Introduction
It has been observed that gastric cancer incidence
is elevated in farm workers involved in the cultivation of citrus
fruits and in workers exposed to high levels of the herbicides
2,4-D and trifluran, the insecticides chlordane and malathion, the
fungicides mancozeb and maneb, the fumigant methyl bromide and the
acaricide propargite (1). Four of
the chemicals associated with elevated gastric cancer incidence
(chlordane, maneb, mancozeb and propargite) are class B2 chemicals
(probable carcinogens) as classified by the United States
Environmental Protection Agency (2006), while trifluran and
simazine are class C chemicals (possible carcinogens).
The gastric cancer subsite distribution was
approximately the same between farm workers and Hispanic males in
California from 1990–1994. Among the farm workers, 14% of cancer
cases were of the gastric cardia compared with 18.7% in Californian
Hispanic males (2). Similarly,
among the farm workers, 61% of cancer cases were classified as
adenocarcinoma, not otherwise specified, and 64% of Californian
Hispanic males were also of this histological type (1).
Dichlorvos has been widely used as an insecticide
for >50 years. Its carcinogenic potential has been studied
extensively and in total there have been 11 long-term studies in
rodents; six in rats and five in mice (3). Numerous studies have been carried out
on the toxicity of dimethoate in non-target animals and humans.
Dimethoate was demonstrated to have an effect on reproductive and
endocrine function, enzymatic changes, immunotoxicity, brain
acetylcholinesterase activity, proteins and carbohydrate metabolism
(4–8).
Few studies have been carried out on the effects of
dimethoate in gastric cancer. The present study aimed to
investigate the changes in p16, Bcl-2 and
c-myc gene expression in the gastric tissue of mice
following perfusion with the organophosphorous pesticides
dichlorvos and dimethoate, which are extensively used in Chinese
agricultural areas.
Materials and methods
Animals and treatment
Male Kunming mice, weighing 180–200 g, were divided
randomly into five equal groups, each containing 6 male mice. The
mice were purchased from Shandong University (Shandong, China). The
mice were routinely screened for common mouse pathogens. The mice
used in this study were housed in groups of 6 in stainless steel
cages (50×40×25 cm) in rooms determined to be free of common
pathogens under standard conditions (24±2°C and 50±5% humidity)
with a 12-h light-dark cycle. The groups were perfused with 0, 5,
10, 20 or 40 mg/kg/day dichlorvos suspended in sterile saline (200
μl, 0.9% NaCl) or 0, 2.5, 5, 10 or 20 mg/kg/day dimethoate
suspended in sterile saline (200 μl, 0.9% NaCl). With the
exception of during treatment, the mice had free access to food and
water.
After three weeks, all mice were killed by
decapitation for the experiments. The esophagus and duodenum were
removed and gastric tissue was excised and washed with sterile
saline. The tissue was rapidly frozen in liquid nitrogen and stored
at −80°C. All animal procedures were approved by Shandong Wanjie
College Animal Investigational Committee and performed in
accordance with the Guide for the Care and Use of Laboratory
Animals published by Ministry of Health People’s Republic of
China.
Hematological investigation
Blood samples were collected from the hearts of the
mice for hematological investigation. The parameters investigated
were the hemoglobin content, hematocrit value (PCV), red blood cell
count and total plasma protein (9–11).
Expression analysis of p16, Bcl-2 and
c-myc by real-time quantitative (qRT)-PCR
Total RNA was isolated from <100 mg of tissue by
using TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. Total RNA was
quantified by determination of the optical density at 260 nm.
First-strand cDNA was produced following instructions for AMV
reverse transcriptase (AMV RT) with slight modifications. In the
reverse transcription reaction system, 2 μg total RNA, 10
μl AMV buffer, 75 pmol oligo(dT18) and 5
μl of each of the four deoxynucleotide triphosphates (10 mM)
were contained in a total 50 μl reaction volume. The mixture
was incubated at 95°C for 5 min and then 40 units RNase, 10 units
AMV RT (Promega, Madison, WI, USA) and 7.5 μl 25 mM
MgCl2 were added. The mixture was incubated at 42°C for
60 min and heated to 95°C for 5 min to inactivate the reverse
transcriptase. The cDNA product was stored at −20°C.
