutive androstane receptor. J Biol Chem.
277:2908–2915. 2002. View Article : Google Scholar
|
|
27
|
Jones SA, Moore LB, Shenk JL, et al: The
pregnane X receptor: a promiscuous xenobiotic receptor that has
diverged during evolution. Mol Endocrinol. 14:27–39. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wentworth JM, Agostini M, Love J, Schwabe
JW and Chatterjee VK: St John’s wort, a herbal antidepressant,
activates the steroid X receptor. J Endocrinol. 166:R11–R16.
2000.
|
|
29
|
Savas U, Wester MR, Griffin KJ and Johnson
EF: Rabbit pregnane X receptor is activated by rifampicin. Drug
Metab Dispos. 28:529–537. 2000.PubMed/NCBI
|
|
30
|
Zhang H, LeCulyse E, Liu L, Hu M, Matoney
L, Zhu W and Yan B: Rat pregnane X receptor: molecular cloning,
tissue distribution, and xenobiotic regulation. Arch Biochem
Biophys. 368:14–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng X and Klaassen CD: Regulation of
mRNA expression of xenobiotic transporters by the pregnane X
receptor in mouse liver, kidney, and intestine. Drug Metab Dispos.
34:1863–1867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bauer B, Hartz AM, Fricker G and Miller
DS: Pregnane X receptor up-regulation of P-glycoprotein expression
and transport function at the blood-brain barrier. Mol Pharmacol.
66:413–419. 2004.PubMed/NCBI
|
|
33
|
Chirulli V, Longo V, Marini S, Mazzaccaro
A, Fiorio R and Gervasi PG: CAR and PXR expression and inducibility
of CYP2B and CYP3A activities in rat and rabbit lungs. Life Sci.
76:2535–2546. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Staudinger JL, Goodwin B, Jones SA, et al:
The nuclear receptor PXR is a lithocholic acid sensor that protects
against liver toxicity. Proc Natl Acad Sci USA. 98:3369–3374. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Masuyama H, Hiramatsu Y, Mizutani Y,
Inoshita H and Kudo T: The expression of pregnane X receptor and
its target gene, cytochrome P450 3A1, in perinatal mouse. Mol Cell
Endocrinol. 172:47–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dubrac S, Elentner A, Ebner S,
Horejs-Hoeck J and Schmuth M: Modulation of T lymphocyte function
by the pregnane X receptor. J Immunol. 184:2949–2957. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Owen A, Chandler B, Back DJ and Khoo SH:
Expression of pregnane-X-receptor transcript in peripheral blood
mononuclear cells and correlation with MDR1 mRNA. Antivir Ther.
9:819–821. 2004.PubMed/NCBI
|
|
38
|
Albermann N, Schmitz-Winnenthal FH,
Z’graggen K, et al: Expression of the drug transporters MDR1/ABCB1,
MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood
mononuclear cells and their relationship with the expression in
intestine and liver. Biochem Pharmacol. 70:949–958. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lamba V, Yasuda K, Lamba JK, Assem M,
Davila J, Strom S and Schuetz EG: PXR (NR1I2): splice variants in
human tissues, including brain, and identification of neurosteroids
and nicotine as PXR activators. Toxicol Appl Pharmacol.
199:251–265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dotzlaw H, Leygue E, Watson P and Murphy
LC: The human orphan receptor PXR messenger RNA is expressed in
both normal and neoplastic breast tissue. Clin Cancer Res.
5:2103–2107. 1999.PubMed/NCBI
|
|
41
|
Miki Y, Suzuki T, Kitada K, et al:
Expression of the steroid and xenobiotic receptor and its possible
target gene, organic anion transporting polypeptide-A, in human
breast carcinoma. Cancer Res. 66:535–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mensah-Osman EJ, Thomas DG, Tabb MM, et
al: Expression levels and activation of a PXR variant are directly
related to drug resistance in osteosarcoma cell lines. Cancer.