Oligonucleotides for qRT-PCR (Table I) were designed using Primer Premier
5.0 software (Premier Biosoft International). A BLAST analysis was
used against other organism genome sequences for specificity
confidence (http://www.ncbi.nlm.nih.gov/BLAST/). The Mfold web
server was applied to avoid positioning on unstable secondary
structures. The primer specificity was analyzed before qRT-PCR.
 | Table IReal-time quantitative PCR
primers. |
Table I
Real-time quantitative PCR
primers.
| Primer | Sequence (5′-3′) |
|---|
| GAPDHF |
TGTGTCCGTCGTGGATCTGA |
| GAPDHR |
CCTGCTTCACCACCTTCTTGA |
| p16F |
GTACCCCGATTCAGGTGATG |
| p16R |
TTAGCTCTGCTCTTGGGATTG |
| Bcl-2F |
AGGAGCAGGTGCCTACAAGA |
| Bcl-2R |
GCATTTTCCCACCACTGTCT |
| c-mycF |
GGTGGAAAACCAGGTAAGCA |
| c-mycR |
CCTTCTCCTCTGCCATCTTC |
The reaction mixture (final volume, 20 μl)
consisted of 10 μl SYBR Ex Taq II (Applied
Biosystems, Carlsbad, CA, USA), 0.4 μl ROX reference dye,
0.8 μl of each primer (final concentration, 250 nM),
7 μl ddH2O and 1 μl cDNA (dilution factor,
1/10). The thermocycling program consisted of two phases; one cycle
at 95°C for 4 min, 40 cycles at 95°C for 15 sec, 52°C for 25 sec
and 72°C for 35 sec. Following completion of these cycles,
melting-curve data were collected to verify the PCR specificity,
contamination and absence of primer dimers. Each sample was tested
in triplicate in a StepOnePlus™ Real-Time PCR apparatus (Applied
Biosystems).
The relative expression levels of p16, Bcl-2
and c-myc were normalized against GAPDH. The normalized
relative gene expression levels (indicated as n-fold induction)
were evaluated by the Real-Time PCR System Cycler software. The
formula was determined as: ΔCttreatment (the threshold
cycle of MT of treated T. thermophila) = CtMT
treatment − Ct17S treatment; ΔCtcontrol
(the threshold cycle of MT of controlled T. thermophila) =
CtMT control − Ct17S control; ΔΔCt =
Cttreatment − Ctcontrol; and the relative
quantity levels of MT mRNA expression were 2−CtΔΔ
(12).
Statistical analysis
All hematological results are presented as the mean
± SD. Data were evaluated using SPSS software (SPSS company, USA).
Statistical differences were determined using Student’s t-test with
a significance level set at P<0.05.
Results
Hematological studies
At the end of the treatment period, no teratogenic
mice were observed. The blood parameters investigated were the
hemoglobin content, PCV, red blood cell counts and total plasma
protein. The results were statistically analyzed using Student’s
t-test and are shown in Table
II.
 | Table IIHematological studies of mice exposed
to dichlorvos. |
Table II
Hematological studies of mice exposed
to dichlorvos.
| Dichlorvos dose
(mg/kg/day)
|
|---|
| Parameter | 0 | 5 | 10 | 20 | 40 |
|---|
| Hb (mg/100 ml) | 13.33±1.97 | 12.83±2.12 | 12.15±2.13 | 10.95±1.59a | 9.85±1.67b |
| PCV (%) | 41.0±2.77 | 40.35±3.17 | 39.86±4.69 | 34.9±3.68a | 32.63±4.41b |
| Total plasma protein
(×1012/l) | 50.52±6.22 | 50.64±6.20 | 50.0±5.98 | 50.02±6.08 | 49.08±7.21 |
| RBC count (mg/100
ml) | 7.41±0.32 | 7.37±0.40 | 7.42±0.22 | 7.38±0.96 | 7.39±0.26 |
The hemoglobin content and PCVs showed significant
decline in mice treated with 20 mg/kg/day dichlorvos (P<0.05)
when compared with control values and the decline was also
significant in mice treated with 40 mg/kg/day dichlorvos
(P<0.001). The plasma protein was investigated quantitatively
and qualitatively. The total plasma protein content was not
significantly different between the mice treated with different
doses of dichlorvos and those from the control group. The
electrophoretic investigation showed no significant differences
between the differential bands of plasma proteins in the treated
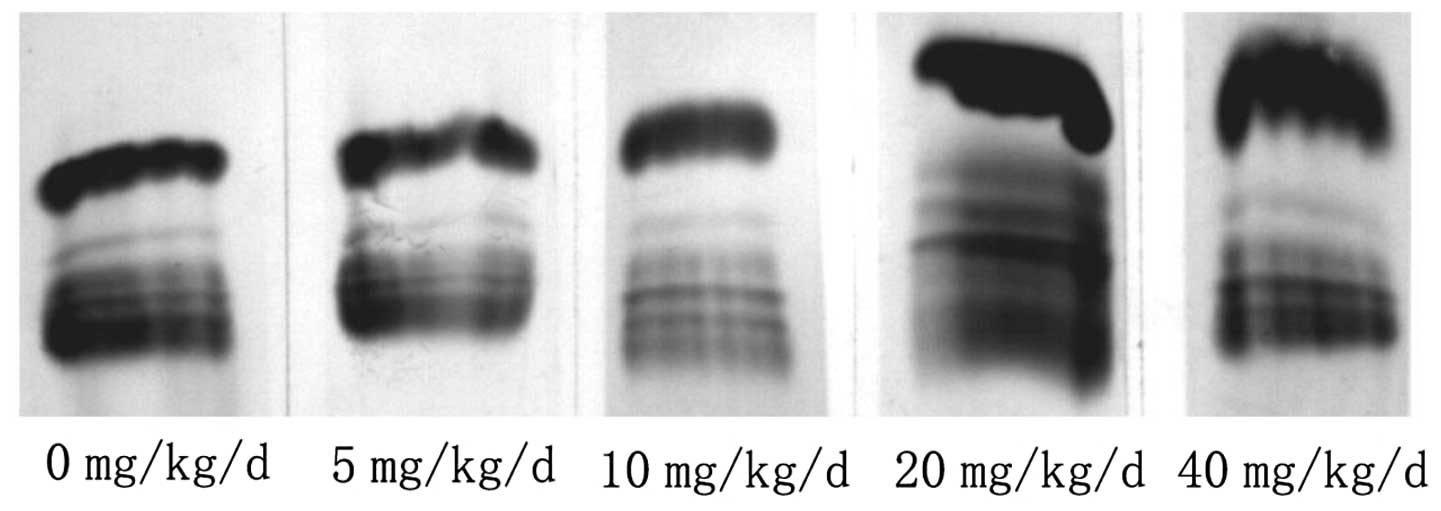
and control mice (Fig. 1). The
fractions that appeared clearly were albumin and α-, β- and
γ-globulins. The red blood cell counts from control and treated
mice are shown in Table III. The
red blood cell counts in mice treated with each of the dichlorvos
concentrations were not significantly different from those of the
controls (P>0.05).
 | Table IIIHematological studies of mice exposed
to dimethoate. |
Table III
Hematological studies of mice exposed
to dimethoate.