109:957–965. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou J, Liu M, Zhai Y and Xie W: The
antiapoptotic role of pregnane X receptor in human colon cancer
cells. Mol Endocrinol. 22:868–880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Masuyama H, Hiramatsu Y, Kodama J and Kudo
T: Expression and potential roles of pregnane X receptor in
endometrial cancer. J Clin Endocrinol Metab. 88:4446–4454. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Masuyama H, Nakatsukasa H, Takamoto N and
Hiramatsu Y: Down-regulation of pregnane X receptor contributes to
cell growth inhibition and apoptosis by anticancer agents in
endometrial cancer cells. Mol Pharmacol. 72:1045–1053. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gupta D, Venkatesh M, Wang H, et al:
Expanding the roles for pregnane X receptor in cancer:
proliferation and drug resistance in ovarian cancer. Clin Cancer
Res. 14:5332–5340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen Y, Tang Y, Wang MT, Zeng S and Nie D:
Human pregnane X receptor and resistance to chemotherapy in
prostate cancer. Cancer Res. 67:10361–10367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takeyama D, Miki Y, Fujishima F, Suzuki T,
Akahira J, Hata S, Miyata G, Satomi S and Sasano H: Steroid and
xenobiotic receptor in human esophageal squamous cell carcinoma: a
potent prognostic factor. Cancer Sci. 101:543–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pfrunder A, Gutmann H, Beglinger C and
Drewe J: Gene expression of CYP3A4, ABC-transporters (MDR1 and
MRP1-MRP5) and hPXR in three different human colon carcinoma cell
lines. J Pharm Pharmacol. 55:59–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang H, Chen K, He J, Pan F, Li J, Chen
J, Chen W and Liang H: Association of pregnane X receptor with
multidrug resistance-related protein 3 and its role in human colon
cancer chemoresistance. J Gastrointest Surg. 13:1831–1838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Harmsen S, Meijerman I, Febus CL,
Maas-Bakker RF, Beijnen JH and Schellens JH: PXR-mediated induction
of P-glycoprotein by anticancer drugs in a human colon
adeno-carcinoma-derived cell line. Cancer Chemother Pharmacol.
66:765–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Habano W, Gamo T, Terashima J, Sugai T,
Otsuka K, Wakabayashi G and Ozawa S: Involvement of promoter
methylation in the regulation of pregnane X receptor in colon
cancer cells. BMC Cancer. 11:812011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Raynal C, Pascussi JM, Leguelinel G, et
al: Pregnane X Receptor (PXR) expression in colorectal cancer cells
restricts irinotecan chemosensitivity through enhanced SN-38
glucuronidation. Mol Cancer. 9:462010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Basseville A, Preisser L, de Carné
Trécesson S, Boisdron-Celle M, Gamelin E, Coqueret O and Morel A:
Irinotecan induces steroid and xenobiotic receptor (SXR) signaling
to detoxification pathway in colon cancer cells. Mol Cancer.
10:802011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zheng XE, Wang Z, Liao MZ, Lin YS, Shuhart
MC, Schuetz EG and Thummel KE: Human PXR-mediated induction of
intestinal CYP3A4 attenuates 1α,25-dihydroxyvitamin D3
function in human colon adenocarcinoma LS180 cells. Biochem
Pharmacol. 84:391–401. 2012.PubMed/NCBI
|
|
56
|
Wang H, Venkatesh M, Li H, et al: Pregnane
X receptor activation induces FGF19-dependent tumor aggressiveness
in humans and mice. J Clin Invest. 121:3220–3232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Khan F, Amatya B, Ng L, Demetrios M, Zhang
NY and Turner-Stokes L: Multidisciplinary rehabilitation for
follow-up of women treated for breast cancer. Cochrane Database
Syst Rev. 12:CD0095532012.PubMed/NCBI
|
|
58
|
Conde I, Lobo MV, Zamora J, Pérez J,
González FJ, Alba E, Fraile B, Paniagua R and Arenas MI: Human
pregnane X receptor is expressed in breast carcinomas, potential
heterodimers formation between hPXR and RXR-alpha. BMC Cancer.
8:1742008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Meyer zu Schwabedissen HE, Tirona RG, Yip
CS, Ho RH and Kim RB: Interplay between the nuclear receptor
pregnane X receptor and the uptake transporter organic anion
transporter polypeptide 1A2 selectively enhances estrogen effects
in breast cancer. Cancer Res. 68:9338–9347. 2008.
|
|
60
|
Sandanaraj E, Lal S, Selvarajan V, Ooi LL,
Wong ZW, Wong NS, Ang PC, Lee EJ and Chowbay B: PXR
pharmacogenetics: association of haplotypes with hepatic CYP3A4 and
ABCB1 messenger RNA expression and doxorubicin clearance in Asian
breast cancer patients. Clin Cancer Res. 14:7116–7126. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Choi HK, Yang JW, Roh SH, Han CY and Kang
KW: Induction of multidrug resistance associated protein 2 in
tamoxifen-resistant breast cancer cells. Endocr Relat Cancer.
14:293–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen Y, Tang Y, Chen S and Nie D:
Regulation of drug resistance by human pregnane X receptor in
breast cancer. Cancer Biol Ther. 8:1265–1272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Masuyama H, Suwaki N, Tateishi Y,
Nakatsukasa H, Segawa T and Hiramatsu Y: The pregnane X receptor
regulates gene expression in a ligand-and promoter-selective
fashion. Mol Endocrinol. 19:1170–1180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Takami N, Sakamoto H and Yamamoto T:
Steroid and xenobiotic receptor (SXR) is a key system for the
acquisition of cisplatin resistance in endometrial cancer cells. J
Int Med Res. 31:59–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yue X, Akahira J, Utsunomiya H, et al:
Steroid and Xenobiotic Receptor (SXR) as a possible prognostic
marker in epithelial ovarian cancer. Pathol Int. 60:400–406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang B, Cheng Q, Ou Z, et al: Pregnane X
receptor as a therapeutic target to inhibit androgen activity.