| Dimethoate dose
(mg/kg/day)
|
|---|
| Parameter | 0 | 2.5 | 5 | 10 | 20 |
|---|
| Hb (mg/100 ml) | 13.33±1.97 | 12.93±2.13 | 11.17±1.11a | 9.55±1.59a | 8.05±1.67b |
| PCV (%) | 41.0±2.77 | 40.45±3.14 | 37.86±1.08a | 36.4±1.68a | 33.67±0.71b |
| Total plasma
protein (mg/100 ml) | 50.52±6.22 | 50.65±6.01 | 50.32±5.78 | 49.02±6.38 | 49.08±7.67 |
| RBC count
(×1012/l) | 7.41±0.32 | 7.40±0.29 | 7.38±0.82 | 7.39±0.86 | 7.37±0.76 |
Compared with the control mice, mice treated with 5,
10 or 20 mg/kg/day dimethoate showed a change in their hemoglobin
content and PCVs. There was a significant decline in the 5mg/kg/day
group, followed by the 10 mg/kg/day group and in the 20 mg/kg/day
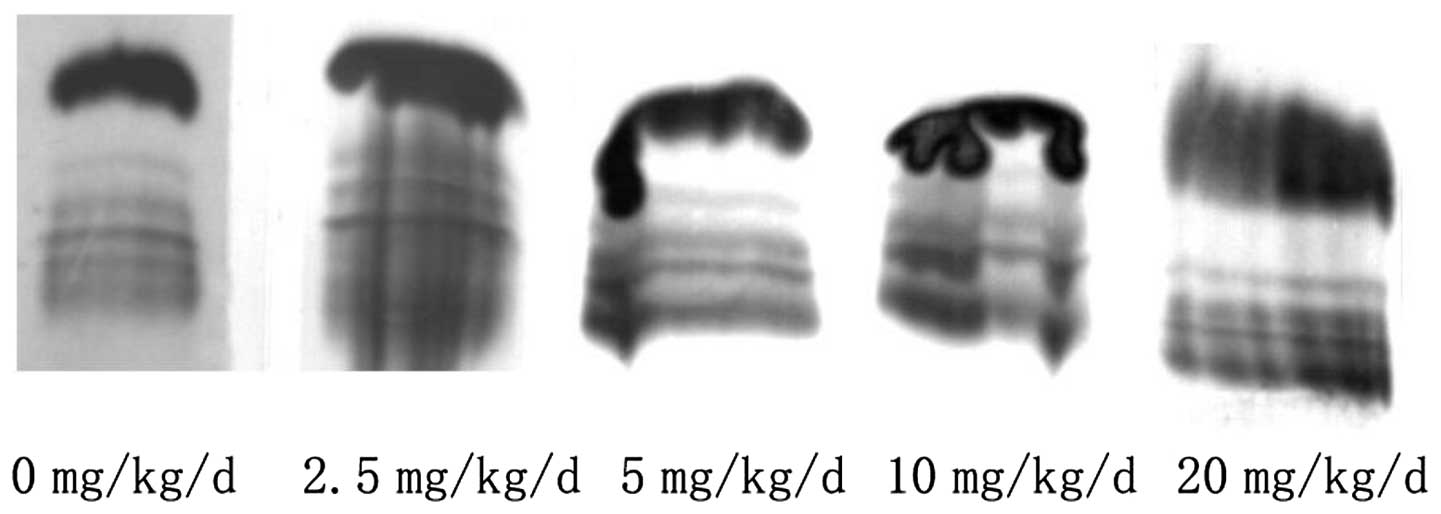
group, the decline was the most evident (Table III). The albumin and α-, β- and
γ-globulins appeared clearly in the electrophoretic fractions for
the total plasma protein and their concentration ratios did not
change significantly (Fig. 2). The
red blood cell counts in mice treated with each of the dimethoate
concentrations were not significantly different from those of the
controls (P>0.05).
Effects of dichlorvos on expression of
the p16, Bcl-2 and c-myc genes
Comparative gene expression analysis of the
p16, Bcl-2 and c-myc genes was carried out
under various concentration stress conditions using qRT-PCR.
Results show mRNA expression levels of the p16, Bcl-2
and c-myc genes induced by various concentration stressors,
normalized against the level of GAPDH (the reference gene). In
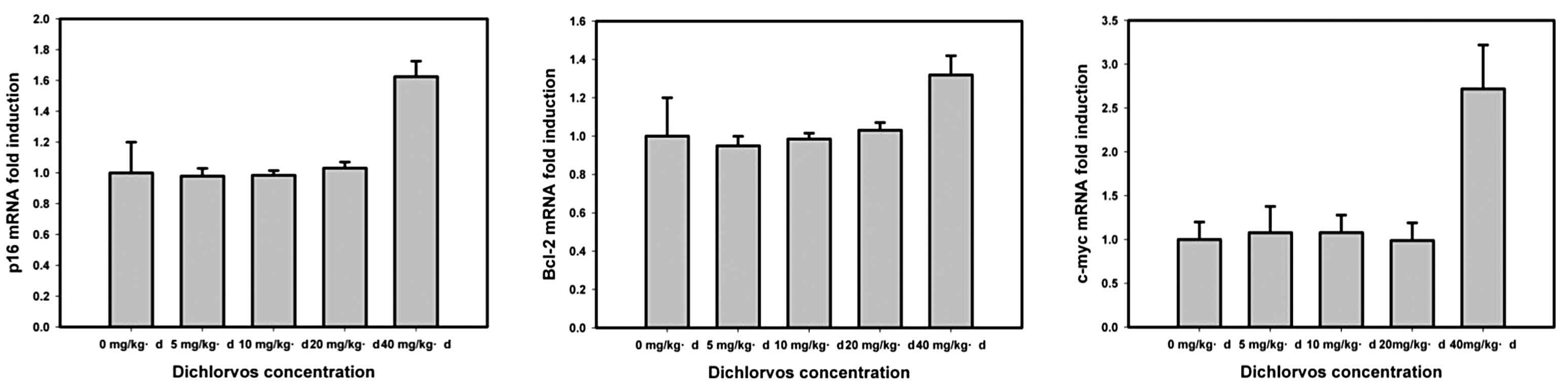
dichlorvos induction, the p16, Bcl-2 and c-myc
expression levels showed no clear change with low dichlorvos
concentrations (<20 mg/kg/day), however, levels subsequently
increased with 20- to 40-mg/kg/day dichlorvos concentrations.
Fig. 3 shows that the effect of
dichlorvos on Bcl-2 (1.3-fold) was the lowest compared with
the upregulated expression fold, the next was p16 (1.7-fold)
and c-myc (2.8-fold) demonstrated the greatest effects.
Effects of dimethoate on expression of
the p16, Bcl-2 and c-myc genes
Expression of the p16, Bcl-2 and
c-myc gene transcripts was assessed after in vivo
exposure to dimethoate. The concentrations used were <1/2 the
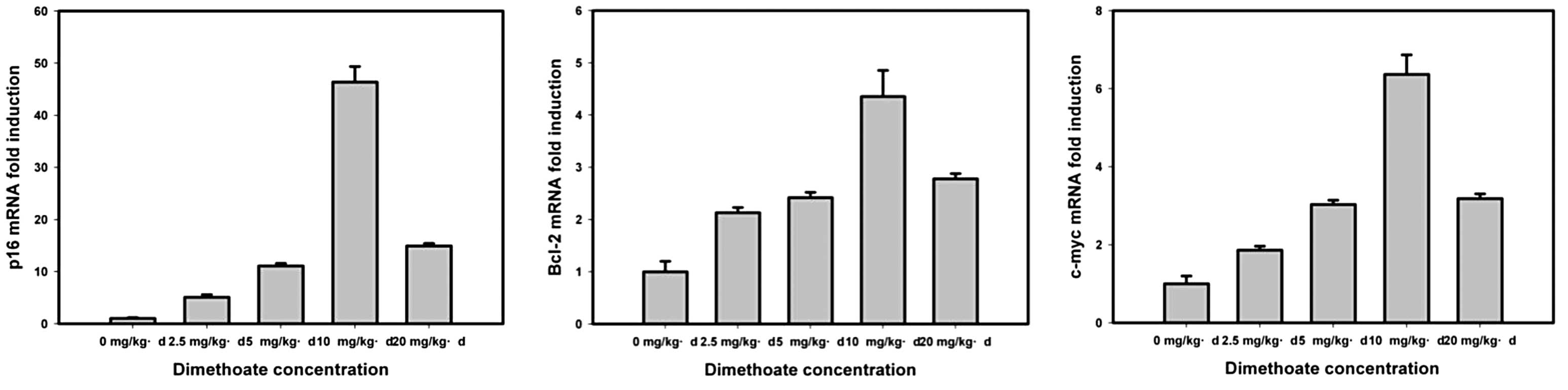
LC50 value of the mouse. In dimethoate induction, the p16,
Bcl-2 and c-myc expression levels gradually increased
with concentrations <10 mg/kg/day and subsequently decreased
with 10- and 20-mg/kg/day dimethoate concentrations (Fig. 4). The effect of dimethoate on the
expression of Bcl-2 (4.5-fold) was the lowest, the next was
c-myc (6.1-fold) and p16 (48-fold) demonstrated the
greatest effects.