Endocrinology. 151:5721–5729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fujimura T, Takahashi S, Urano T, et al:
Clinical significance of steroid and xenobiotic receptor and its
targeted gene CYP3A4 in human prostate cancer. Cancer Sci.
103:176–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Maruyama M, Matsunaga T, Harada E and
Ohmori S: Comparison of basal gene expression and induction of
CYP3As in HepG2 and human fetal liver cells. Biol Pharm Bull.
30:2091–2097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Azuma K, Urano T, Ouchi Y and Inoue S:
Vitamin K2 suppresses proliferation and motility of hepatocellular
carcinoma cells by activating steroid and xenobiotic receptor.
Endocr J. 56:843–849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kodama S and Negishi M: Pregnane X
receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK
signal and cell migration. J Biol Chem. 286:3570–3578. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
van de Winkel A, Menke V, Capello A, et
al: Expression, localization and polymorphisms of the nuclear
receptor PXR in Barrett’s esophagus and esophageal adenocarcinoma.
BMC Gastroenterol. 11:1082011.PubMed/NCBI
|
|
72
|
van de Winkel A, van Zoest KP, van Dekken
H, Moons LM, Kuipers EJ and van der Laan LJ: Differential
expression of the nuclear receptors farnesoid X receptor (FXR) and
pregnane X receptor (PXR) for grading dysplasia in patients with
Barrett’s oesophagus. Histopathology. 58:246–253. 2011.PubMed/NCBI
|
|
73
|
Kawai M, Chen J, Cheung CY and Chang TK:
Transcript profiling of cytochrome P450 genes in HL-60 human
leukemic cells: upregulation of CYP1B1 by all-trans-retinoic acid.
Mol Cell Biochem. 248:57–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang T, Ma X, Krausz KW, Idle JR and
Gonzalez FJ: Role of pregnane X receptor in control of all-trans
retinoic acid (ATRA) metabolism and its potential contribution to
ATRA resistance. J Pharmacol Exp Ther. 324:674–684. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zucchini N, de Sousa G, Bailly-Maitre B,
Gugenheim J, Bars R, Lemaire G and Rahmani R: Regulation of Bcl-2
and Bcl-xL anti-apoptotic protein expression by nuclear receptor
PXR in primary cultures of human and rat hepatocytes. Biochim
Biophys Acta. 1745:48–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang K, Damjanov I and Wan YJ: The
protective role of pregnane X receptor in
lipopolysaccharide/D-galactosamine-induced acute liver injury. Lab
Invest. 90:257–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ouyang N, Ke S, Eagleton N, Xie Y, Chen G,
Laffins B, Yao H, Zhou B and Tian Y: Pregnane X receptor suppresses
proliferation and tumourigenicity of colon cancer cells. Br J
Cancer. 102:1753–1761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Verma S, Tabb MM and Blumberg B:
Activation of the steroid and xenobiotic receptor, SXR, induces
apoptosis in breast cancer cells. BMC Cancer. 9:32009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu C, Li J, Wang L, Wu F, Huang L, Xu Y,
Ye J, Xiao B, Meng F, Chen S and Yang M: Analysis of tanshinone IIA
induced cellular apoptosis in leukemia cells by genome-wide
expression profiling. BMC Complement Altern Med. 12:52012.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zimmermann K, Wittman MD, Saulnier MG, et
al: SAR of PXR transactivation in benzimidazole-based IGF-1R kinase
inhibitors. Bioorg Med Chem Lett. 20:1744–1748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Healan-Greenberg C, Waring JF, Kempf DJ,
Blomme EA, Tirona RG and Kim RB: A human immunodeficiency virus
protease inhibitor is a novel functional inhibitor of human
pregnane X receptor. Drug Metab Dispos. 36:500–507. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang H, Wang H, Sinz M, et al: Inhibition
of drug metabolism by blocking the activation of nuclear receptors
by ketoconazole. Oncogene. 26:258–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhou C, Poulton EJ, Grün F, Bammler TK,
Blumberg B, Thummel KE and Eaton DL: The dietary isothiocyanate
sulforaphane is an antagonist of the human steroid and xenobiotic
nuclear receptor. Mol Pharmacol. 71:220–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kliewer SA, Goodwin B and Willson TM: The
nuclear pregnane X receptor: a key regulator of xenobiotic
metabolism. Endocr Rev. 23:687–702. 2002. View Article : Google Scholar : PubMed/NCBI
|