Discussion
In the past decade, the incidence of gastric cancer
has been declining, however it remains the fourth most common
cancer and the second most frequent cause of cancer-associated
mortalities (13,14). The reason for the decline in gastric
cancer incidence is possibly due to the recognition of specific
risk factors, including H. pylori, and dietary and
environmental risks. However, gastric cancer is the predominant
type of cancer in farmers in the developing world, particularly
China, and remains a significant cancer burden (15).
In this study, the risks to mouse gastric tissue
were analyzed by continuous exposure to low doses of dichlorvos and
dimethoate. We showed that red blood cell counts and total plasma
protein in these mice were not affected by dichlorvos and
dimethoate, while hemoglobin content and PCVs demonstrated a
significant decline in treated mice when exposed to higher doses of
dichlorvos (20–40 mg/kg/day) and dimethoate (5–20 mg/kg/day). These
results suggest that the pesticides in this study were able to
affect the physiological functions of mice, however, the impacts
were small.
A number of biological markers, including oncogenes,
tumor suppressor genes, cell cycle regulators and DNA repair genes,
were correlated with the genesis, growth, invasion and metastasis
of tumors and they may be used as prognostic factors for tumors
(16). Currently, numerous studies
have evaluated the prognostic significance of markers, such as the
p16, Bcl-2, MTDH and c-myc genes in
gastric cancer (17–19). This study also evaluated expression
of the p16, Bcl-2 and c-myc genes. Results
showed that expression of the p16, Bcl-2 and
c-myc genes in mouse gastric tissue was not affected when it
was exposed to low concentrations of dichlorvos (5, 10 or 20
mg/kg/day), however, there was a significant increase in expression
levels with a 40-mg/kg/day dose of dichlorvos. These results
suggest the risk of tumorigenesis caused by dichlorvos was
substantial, but not high, when mice were continuously exposed to
dichlorvos. The risk of tumorigenesis induced by dimethoate was
higher than for dichlorvos. Low doses of dimethoate were able to
induce expression of the p16, Bcl-2 and c-myc
genes in mouse gastric tissue. There was a positive correlation
between dimethoate concentration and expression levels of the
p16, Bcl-2 and c-myc genes. In the 20
mg/kg/day group, the cause of the apparent decline in expression
levels of these genes may be due to the function of cells being
affected.
In conclusion, cancer hazards from pesticide
residues in food have been much discussed in decades past. Our
results suggest that mouse gastric tissue exposed long-term to low
dose dichlorvos and dimethoate has the potential to become
cancerous. However, the mechanisms of gastric tissue cancerization
induced by pesticide remains to be elucidated by further studies in
the future.
References
|
1
|
Mills PK and Yang RC: Agricultural
exposures and gastric cancer risk in Hispanic farm workers in
California. Environ Res. 104:282–289. 2007. View Article : Google Scholar
|
|
2
|
Perkins CI, Cohen R, Young JL, Schlag R
and Wright WE: Cancer in California: Detailed Site and Histology,
1990–1994. Sacramento: California Cancer Registry; 1997
|
|
3
|
Ishmael J, MacGregor JA and Manley A:
Dichlorvos - a comprehensive review of 11 rodent carcinogenicity
studies. Regul Toxicol Pharmacol. 44:238–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rawlings NC, Cook SJ and Waldbillig D:
Effects of the pesticides carbofuran, chlorpyrifos, dimethoate,
lindane, triallate, trifluralin, 2,4-D, and pentachlorophenol on
the metabolic endocrine and reproductive endocrine system in ewes.
J Toxicol Environ Health A. 54:21–36. 1998. View Article : Google Scholar
|
|
5
|
Begum G and Vijayaraghavan S: Alterations
in protein metabolism of muscle tissue in the fish Clarias
batrachus (Linn) by commercial grade dimethoate. Bull Environ
Contam Toxicol. 57:223–228. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Institóris L, Siroki O and Dési I:
Immunotoxicity study of repeated small doses of dimethoate and
methylparathion administered to rats over three generations. Hum
Exp Toxicol. 14:879–883. 1995.PubMed/NCBI
|
|
7
|
Dell’Omo G and Shore RF: Behavioral
effects of acute sublethal exposure to dimethoate on wood mice,
Apodemus sylvaticus: II - Field studies on radio-tagged mice
in a cereal ecosystem. Arch Environ Contam Toxicol. 31:538–542.
1996.
|
|
8
|
Begum G and Vijayaraghavan S: In vivo
toxicity of dimethoate on proteins and transaminases in the liver
tissue of fresh water fish Clarias batrachus (Linn). Bull
Environ Contam Toxicol. 54:370–375. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silva MA, Ronconi A, Cordeiro N, et al:
Blood parasites, total plasma protein and packed cell volume of
small wild mammals trapped in three mountain ranges of the Atlantic
Forest in Southeastern Brazil. Braz J Biol. 67:531–535. 2007.
|
|
10
|
Spiess BD, Ley C, Body SC, et al:
Hematocrit value on intensive care unit entry influences the
frequency of Q-wave myocardial infarction after coronary artery
bypass grafting. The Institutions of the Multicenter Study of
Perioperative Ischemia (McSPI) Research Group. J Thorac Cardiovasc
Surg. 116:460–467. 1998. View Article : Google Scholar
|
|
11
|
Tilak KS, Veeraiah K and Raju JM: Effects
of ammonia, nitrite and nitrate on hemoglobin content and oxygen
consumption of freshwater fish, Cyprinus carpio (Linnaeus).
J Environ Biol. 28:45–47. 2007.PubMed/NCBI
|
|
12
|
Applied Biosystems: User Bulletin No. 2.
ABI Prism 7700 Sequence Detection System, 1997.
|
|
13
|
Shibata A and Parsonnet J: Stomach cancer.
Cancer Epidemiology and Prevention. Schottenfeld D and Fraumeni JF:
3rd edition. Oxford University Press; New York: pp. 707–720. 2006,
View Article : Google Scholar
|
|
14
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
15
|
Shi J, Zhang G, Yao D, et al: Prognostic
significance of aberrant gene methylation in gastric cancer. Am J
Cancer Res. 2:116–129. 2012.PubMed/NCBI
|
|
16
|
Bashir SA, Pandith AA, Yousuf A, et al:
Lack of p16 gene mutations in gastric cancers in Kashmir. Asian Pac
J Cancer Prev. 11:339–342. 2010.PubMed/NCBI
|
|
17
|
Liu X, Cai H, Huang H, Long Z, Shi Y and
Wang Y: The prognostic significance of apoptosis-related biological
markers in Chinese gastric cancer patients. PLoS One. 6:e296702011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang P, Mei J, Zhang N, Tao J, Tian H and
Fu GH: Helicobacter pylori upregulates the expression of
p16(INK4) in gastric cancer cells. Hepatogastroenterology.
58:846–853. 2011. View
Article : Google Scholar
|
|
19
|
Mitomi H, Fukui N, Kishimoto I, et al:
Role for p16(INK4a) in progression of gastrointestinal stromal
tumors of the stomach: alteration of p16(INK4a) network members.
Hum Pathol. 42:1505–1513. 2011. View Article : Google Scholar : PubMed/NCBI
|